2020 Update to classification of chronic venous disorders
of Vascular Surgery at the University
of Michigan, Ann Arbor,
Michigan, USA.
Abstract
In 2017, the American Venous Forum (AVF) created a task force to determine if the CEAP classification needed a revision. An extensive literature review led the task force to conclude that there was sufficient evidence to update it to align with the newest knowledge of chronic venous disorder (CVD) and to clarify terminology. Using the modified Delphi methodology, the AVF task force concluded its 2-year project by publishing the CEAP 2020 update, which also became a reporting standard for studies of patients with CVD. The updated CEAP classification remains a discriminative instrument designed to describe the signs and symptomatic status of each limb of a patient with CVD at a specific time point. The CEAP 2020 update added a subclass C4c for corona phlebectatica. This modification reflects current understanding that corona phlebectatica has a similar natural history to the C4a and C4b subclasses. Another update for the “C” component is a modifier “r” describing recurrent varicose veins (C2r) or recurrent venous ulcer (C6r). The update for the “E” component of CEAP includes creation of two subclasses for secondary CVD (Es) as follows: (i) Esi–intravenous causes; and (ii) Ese–extravenous causes. Finally, the numbering of the venous segments in the “A” component of the CEAP is replaced by commonly used anatomical abbreviations.
Introduction
Classifications of diseases and pathological conditions have a very long history. Perhaps the first practical classification was developed in 1662 by John Graunt who published an index of causes of mortality. A century later in 1768, François Boissier de Lacroix developed a systematic classification of all known diseases at that time. Around the same time, in 1780, William Cullen published the classification of disease that became widely used by clinicians, especially in the United Kingdom. As multiple classifications began to emerge, the need for a unified single classification became apparent. William Farr wrote in 1839, “the advantages of a uniform nomenclature, however imperfect, are so obvious as weights and measures in the physical sciences. It should be settled without delay and kept without change.” This need was addressed in 1855 at the International Statistical Congress in Paris, where Mark D’Espine and William Farr established the first international classification of diseases–a compromise between Farr’s phenotypical approach and d’Espine’s pathological approach to classification.
This international classification of diseases is an example of a descriptive classification that defines distinct diseases and conditions for public health and statistical purposes. Clinical classification is similar to descriptive classification in that it defines distinct diseases or conditions, but perhaps its greater purpose is to standardize communication among practitioners and clinical researchers. As a descriptive tool, clinical classification defines diseases based on their phenotypical manifestations, such as symptoms and signs. However, to address the needs of clinical practice, these disease definitions should be connected to treatment options. Evolving knowledge of the pathological mechanisms of diseases does not justify a change in a clinical classification until the treatment options targeting specific mechanisms become available. Oncologic classifications exemplify a transition from empirical phenotypical clinical classification to molecular classifications of cancer that are based on both an understanding of pathological mechanisms and the availability of therapeutics targeting these mechanisms.
CEAP classification of CVD
The current understanding of CVDs includes knowledge of key pathological mechanisms, such as reflux and obstruction, that can be targeted by interventions in some anatomical locations. It also includes empirical knowledge that some of the CVD phenotypes have a similar natural history and impact on a patient’s quality of life. However, the biological and pathological basis for these phenotypes is poorly understood. This complex situation has required a different classification. First introduced in 1996, the Clinical-Etiological-Anatomical-Pathophysiological (CEAP) classification addressed the complexity of CVD by incorporating four different taxonomical approaches. The clinical class “C” is a description of signs and the symptomatic status of a lower extremity (LE). These clinical classes are based on the most frequently seen manifestations of CVD that also have a similar natural history. The “E” (etiology) of the CEAP reflects the current understanding of what causes the signs and symptoms in an affected LE. The “A” of CEAP describes which anatomical segments of the LE venous systems are affected. Finally, the “P” (pathophysiology) describes identified hemodynamic abnormalities in the affected anatomical segments. Because of the complexity associated with CVD, an individual component of the CEAP classification alone cannot provide an appropriate clinical description of an affected LE, but a combination of the components gives the clinician a more complete understanding of each patient’s disease and guides the subsequent clinical management.
Evolving CEAP classification: 2004 revision
The introduction and wide use of the CEAP classification has made it possible to conduct large epidemiological studies and develop a set of clinical practice guidelines.1-4 Global use of this uniform classification has led to multiple comparable studies that have provided new evidence and improved our understanding of CVD. As new knowledge has developed, the classification itself has required revisions and updates. Thus, significant revision of the CEAP was done in 2004.5 Although that revision substantially improved the classification, the transition to a new version of CEAP took several years. Studies that were initiated before the revision continued to report their findings using the previous version, whereas some publications were utilizing the revised classification. The experience suggested that future revisions of CEAP should be backward compatible, so the revised version of the CEAP may add more specific subcategories but leave the previous categories unchanged.
Evolving CEAP classification: 2020 update
In 2017, the American Venous Forum (AVF) created a task force to determine if the CEAP needed further revision. An extensive literature review led the task force to conclude that there was sufficient evidence to update it to align with the newest knowledge of CVD and to clarify terminology. The task force was extended to include four groups, each group to focus on one of the four components of the CEAP. The advisory group of experts who participated in the creation and previous revision of CEAP was assembled to ensure continuity of the process (Table I). Realizing that revision of the CEAP is essentially a consensus process, the modified Delphi methodology was used.6 During a 2-year process with multiple discussions, several proposed changes were rejected because they either lacked supportive evidence, violated one of the predefined revision criteria, or affected practicality of using the CEAP. These rejected proposals are described in the CEAP 2020 publication.7
Table I. The CEAP (clinical, etiological, anatomical, and pathophysiological classification) Task Force of the American Venous Forum.
The updated CEAP classification remains a discriminative instrument designed to describe the signs and symptomatic status of each limb of a patient with CVD at a specific time point. Manifestation of CVD changes significantly over time, so the same patient may have a different CEAP description at different time points. The interpretation of such changes is beyond the ability of discriminatory instruments, and the CEAP cannot and should not be used to interpret these changes as improvement or deterioration. These terms require evaluatory instruments capable of measuring the disease severity and its change over time or as a result of an intervention. The Venous Clinical Severity Score (VCSS) is an example of such an instrument.
All four components of the CEAP should be treated as nominal variables. This includes the clinical class “C” and its subclasses. It is not appropriate to state that a patient with a manifestation of CVD classified as C4 has a more severe condition than a patient classified as C2. This also applies to the subclasses of the CEAP. The CEAP 2020 update added a subclass C4c for corona phlebectatica. This modification reflects current understanding that corona phlebectatica has a similar natural history to the C4a and C4b subclasses. It was assigned to “c” subclass of C4 in order to preserve the previous version of CEAP, so the C4a and C4b subclasses remain unchanged. This order of subclasses reflects neither the severity of disease nor a different prognosis. Another update for the “C” component is a modifier “r” describing recurrent varicose veins (C2r) or recurrent venous ulcer (C6r).
The update for the “E” component of CEAP includes creation of two subclasses for secondary CVD (Es). The CEAP 2020 separates intravenous and extravenous causes of the Es. Intravenous causes are conditions that are caused by venous wall or valve damage. Intravenous subclass Esi includes venous wall and/or valve damage caused by deep-vein thrombosis (DVT), primary intravenous sarcoma, or other intravenous lesions. Extravenous causes are pathological conditions that affect venous hemodynamics locally or systematically but are not located in the venous wall or venous lumen. The extravenous subclass of the Es includes CVD caused by congestive heart failure, external vein compression, perivenous fibrosis, muscle pump dysfunction (paraplegia, arthritis, chronic immobility, frozen ankle, or severe sedentary state), and obesity.
CEAP: classification of CVD, not syndromes
The CEAP is a classification of CVDs, not syndromes. The difference becomes clear when comparing the CEAP definition of the secondary etiology of CVD and the definition of the post-thrombotic syndrome (PTS). Acute DVT can damage venous valves causing reflux–which will be classified as Esi; Ad; Pr by the CEAP–or cause an obstruction to venous flow by intravenous organized thrombus or synechia–which will be classified as Esi; Ad; Po. Each of these descriptions are specific to the sequelae of the DVT. In contrast, the definition of the PTS is based on a combination of symptoms and signs that are not specific, and in more than 50% of patients, are not related to the sequelae of DVT but are caused by preexisting primary CVD.8,9 This means that studies that use the PTS as an outcome, such as the SOX (Compression Stockings to Prevent Post-Thrombotic Syndrome) and ATTRACT (Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis) trials, are subject to significant misclassification bias.
Use of CEAP 2020
As with the previous versions, CEAP 2020 can be used in two different ways. The abbreviated CEAP lists the highest clinical class with the symptomatic status (“s” for symptomatic, “a” for asymptomatic). This is followed by the description of etiology (congenital, primary, or secondary), anatomy (superficial, deep, perforators, or their combination), and pathology (reflux, obstruction, or their combination). Such descriptions provide minimum information about the patient but still may be sufficient for some purposes. The complete CEAP provides more specific information that is frequently sufficient for clinical management decision.
For example, two patients (Figure 1 A, B) with a healed ulcer in the left leg can be described as LLE (left LE): C5s; Es; Ad; Po by the abbreviated CEAP. Such description indicates that both patients have a healed ulcer, are symptomatic, and have secondary venous disease caused by obstruction in the deep veins. However, the complete CEAP description of these patients may be very different. The first patient is described as LLE: C3,5s; Ese; Ad; PoCIV. This patient has edema and a healed ulcer caused by extravenous obstruction of the left common iliac vein (May-Thurner Syndrome) and requires a work-up for possible iliac vein angioplasty and stenting. The second patient is described as LLE: C4b,5s; Esi; Ad; PoFV,POPV. He has lipodermatosclerosis and post-thrombotic obstruction of the left femoral and popliteal veins and is unlikely to be treated surgically. A complete CEAP provides all the information that otherwise would be missed.
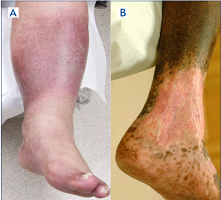
Figure 1. Left lower extremities (LLE) of two patients who can be described as LLE: C5s; Es; Ad; Po. The complete clinical, etiological, anatomical, and pathophysiological (CEAP) classification for patient A is LLE: C3,5s; Ese; Ad; PoCIV (edema and a healed ulcer caused by extravenous obstruction of the left common iliac vein; May-Thurner Syndrome). The complete CEAP classification for patient B is LLE: C4b,5s; Esi; Ad; PoFV, PoPV (lipodermatosclerosis and post-thrombotic obstruction of the left femoral and popliteal veins).
As with any other instrument, the CEAP has a number of limitations. Future revisions and updates on the CEAP classification may include some of the proposed modifications that have been rejected by the task force. It may be considered, for example, that some of the CEAP classes should include subcategories for the complications. A sufficient level of evidence is required for such revisions, including establishing the incidence of such complications in each of the specific CEAP classes and how they change the natural history of the CVD.
Conclusions
Although an imperfect instrument, the CEAP has proven to be an essential tool for practitioners and clinical researchers. Its worldwide utilization since 1996 has contributed to substantial progress in our understanding of CVD and development of new treatment options. Ultimately it has led to improved outcomes in the management of patients with venous disorders. CEAP 2020 is the evidence-based update of the CEAP classification that reflects the progress of the field of phlebology during the last two decades.
REFERENCES
1. Wrona M, Jöckel KH, Pannier F, Bock E, Hoffmann B, Rabe E. Association of venous disorders with leg symptoms: results from the Bonn Vein Study 1. Eur J Vasc Endovasc Surg. 2015;50(3):360- 367.
2. Robertson L, Lee AJ, Evans CJ, et al. Incidence of chronic venous disease in the Edinburgh Vein Study. J Vasc Surg Venous Lymphat Disord. 2013;1(1):59-67.
3. Gloviczki P, Comerota AJ, Dalsing MC, et al; Society for Vascular Surgery; American Venous Forum. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 suppl):2S-48S.
4. O’Donnell TF Jr, Passman MA, Marston WA, et al; Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014;60(2 suppl):3S- 59S.
5. Eklöf B, Rutherford RB, Bergan JJ, et al; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40(6):1248-1252.
6. Dalkey N, Helmer O. An experimental application of the Delphi method to the use of experts. Manag Sci. 1963;9(3):458-467.
7. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-352.
8. Galanaud JP, Holcroft CA, Rodger MA, et al. Comparison of the Villalta post-thrombotic syndrome score in the ipsilateral vs. contralateral leg after a first unprovoked deep vein thrombosis. J Thromb Haemost. 2012;10(6):1036- 1042.
9. Ning J, Ma W, Fish J, Trihn F, Lurie F. Biases of Villalta scale in classifying post-thrombotic syndrome in patients with pre-existing chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2020;21:S2213-S2333.hypertension and the inflammatory cascade: major manifestations and trigger mechanisms. Angiology. 2005;56:S3-S10.

