Atrophie blanche: a diagnostic and therapeutic challenge
Elena Conde Montero, MD, PhD
Dermatology Department, Hospital Universitario Infanta Leonor y Virgen de la Torre, Madrid, Spain
Alejandro Vivero, MD
Wound Clinic, Santo Cuore, Córdoba, Argentina
ABSTRACT
Atrophie blanche (AB) is a distinctive dermatological finding characterized by stellate, porcelain-white, atrophic plaques, commonly located on the lower extremities. Although traditionally regarded as a permanent sequela of chronic venous insufficiency (CVI), AB may also arise from diverse etiologies including livedoid vasculopathy, vasculitis, and medicationinduced vascular damage. This review aims to clarify the clinical significance of AB, emphasizing the importance of accurate diagnosis and etiology-based management. AB is not a diagnosis itself but a marker of underlying vascular pathology; thus, a thorough clinical assessment— including duplex ultrasound and, in some cases, histopathological and immunological studies—is essential. Management strategies differ depending on the cause: compression therapy and venous interventions for CVI; anticoagulation for livedoid vasculopathy; and drug cessation in cases of hydroxyurea-induced ulcers. Adjunctive treatments such as punch grafting and advanced wound care can enhance healing and reduce pain of ulcerated AB. Multidisciplinary care is critical for optimal outcomes. Although AB is often associated with difficult-to-heal ulcers, emerging evidence suggests that, with targeted treatment, reversal of lesions might be possible. Further research is needed to better understand the pathophysiology and to establish standardized therapeutic protocols.
Introduction
Among practitioners, there is significant confusion regarding the concept of atrophie blanche, which frequently results in misdiagnosis and, consequently, therapeutic failure. For many professionals, due to a lack of awareness, it is erroneously regarded as synonymous with livedoid vasculopathy.
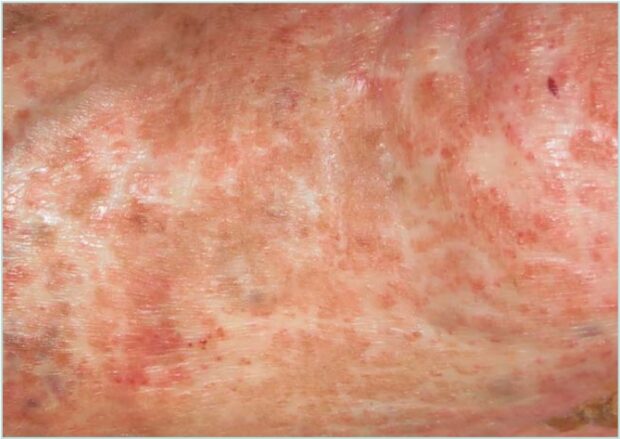
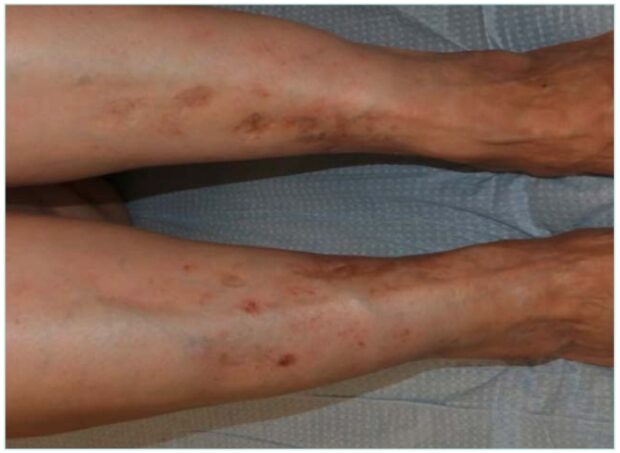
Atrophie blanche (AB) is the clinical term used to describe stellate, ivory-white atrophic plaques with central punctate erythema representing dilated capillaries (Figure 1). These lesions typically occur on the lower extremities, particularly the legs and feet, and may result from various underlying etiologies. It is a descriptive dermatological finding rather than a pathognomonic feature of a specific condition, as it can be associated with multiple clinical scenarios, including chronic venous insufficiency (CVI), livedoid vasculopathy, and vasculitis. As such, a thorough clinical history and examination are essential upon identification.1 An early adequate diagnosis is essential considering that AB lesions are often painful, and the presence of ulcers within these areas is associated with impaired wound healing and a chronic course. Understanding the link between the characteristic scar-like lesions and their potential to ulcerate is crucial for timely diagnosis and effective management.
Due to the limited number of studies available, this review does not follow a systematic approach and, to enhance its applicability in daily clinical practice, incorporates the authors’ own observations.
Historical background
AB was initially identified in 1929 by Milian,2 who described it as a unique type of skin atrophy known as “atrophie blanche en plaque.” This first description highlighted that it typically presents as smooth, porcelain-white patches or plaques located on the lower limbs, often surrounded by a pigmented border and visible dilated capillaries (telangiectasias).2,3 Milian originally associated these lesions with infectious diseases such as syphilis and tuberculosis. However, further research by Gonin4 demonstrated that approximately 70% of cases were more accurately linked to vascular disorders.
Later, Gougerot and Hamburger proposed that these lesions resulted from chronic capillaritis and categorized AB as a variant of stasis dermatitis.5 In 1950, Nelson6 contributed a histological perspective, describing the presence of fibrin occlusions within small blood vessels, reinforcing the idea of an underlying microvascular pathology.
Pathogenesis
The development of AB is primarily attributed to microvascular injury, particularly thrombosis of the capillaries within the subpapillary vascular plexus. This occlusion leads to localized ischemia, resulting in the characteristic ivory-white patches seen in affected skin. As a compensatory response, dilated capillaries or megacapillaries—often observed as red dots—may emerge within these hypoxic areas as part of a dysfunctional repair mechanism. Consequently, any disorder that produces microcirculatory flow diminution can cause tissue infarction and these consecutive scar areas.7
Several underlying conditions can contribute to or exacerbate this vascular damage. CVI is one of the most common associated disorders, where persistent venous hypertension leads to endothelial dysfunction, leukocyte trapping, and microthrombi formation.1 Moreover, a scar from a previous venous leg ulcer can also be considered AB.1
It is not uncommon to find erythematous-purpuric or brownish macules adjacent to areas of AB. These may evolve into papules or plaques, which can become verrucous and eventually ulcerate. Such lesions are referred to as acroangiodermatitis secondary to CVI (Figure 2). They represent a reactive proliferation of small blood vessels in response to longstanding vascular dysfunction, most commonly poorly controlled venous hypertension.8
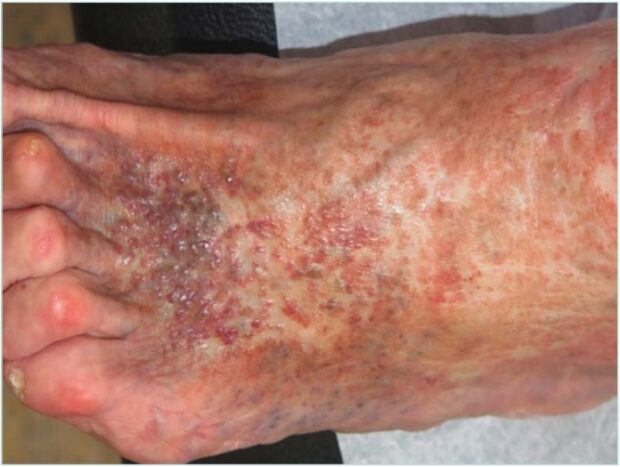
Figure 2. Clinical picture of acroangiodermatitis associated with atrophie blanche in patients with chronic venous insufficiency.
It is essential to provide a more in-depth analysis to understand the role of microangiopathy secondary to CVI in the pathogenesis of AB. The severity of microangiopathy in patients with CVI determines the extent of the trophic disturbances of the skin. In the initial stages of CVI, the microangiopathy is characterized by dilatation of the blood capillaries. In more severe stages of CVI, microthrombosis of capillaries can be found contributing to the locally reduced capillary density. Most likely, these microthromboses are the consequence of interactions between leukocytes and other blood cells with the vessel wall of postcapillary venules. The capillaries are stuffed with red blood cell aggregates, which would not be the case if thrombosis were to start in the terminal arterioles or at the arterial side of the capillary loop.9 In such cases, the capillaries are void of corpuscular blood elements. At the border zones of AB spots, which are potential sites for venous ulcer formation, the capillary density is significantly reduced, and the capillaries are even more tortuous than in the mild stages of the disease. In these cases, the increase in capillary size is most likely necessary to increase the exchange area between intra- and extravascular compartments for fluids and soluble substances. Similar changes in capillary morphology are observed at cutaneous scar sites after injuries.9
In the center of AB, avascular fields are present, which result in enlarged diffusion distances and in significantly prolonged diffusion times.10
A condition that often comes to mind—and, as previously mentioned, is sometimes mistakenly used synonymously with AB—is livedoid vasculopathy, a rare thrombo-occlusive disorder primarily affecting the superficial dermal vasculature. Livedoid vasculopathy has been given multiple names in the literature including livedo vasculitis, segmental hyalinizing vasculitis, livedo reticularis with summer ulceration, Milian white atrophy, AB en plaque, and PURPLE (painful Purpuric Ulcers with Reticular Pattern of Lower Extremities), among others.1
It typically presents with recurrent, painful ulcerations on the lower extremities that eventually heal into ivorywhite, atrophic scars resembling those seen in AB. Although the clinical appearances can be similar, the underlying pathophysiology of livedoid vasculopathy is more strongly associated with hypercoagulable states and primary vascular occlusion, rather than venous insufficiency.7
The occlusion of blood vessels by fibrin thrombi is a primary event in livedoid vasculopathy. There is evidence for a decrease in fibrinolytic activity and an increase in thrombogenic activity. Several cases have been reported in association with other thrombophilias, such as with the lupustype anticoagulant, hyperhomocysteinemia, increased levels of anticardiolipin, protein C deficiency, cryoglobulinemia, factor V Leiden mutation, plasminogen activator inhibitor 1 promoter mutation, and antithrombin III deficiency.11
Additionally, certain medications, such as hydroxyurea, have been implicated in the pathogenesis of AB. The underlying mechanisms behind the development of ulcerated AB in patients treated with hydroxyurea remain unclear. However, this antineoplastic agent, commonly used in myeloproliferative syndromes, also inhibits epidermal cell turnover and may induce microvascular alterations.
Clinical features of ulcerated atrophie blanche
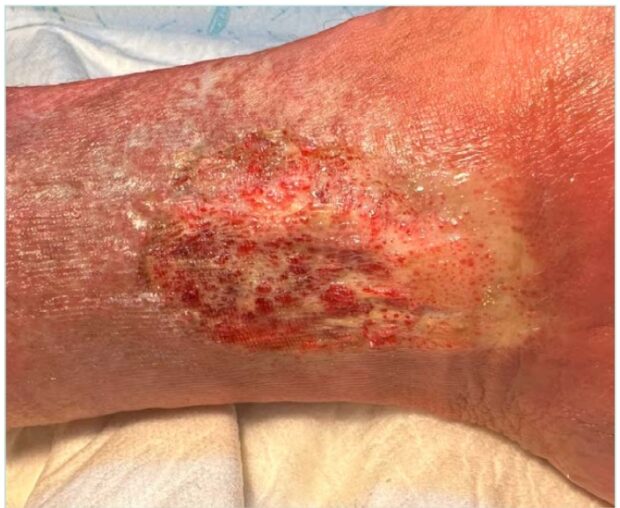
Discussing AB inevitably involves addressing skin ulcers, as these lesions not only represent the possible sequelae of previous ulceration—as observed in livedoid vasculopathy— but also constitute a clinical context in which new ulcers may develop. This is particularly evident in CVI, where AB often precedes the onset of ulcers that are typically very painful and resistant to treatment (Figure 3).
Although ochre dermatitis, due to its dark coloration, may appear more severe than AB, the opposite is actually true. The appearance of AB indicates a more advanced stage of venous insufficiency, and the ulcers that develop in this context tend to be more painful and resistant to treatment than those on legs with ochre dermatitis.
In the context of CVI, ulcerated AB typically presents as a superficial wound with a sclerotic, whitish-yellow base, often punctuated by small, highly vascularized areas.
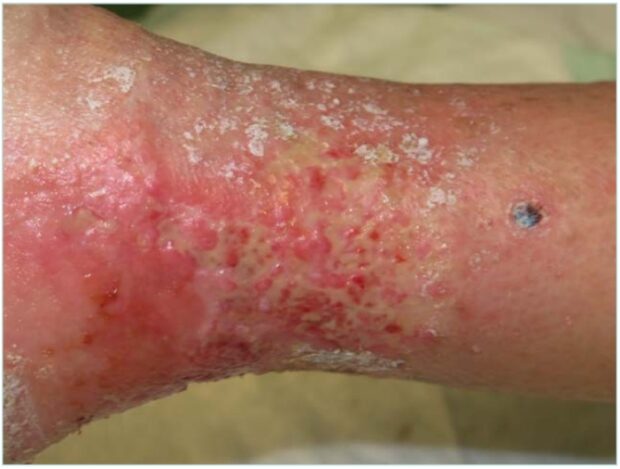
Regarding livedoid vasculopathy, the typical clinical presentation consists of painful purpuric reticular lesions, most commonly affecting middle-aged women. These lesions typically appear on the dorsum of the feet and the inframalleolar region, and often progress to ulceration and the formation of AB.1 Since livedoid vasculopathy progresses in flare-ups, it is typical to find small pinpoint-crusted ulcers mixed with areas of AB patches in the periwound areas with associated telangiectasia (Figure 4). The clinical appearance of livedo reticularis may relate to dilated or contracted blood vessels in addition to hyalinizing disorders.1
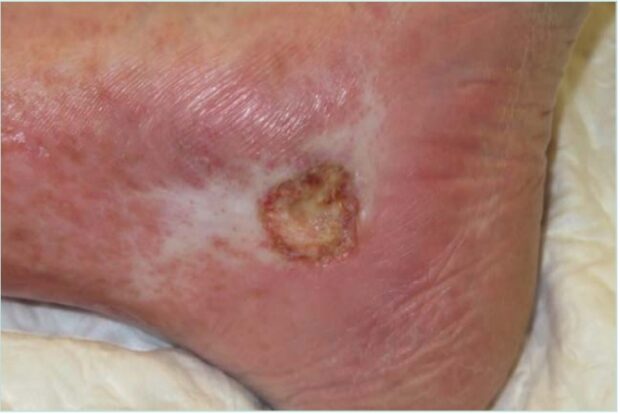
Considering patients receiving hydroxyurea treatment, resulting ulcers are typically very painful, tend to appear after several years of treatment, and may sometimes be triggered by minor trauma.12 They are often multiple and bilateral, typically developing in the perimalleolar region. They are generally well-defined and shallow with an adherent, yellow, fibrinous base (Figure 5).
Diagnosis of ulcerated atrophie blanche and its underlying conditions
The diagnosis of ulcerated AB requires a comprehensive approach. Whereas the patient’s medical history—including current and past medications—is important, the cornerstone of diagnosis is clinical examination. The presence of clinical signs of venous insufficiency typically suggests an underlying venous etiology, which is the most common cause of AB. Clinically, these patients fall under stage C4b of the CEAP (clinical, etiological, anatomical, pathophysiological) classification, which includes skin changes such as AB.13 In this context, a venous duplex ultrasound (Doppler ultrasound) is strongly recommended to assess for underlying chronic venous disease and guide further management. A skin biopsy is not necessary for diagnosis, but it would typically reveal dermal fibrosis, dilated and proliferated capillaries in the upper dermis, and hyperpigmentation of the basal layer of the epidermis or melanin-laden melanophages in the dermal papillae.1
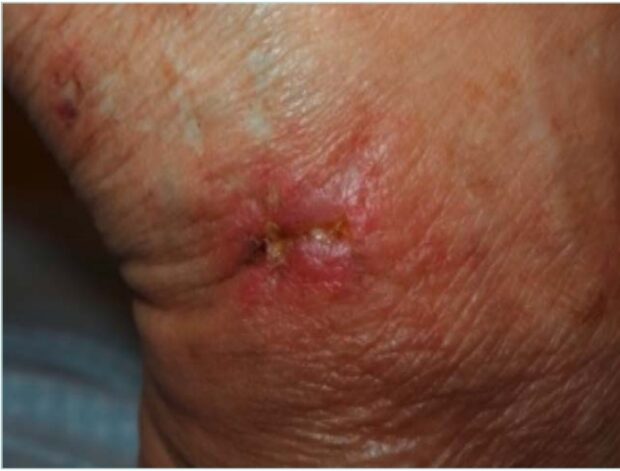
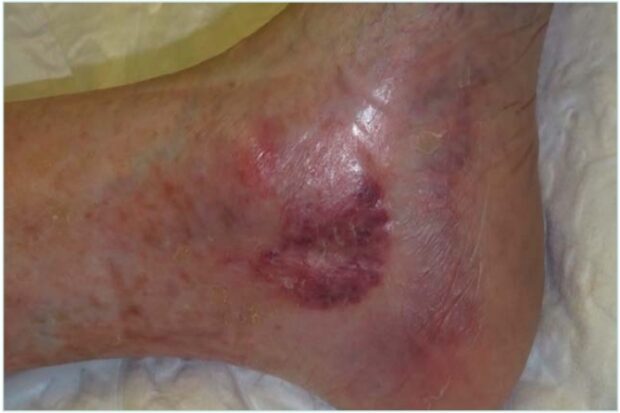
However, in middle-aged women, when AB appears following previous ulcers located in the distal third of the lower extremities, livedoid vasculopathy should be strongly considered (Figure 6). This condition can mimic other forms of chronic ulceration and warrants a more specific diagnostic approach.7
Consequently, in patients presenting with AB in the absence of CVI, a comprehensive diagnostic work-up is essential to identify potential underlying conditions and guide appropriate treatment.14 A skin biopsy remains the gold standard for confirming the diagnosis, typically revealing intraluminal fibrin deposition, thrombosis, segmental hyalinization, and endothelial proliferation.7,14 Given the thrombotic nature of livedoid vasculopathy, thrombophilia screening is recommended and should include assessment of protein C and S, antithrombin III, Factor V Leiden mutation, prothrombin gene mutation, antiphospholipid antibodies (including anticardiolipin antibodies and lupus anticoagulant), and homocysteine levels. Di Giacomo et al demonstrated procoagulable laboratory abnormalities in 52% of their study population.15
Autoimmune markers such as antinuclear antibodies (ANA), anti-SSA/SSB antibodies, and rheumatoid factor should be considered due to potential overlap with autoimmune diseases. Direct immunofluorescence (DIF) may also assist in distinguishing livedoid vasculopathy from other vasculopathies, with findings including vascular deposition of immunoreactants such as C3 and immunoglobulin M (IgM). Collectively, these investigations support a thorough and targeted approach to the diagnosis and management of livedoid vasculopathy.14-16
Deep and multiple biopsies may be necessary to exclude other entities such as medium-vessel vasculitis, particularly polyarteritis nodosa (PAN), which may present with similar features. A study by Mimouni et al highlighted that medium-sized vasculitides, such as PAN, can present with ulceration leading to AB-like scarring. In their cohort, 6 out of 29 patients with AB had underlying PAN, emphasizing the importance of considering vasculitis in the differential diagnosis, especially in the absence of venous insufficiency.17
Dermoscopy can also aid in the diagnostic process. Common dermoscopic findings include centrally located crusted ulcers or ivory-white areas, often surrounded by reticular pigmentation and increased vascular structures. These features correlate histopathologically with dermal fibrosis, epidermal basal layer hyperpigmentation, and capillary proliferation.18
Treatment strategies for ulcerated atrophie blanche
The management of AB and ulcerated AB depends on its underlying cause. Strategies differ significantly due to their distinct pathophysiological mechanisms.
The treatment of AB secondary to CVI focuses on reducing venous hypertension and promoting wound healing. Compression therapy must be adjusted to the patient’s tolerance, as these lesions are usually very painful. Pharmacological interventions, including venoactive agents such as micronized purified flavonoid fraction (MPFF), pentoxifylline, and sulodexide, can help reduce inflammation and improve microcirculation; combining these with compression may enhance healing.19
Lifestyle modifications, such as leg elevation, regular physical activity, and weight management, are also recommended to reduce venous hyperpressure. In case of superficial venous system insufficiency, interventional or surgical options like endovenous ablation are indicated.19
Although AB has traditionally been considered a permanent skin change associated with CVI, appropriate targeted treatments, such as endovenous procedures, may lead to its reversal, as has been published in a case after radiofrequency ablation and phlebectomies, followed by additional foam sclerotherapy.20
Despite an extensive review during which no previously published cases were identified, in our clinical practice we have observed reversal of the perilesional AB 6 weeks after punch grafting, as shown in this case depicted in Figures 7 and 8.
The treatment for livedoid vasculopathy involves a multifaceted approach aimed at managing pain, promoting ulcer healing, and preventing recurrence. There is a need for randomized clinical trials to better establish these treatments in clinical practice due to the current reliance on low levels of evidence, primarily from case reports and case series.21
Anticoagulants are the cornerstone of livedoid vasculopathy therapy, with rivaroxaban—a direct oral anticoagulant— demonstrating significant efficacy in reducing pain and facilitating ulcer healing.22 Low molecular weight heparin is also commonly used and associated with favorable outcomes.23
Antiplatelet agents such as aspirin and clopidogrel are frequently employed to inhibit platelet aggregation and thrombus formation, often in combination with anticoagulants. Corticosteroids have also been used to reduce inflammation.21 In refractory cases, intravenous immunoglobulins (IVIg) have proven effective, leading to rapid and notable improvement in both pain and ulceration.24,25 Although the mode of action of IVIg is not completely understood, it induces modulation of cytokine production, neutralization of pathogens, and inhibition of complement-mediated damage. Multiple case studies have demonstrated the response of patients with livedoid vasculopathy to IVIg with minimal complications. Cessation of smoking may also be beneficial.26
A novel combination regimen referred to as “CHAP” has been recently described and consists of cilostazol, hydroxychloroquine, aspirin, and pentoxifylline.27 This combination aims to address the thrombotic and inflammatory components of the disease, leveraging the antiplatelet and vasodilatory effects of cilostazol and aspirin, the immunomodulatory properties of hydroxychloroquine, and the hemorheological benefits of pentoxifylline.
The treatment for ulcers due to hydroxyurea primarily involves discontinuation of hydroxyurea. The ulcers may resolve after stopping the drug. Nevertheless, it is not unusual to encounter wounds that remain despite its discontinuation. For patients who require ongoing treatment for their underlying condition, alternative therapies to hydroxyurea should be considered. These alternatives may include other cytoreductive agents such as anagrelide, depending on the specific indication and patient tolerance.12,28
Despite the lack of research, compression therapy, due to its anti-inflammatory effect, might be considered an adjuvant treatment, and preventive measure, for all cases of ulcerated AB.23 In addition, it should be taken into account that venous insufficiency may be also present in these patients.
The ulcers in the context of AB, regardless of their underlying cause, tend to be very painful and resistant to treatment as they appear in scar areas. Taking into account that ulcerated AB can be considered a wound on scar tissue, it must be treated as a hard-to-heal wound. Punch grafting, associated with compression therapy may accelerate wound healing and decrease pain.29 Even when the wound bed is not in optimal condition for grafting, skin grafts may still take. And even if they do not fully adhere to the wound bed, they can still contribute to healing and pain reduction by releasing growth factors and beneficial cellular components.30 The key is to minimize dressing changes as much as possible and perform only minimal wound cleansing, in order to preserve the local healing microenvironment.
Pain control is essential in all conditions in the context of ulcerated AB though often more challenging in livedoid vasculopathy due to the neuropathic component of ulcer-associated pain.
Multidisciplinary care is key in the effective management of AB, particularly when associated with complex conditions such as livedoid vasculopathy. Given its multifactorial etiology and potential complications, a collaborative approach involving multiple specialties ensures comprehensive evaluation and treatment, including dermatologists, vascular surgeons, phlebologists, hematologists, pain specialists, and wound care specialists. This coordinated, multidisciplinary strategy allows a more personalized and effective management plan, improving both clinical outcomes and patient quality of life.1
Key takeaways for clinicians
• Patients with AB commonly present in wound care clinics either following a healed ulcer or without preceding ulceration.
• Though often associated with CVI, AB is also observed in thrombotic and inflammatory dermatoses, and recognizing these contexts is critical for timely, individualized care.
• Early and accurate diagnosis, aided by dermoscopy and, when needed, histopathology, is essential to prevent complications like painful, recurrent ulcers.
• Management must be tailored to the etiology: compression therapy and venous interventions for CVI, anticoagulation for livedoid vasculopathy, and drug discontinuation for hydroxyurea-induced ulcers. Adjunctive strategies like punch grafting and proper wound care can accelerate healing and improve quality of life. Multidisciplinary care is often necessary for optimal outcomes.
• Much of the current literature is based on case reports and small observational studies. Randomized controlled trials are urgently needed to define the most effective therapies for each subtype of ulcerated AB. Additionally, the reversibility of AB lesions, as suggested by recent studies, warrants further investigation.
CORRESPONDING AUTHOR
Elena Conde Montero, MD, PhD
Hospital Virgen del la Torre c/ Puerto Lumbreras n5 Madrid, Spain
e-mail: elenacondemontero@gmail.com
References
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27(11):518-524; quiz 525-526.
2. Milian G. Les atrophies cutanees syphilitiques. Bull Soc Franc Derm Syph. 1929;36:865-871.
3. Gray HR, Graham JH, Johnson W, Burgoon CF Jr. Atrophie blanche: periodic painful ulcers of lower extremities. A clinical and histopathological entity. Arch Dermatol. 1966;93:187-193.
4. Gonin R. Treatment of painful ulcers of the leg following white atrophy of the skin [article in French]. Bull Soc Fr Dermatol Syph. 1963;70:633-637.
5. Gougerot HL, Hamburger J. Capilarite telangiectasique et atrophiante (atrophie blanche). Bull Soc Fr Dermatol Syph. 1936;43:1792-1794.
6. Nelson LM. Atrophie blanche en plaque. AMA Arch Derm. 1955;72:242-251.
7. Alavi A, Hafner J, Dutz JP, et al. Livedoid vasculopathy: an in-depth analysis using a modified Delphi approach. J Am Acad Dermatol. 2013;69(6):1033-1042.e1.
8. Badahdah HM, Edrees KM, Alnasr L, Junainah E. Acroangiodermatitis of Mali (pseudo-Kaposi sarcoma) associated with chronic venous insufficiency and obesity: a case report. Wounds. 2018;30(11):E105-E107.
9. Franzeck UK, Haselbach P, Speiser D, Bollinger A. Microangiopathy of cutaneous blood and lymphatic capillaries in chronic venous insufficiency (CVI). Yale J Biol Med. 1993;66(1):37-46.
10. Bollinger A, Jager K, Geser A, Sgier F, Seglias J. Transcapillary and interstitial diffusion of Na-fluorescein in chronic venous insufficiency with white atrophy. Int J Microcirc Clin Exp. 1982;1(1):5-17.
11. Apti Sengun O, Ergun T, Guctekin T, Alibaz Oner F. Endothelial dysfunction, thrombophilia, and nailfold capillaroscopic features in livedoid vasculopathy. Microvasc Res. 2023;150:104591.
12. Dissemond J, Hoeft D, Knab J, Franckson T, Kroger K, Goos M. Leg ulcer in a patient associated with hydroxyurea therapy. Int J Dermatol. 2006;45(2):158-160.
13. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-352.
14. Seguí M, Llamas-Velasco M. A comprehensive review on pathogenesis, associations, clinical findings, and treatment of livedoid vasculopathy. Front Med (Lausanne). 2022 8;9:993515.
15. Di Giacomo TB, Hussein TP, Souza DG, Criado PR. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24(11):1340-1346.
16. Eswaran H, Googe P, Vedak P, Marston WA, Moll S. Livedoid vasculopathy: a review with focus on terminology and pathogenesis. Vasc Med. 2022;27(6):593- 603.
17. Mimouni D, Ng PP, Rencic A, Nikolskaia OV, Bernstein BD, Nousari HC. Cutaneous polyarteritis nodosa in patients presenting with atrophie blanche. Br J Dermatol. 2003;148(4):789-794.
18. Hu SC, Chen GS, Lin CL, Cheng YC, Lin YS. Dermoscopic features of livedoid vasculopathy. Medicine (Baltimore). 2017;96(11):e6284.
19. McVittie E, Holloway S. Aetiology and management of atrophie blanche in chronic venous insufficiency. Br J Community Nurs. 2015;20(Suppl 12):S8- S13.
20. van Montfrans C, De Maeseneer MGR. Atrophie blanche (C4b) can be reversible after targeted treatment. Eur J Vasc Endovasc Surg. 2019;58(3):435.
21. Micieli R, Alavi A. Treatment for livedoid vasculopathy: a systematic review. JAMA Dermatol. 2018;154(2):193-202.
22. Weishaupt C, Strölin A, Kahle B, et al. Anticoagulation with rivaroxaban for livedoid vasculopathy (RILIVA): a multicentre, single-arm, open-label, phase 2a, proof-of-concept trial. Lancet Haematol. 2016;3(2):e72-e79.
23. Gardette E, Moguelet P, Bouaziz JD, et al. Livedoid vasculopathy: a French observational study including therapeutic options. Acta Derm Venereol. 2018;98(9):842-847.
24. Monshi B, Posch C, Vujic I, Sesti A, Sobotka S, Rappersberger K. Efficacy of intravenous immunoglobulins in livedoid vasculopathy: long-term follow-up of 11 patients. J Am Acad Dermatol. 2014;71(4):738-744.
25. Kim EJ, Yoon SY, Park HS, Yoon HS, Cho S. Pulsed intravenous immunoglobulin therapy in refractory ulcerated livedoid vasculopathy: seven cases and a literature review. Dermatol Ther. 2015;28(5):287- 290.
26. Callen JP. Livedoid vasculopathy: what it is and how the patient should be evaluated and treated. Arch Dermatol. 2006;142:1481-1482.
27. Coromilas AJ, Micheletti RG. A novel combination (“CHAP”) regimen for management of livedoid vasculopathy in 12 patients. J Am Acad Dermatol. 2023;88(3):672-674.
28. Ammad Ud Din M, Hussain SA, Jamshed S. Leg ulcer with long-term hydroxyurea use. Clin Case Rep. 2021;9(4):2487-2488.
29. Orbea Sopeña A, Conde Montero E. Punch grafting for the treatment of ulcerated atrophie blanche. Phlebology. 2023;38(10):695-697.
30. Atkin L, Bućko Z, Conde Montero E, et al. Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care. 2019;23(Suppl 3a):S1-S50.