Benefit of MPFF at a dose of 500 mg in chronic venous disease-related edema
Paris – France
INTRODUCTION
Lower-extremity edema is often encountered in clinical practice, and represents a manifestation of a variety of possible disease processes. Edema results from fluid accumulation in the interstitial compartment of the extravascular space.1 This fluid retention clinically is shown by the “pitting” test.
The underlying cause should be actively investigated to optimize treatment. The etiology is multifactorial, revolving around the intricate balance of capillary blood and oncotic pressures, tissue pressures, lymphatic flow, and capillary permeability. Changes in any of these factors can offset the extravascular fluid balance. Hormonal impregnation, plasma and interstitial protein concentration, as well as leukocyte activation, play a role in edema formation. There is increasing evidence that the leukocyte is a key cell in the chronic inflammation which leads to valve damage. This might subsequently increase venous hypertension, which has direct consequences on increased capillary permeability and edema formation.
PREVALENCE OF CHRONIC VENOUS DISEASE-RELATED EDEMA
The edema associated with chronic venous disease (CVD) is the most common type of edema (90%) and is the first sign of microangiopathy.2 The place of edema in the natural course of CVD has not been clearly elucidated.3 CVDrelated edema is assigned to class 3 in the CEAP clinical classification (Table I), which is made up of 7 clinical classes.4,5 Venous edema is considered in the Venous Clinical Severity Score (VCSS) which extents the descriptive clinical CEAP classification and allows scoring of the disease (Table II).6
![]()
Table I. Description of chronic venous disease-related edema according to the CEAP
classification (Adapted from ref 4).
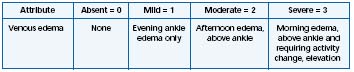
Table II. Scoring of chronic venous disease-related edema according to the Venous Clinical
Severity Score (VCSS) (Adapted from ref 6).
PATHOPHYSIOGENESIS OF EDEMA
Four major mechanisms may be responsible for edema formation:3
– blood pressure increase in the venous system (chronic venous disease or chronic venous insufficiency, and heart failure),
– capillary hyperpermeability with high molecular weight protein and water leakage (endocrine abnormalities, inflammation, and anaphylaxis),
– decrease in lymphatic drainage,
– diminution of plasma protein concentration (in particular hypoalbuminemia) due to proteinuria.
THE DIFFERENT TYPES OF EDEMA1
Lower-limb edema may be unilateral or bilateral. Physical examination and diagnostic evaluation are mandatory to differentiate between different types of lower-extremity edema.
Unilateral lower-extremity edema may be due to
– Lymphedema: the development of lymphedema is a slow, insidious process. Primary lymphedema which may occur as a hereditary (eg, Milroy’s disease) or a sporadic disease starts frequently in the distal part of the extremity. The causes for secondary lymphedema are related to traumatic or surgical disruption of the lymphatic system, or lymphatic obstruction (eg, filaria invasion).
– Other causes:
– Venous abnormalities: acute DVT, post-thrombotic syndrome. The thrombosis often damages the valves with subsequent development of chronic venous disease. The incompetent valves result in transmission to capillaries of high venous pressure, promoting both fluid and protein loss into the interstitial tissues.
– Arterial abnormalities: popliteal artery aneurysms often are associated with lower-extremity edema and congenital or acquired vascular anomalies with arteriovenous fistulas may be first noted as lower-extremity swelling.
– Infection: cellulitis (erysipelas) is frequently associated with some swelling of the affected extremity.
– Trauma: fractures, skin and soft-tissue disruptions, and muscular injuries associated with trauma frequently result in edema of the affected limb.
– Tumors: popliteal cysts, otherwise known as Baker’s cysts, may produce localized and distal swelling of the extremity. The cyst compresses the popliteal vein and subsequently the venous return, which can result in an acute DVT.
Bilateral or unilateral lower-extremity edema may be due to
– Chronic venous disease (CVD): Edema related to CVD generally occurs after prolonged standing, at the end of the day, and is diminished in the morning, by supine position, or with the legs elevated. Also this type of edema may form due to warmth, the summertime season, hot baths, and floor-based heating systems, and improve in winter and with cold temperatures. The CVD related edema is characterized by its diurnal variation, mainly worsened at the end of the day and relieved in the morning, after rest or elevation of the legs. The venous, lymphatic, and capillary networks are often intricate and involved in the appearance of such an edema. A classification has been proposed depending on which system is at the origin of edema formation7 (Table III).
– Other causes:
– Congestive heart failure: bilateral edema can be the early manifestation of right-sided congestive heart failure, which is common with myocardial infarctions.
– Systemic and metabolic abnormalities: bilateral edema may result from secondary liver failure, and in protein deficiency states such as protein-losing gastroenteropathy and severe malnutrition. Acute glomerulonephritis, due to damage to the renal glomerulus, results in altered renal function. The kidney becomes unable to excrete sodium, leading to salt and water retention.
– Endocrine abnormalities: Cushing’s syndrome often results in edema formation.
– Lipedema: predominantly affects women, producing a bilateral deposition of fat in the lower limbs. The feet are typically excluded. The edema is non-pitting and not relieved by leg elevation, contrary to chronic venous disease- related edema.
– Iatrogenic edema: many drugs can cause edema in lower limbs. These include corticosteroids, contraceptive pills, nonsteroidal anti-inflammatory drugs, certain antibiotics, etc.
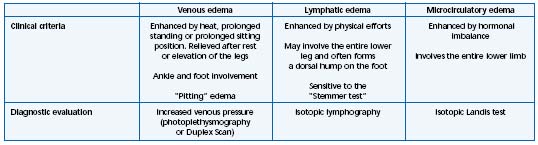
Table III. The different types of nonsystemic edema (Adapted from ref 7).
CLINICAL EVALUATION AND DIAGNOSIS OF EDEMA
The time of onset is an important factor in determining the cause of edema. Sudden onset suggests an acute process such as deep vein thrombosis (DVT), trauma, or infection. Whereas the gradual appearance of edema over weeks or months suggests chronic causes such as chronic venous disease, medications, or a progressive systemic process. Intermittent episodes of edema often occur with recurring erysipelas (cellulitis) or lymphangitis. Systemic disease must be excluded as a cause of peripheral edema. The history and investigations should focus especially on the cardiac, hepatic, and renal functions.
Edema, especially when it is related to chronic venous disease, worsens after prolonged standing and improves after rest. Redistribution of the extracellular fluid occurs after rest or sleeping in a horizontal position. This diurnal variation is typical of the chronic venous disease-related edema.
ASSESSMENT OF CHRONIC VENOUS DISEASE-RELATED EDEMA
– Methods based on volumetry:
• Volumetry by water displacement8 (Figure 1): Archimedes’ principle is applied in this method which postulates that the leg volume is equal to the volume of water displaced. The patient stands up and is requested to place her (his) lower limb in a plexiglas container filled in with water. Usually the dimension of the container, around 50 cm in height, allows the measurement of a volume of 2500 to 4000 mL including foot, ankle, and calf. All sorts of lower-limb edema including those related to CVD and lymphedema can be assessed using this method.
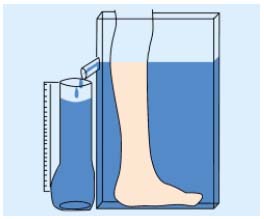
Figure 1. Schematic presentation of the volumeter by water
displacement.
• Optoelectronic volumetry9 (Figure 2): this is the most sophisticated method for assessing edema. The leg passes through a four-sided rigid frame which can be moved along a rail in the long axis of the limb. The frame is equipped with infrared-detecting diodes emitting an infrared beam which allows precise measurement of the lower limb volume. Markers placed on the leg allow the identification of the upper and the lower reference point.
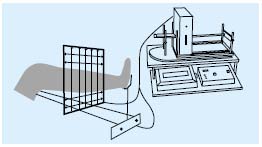
Figure 2. Schematic presentation of the optoelectronic volumeter.
– Methods based on circumference measurement:
The most used methods are the following:
• Spring tape10 consists of a conventional tape-measure locked in a small box. The tape is pulled out of the box, put around the limb to measure and the end of the tape hooked to the box. The tape is automatically tightened by a spring mechanism, guaranteeing that the tightening force is similar with all measurements.
• Leg-O-Meter®11 (Figure 3) is derived from the spring tape. This device takes into account the height at which the measurement is taken, which greatly increases the precision and the reproducibility of the measurement.
• Assessment of lymphatic drainage.
• Indirect lymphography and lymphoscintigraphy are currently used to confirm the involvement of the lymphatic system in the edema formation.
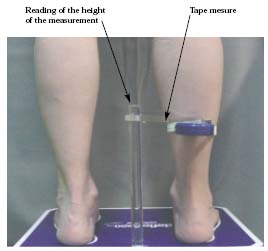
Figure 3. Assessment of ankle circumference with a Leg-O-Meter®.
THE BENEFIT OF MPFF at a dose of 500 mg IN CHRONIC VENOUS DISEASE-RELATED EDEMA
Evaluation of the edema associated with chronic venous disease and the expected benefit of therapy with MPFF at a dose of 500 mg has been evidenced in randomized, controlled studies as well as in open prospective trials.
– Placebo-controlled studies
Ankle and calf circumference measurement was used in the two double-blind, randomized, placebo-controlled studies with MPFF at a dose of 500 mg. In the study by Chassignolle et al,12 a significant decrease in ankle and calf circumferences was observed (P<0.001 for both levels of measurement and for both legs). The protocol used by Gilly et al13 included the measurements of the calf (maximum circumferences) and ankle (minimum supramalleolar circumference) on each affected leg using a spring tape measure. The changes in these parameters were significantly greater in the MPFF at a dose of 500 mg group (-7.15 mm) compared with placebo (-1.15 mm) after 8 weeks of treatment (Figure 4). The decrease in supramalleolar circumference correlated well with the improvement in the sensation of swelling (r=0.56; P<0.001).
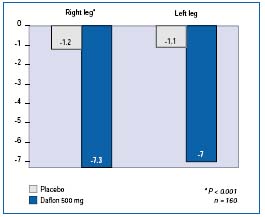
Figure 4. Ankle circumference of affected legs. Mean decrease
(in mm) after 2 months of treatment with MPFF at a dose of 500 mg (in blue)
or placebo (in grey), 2 tablets/day (Adapted from ref 13).
– Open trials
In the multicenter RELIEF study14 in which 5052 patients assigned C0s to C4 according to the clinical CEAP classification, edema was assessed by the Leg-O-Meter®. A significant improvement (P<0.0001) was observed in patients with and without venous reflux (Figure 5).
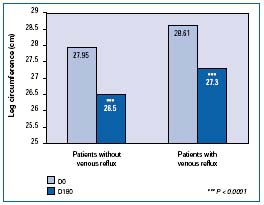
Figure 5. Leg circumference measured with Leg-O-Meter® in
the Reflux assEssment and quaLity of lIfe improvEment with
micronized Flavonoids (RELIEF) study. Comparison between
patients with a reflux and patients without reflux
(Adapted from ref 14).
In 20 patients with CVD stage I-II of the Widmer’s classification, the optoelectronic method was used for assessing edema.15 Nine patients had post-thrombotic syndrome and 11 had varicose veins. Patients with varicose veins showed a significant decrease in the volume of the leg that was more affected (-392 mL; P<0.001). For all patients and for the more affected leg, the decrease was of 263 mL (P<0.05).
– Comparative study
Cospite compared MPFF at a dose of 500 mg with diosmin and evaluated edema with a simple tape measure.16 The measurements of ankle and calf circumferences revealed significantly better efficacy in the MPFF at a dose of 500 mg group (P<0.001) (Figure 6). This difference was also found for several parameters of strain-gauge plethysmography (with P ranging from P=0.05 to P<0.001).
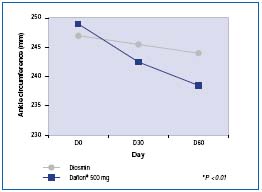
Figure 6. Decrease in ankle circumference (in mm: comparison
between two treatment groups MPFF at a dose of 500 mg and diosmin
at the daily dose of 2 tablets (Adapted from ref 16).
MPFF at a dose of 500 mg: THE REFERENCE TREATMENT QUOTED IN GUIDELINES
In recent guidelines17,18 or extensive reviews19 on the treatment of chronic venous disease, MPFF at a dose of 500 mg has been quoted as a better-established and well-tolerated anti-edema drug. MPFF at a dose of 500 mg is indicated as the firstline treatment of edema and the associated chronic venous disease-related symptoms (eg, edema, fatigue, nocturnal cramps, and heaviness) at any stage of the disease.
REFERENCES
2. Vayssairat M. The causes of edema in chronic venous insufficiency. Phlebolymphology. 2003;41:168-176.
3. Garde C. First consensus meeting on venoactive agents and review of their clinical benefits. Phlebolymphology. 2005;49:384-396.
4. Allegra C, Antignani PL, Bergan JJ, et al. The “C” of CEAP: Suggested definitions and refinements: An International Union of Phlebology conference of experts. J Vasc Surg. 2003;37:129-131.
5. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg. 2004;40:1248-1252.
6. Rutherford RB, Padberg FT Jr, Comerota AJ, et al. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307-1312.
7. Blanchemaison P. Physiopathologie et classification des oedèmes vasculaires [in French]. Angeiologie. 2000;52:47-50.
8. Vayssairat M, Maurel A, Gouny P, et al. La volumétrie à eau : une méthode précise de quantification en phlébologie. J Mal Vasc. 1994;19:108-110.
9. Tierney S, Aslam M, Rennie K, et al. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12:412-417.
10. Pani SP, Vanamail P, Yuvaraj J. Limb circumference measurements for recording edema volume in patients with filarial lymphedema. Lymphology. 1995;28:57-63.
11. Berard A, Kurz X, Zuccarelli F, et al. Validity of the Leg-O-Meter®, an instrument to measure leg circumference. Angiology. 2002;53:21-28.
12. Chassignolle JF, Amiel M, Lanfranchi G, et al. Activité thérapeutique de MPFF at a dose of 500 mg dans l’insuffisance veineuse fonctionnelle. JIM. 1987;99(suppl):32-35.
13. Gilly R, Pillion G, Frileux C. Evaluation of a new vasoactive micronized flavonoid fraction (S 5682) in symptomatic disturbances of the venolymphatic circulation of the lower limb: a doubleblind, placebo-controlled study. Phlebology. 1994;9:67-70.
14. Jantet G, and the RELIEF Study Group. Chronic venous insufficiency – worldwide results of the RELIEF study. Angiology. 2002;53:245-256.
15. Blume J, Langenbahn H, De Champvallins M. Quantification of oedema using the volometer technique: therapeutic application of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology. 1992;(suppl 2):37-40.
16. Cospite M, Dominici A. Advantage of micronisation of MPFF at a dose of 500 mg compared with a simple diosmin in the treatment of venous insufficiency. Double blind study. Phlébologie. 1998;51:243-247.
17. Italian College of Phlebology. Guidelines for the diagnosis and treatment of chronic venous insufficiency. Int Angiol. 2001;20(2 Suppl 2):1-37.
18. Coleridge Smith PD. The drug treatment of chronic venous insufficiency and venous ulceration. In: Gloviczki P, Yao JST, editors. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 2nd ed. London: Arnold; 2001:309-321.
19. Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
