A comprehensive review of the history, pathophysiology, diagnosis, and treatment of iliac vein entrapment syndrome, also known as May-Thurner syndrome
Domenico Baccellieri,MD, Associate Professor
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Ferdinando B. A. Valente, MD
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Vincenzo Ardita, MD, PhD
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Mert Dumantepe, MD, PhD
Florence Nightingale Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey
ABSTRACT
May-Thurner syndrome (MTS), also known as iliac vein entrapment syndrome, is caused by extrinsic compression of the left common iliac vein—most often by the right common iliac artery—leading to venostasis and potential development of deep vein thrombosis (DVT). Though historically underdiagnosed, its recognition has increased with the routine use of cross-sectional imaging and intravascular ultrasound (IVUS), which now serves as the gold standard for diagnosis and procedural guidance.
MTS primarily affects young women and often presents with unilateral leg swelling, recurrent DVT, or chronic pelvic symptoms. Diagnosis relies on duplex ultrasound, computed tomography or magnetic resonance venography, venography, and IVUS, which can detect subtle intraluminal abnormalities and guide precise stent placement. Endovascular treatment with dedicated venous stents has largely replaced open surgical options due to superior safety, efficacy, and durability.
Proper stent selection and IVUS-guided sizing are essential to avoid complications such as restenosis or migration. Postprocedural management includes anticoagulation and patient-specific follow-up. Ongoing research focuses on novel stent technologies, AI-enhanced imaging, and long-term outcome data from registries like the European Venous Registry. Early identification and intervention improve long-term outcomes and quality of life, underscoring the importance of clinical awareness and multidisciplinary care.
Introduction
Extrinsic compression of the left common iliac vein (LCIV) by the arterial system against bony structures in the iliocaval area is commonly known as May-Thurner syndrome (MTS) or Cockett syndrome. Commonly, the LCIV is compressed between the spine and the right common iliac artery (RCIA). This condition may represent a cause of obstructive venous disease due to venostasis in the proximal venous system and unfortunately is rarely considered and diagnosed. Obstructed outflow evolving from this syndrome may progress to severe venous disorders involving the leg or pelvis or in worse cases may increase the risk of deep vein thrombosis (DVT). With increasing use of cross-sectional imaging and heightened awareness among vascular specialists, this syndrome is being diagnosed more frequently today, particularly in patients with unexplained lower-limb swelling or recurrent thrombosis.1-2
History of iliac vein entrapment syndrome
The origins of this syndrome trace back to 1851, when Rudolf Virchow hypothesized that increased incidence of left-sided venous thrombosis in left lower extremities was the result of the RCIA compression by the LCIV. The latter finding was later supported by McMurrich reporting 32% of intravascular obstruction or adhesion in the LCIV in an unselected population of 107 cadavers, concluding for a congenital disease. Some years after, other authors reported the same incidence in 399 cadavers but in relationship to “elastine and collagene” findings inside the diseased vein, concluding for acquired diseases due to extrinsic compression over time.3
In 1957, May and Thurner reported a fibrous intraluminal band outflow evolving from this syndrome may progress to severe venous disorders involving the leg or pelvis or in worse cases may increase the risk of deep vein thrombosis (DVT). With increasing use of cross-sectional imaging and heightened awareness among vascular specialists, this syndrome is being diagnosed more frequently today, particularly in patients with unexplained lower-limb swelling or recurrent thrombosis.1-2 or “spur” in 22% of 430 cadavers on the LCIV, postulating this was acquired from chronic and pulsatile compression causing local trauma, inflammation, and endothelial proliferation.4 Following this finding, Cockett in 1965 began to study the clinical implications in living patients with DVT and iliac vein compression through the use of venography, reporting typical symptoms such as swelling and pain associated with edema, hyperpigmentation, induration, and ulceration. DVT was diagnosed in young patients (mean age, 23 years old), always in the LCIV below the arterial compression after a period of immobilization. Therefore, he also concluded that compression often can be asymptomatic due to the possibility to develop collateral circulation.4
Epidemiology
The true prevalence of MTS is difficult to ascertain due to the asymptomatic nature of many cases. Imaging and autopsy studies have reported LCIV compression in up to 66% of individuals, yet only a small percentage develop symptoms. Among patients presenting with left-sided lower-extremity DVT, MTS has been identified as the underlying cause in 2% to 5% of cases. Women between the ages of 20 and 40 are disproportionately affected, especially those with risk factors such as hormonal therapy, pregnancy, or prolonged immobilization.1-4
Pathophysiology
Venous compression can be permissive and asymptomatic, but 3 factors may lead to the development of symptoms; these are as follows: i) chronic inflammation and fibrotic response due to persistent extrinsic trauma may cause formation of spurs, webs, channels, and diaphragms inside the vessel; ii) external inflammation may lead to increased vessel rigidity; and iii) flow alteration secondary to lower-limb reflux and loss of volume during backflow to the heart may lead to vein collapse and gradually to thrombosis.3
Types of compression
Several variations have been described; nowadays, we recognize the following groups5-7: i)LCIV compressed by RCIA; ii) right common iliac vein (RCIV) compressed by RCIA/ left common iliac artery (LCIA)/right internal iliac artery (RIIA)/right external iliac artery (REIA); iii) left external iliac vein (LEIV) compressed by left external iliac artery (LEIA); iv) right external iliac vein compressed by REIA; v) RCIV compressed by aortic bifurcation; and vi) inferior vena cava (IVC) compressed by RCIA.
Clinical manifestation
Patients with MTS can present extreme heterogeneity of symptoms from leg swelling with persistent edema, venous claudication, symptomatic varicose veins, and phlebitis. Most rarely, in case of primary DVT presentation, phlegmasia cerulea dolens can be the first presentation.4
All patients with left-sided chronic venous disorders need to be investigated for MTS, particularly patients with a history of isolated or recurrent DVT. All causes of extrinsic compression need to be excluded during differential diagnosis, such as pelvic mass, iliac artery aneurysm, bladder distension, spinal lithiasis or lumbar discopathy, lymphadenopathies, and tumors.1,4
Clinical stages can be classified as follows: i) stage 1, asymptomatic LCIV compression; ii) stage 2, formation of intraluminal spurs; and iii) stage 3, occurrence of left iliac DVT.
Diagnosis
Persistent narrowing of a vein compressed with stenosis of more than 50% is adequate for suspicion of MTS; other indicators include venous collaterals, identified intraluminal changes, diameters of proximal vessels, and flow patterns.5,6
Imaging modalities include: i) nonivasive venous imaging— such as duplex ultrasound (DUS), plethysmography, and computed tomography venography (CTV)/magnetic resonance venography (MRV); and ii) invasive venous imaging—such as venography and intravascular ultrasound (IVUS). Before being subjected to any diagnostic imaging modality, it is important that patients are well hydrated to avoid false positive results.
Duplex ultrasound
DUS is currently the initial modality of diagnosis of all venous disorders; it’s a full noninvasive, repeatable examination of the whole venous system. Although it provides high resolution and sensitivity and specificity, the abdominal ultrasound scan may present some limitations for use with compressive syndromes. The deep location of veins and standard prone position of patients can be a limitation for correct evaluation and may lead to overdiagnosis of compression.
To solve this limitation, this examination needs to be performed in supine and semi-sitting positions.
After DUS in the standing position, focused on reflux, the patient is invited to lay in the supine position, and the femoral vein evaluation is performed using a 4–7 MHz linear array transducer. In nonocclusive disease such as MTS, the flow patterns can be absolutely normal with regular phasicity of the common femoral vein, but other cases can reveal reduced phasicity of flow, reduction in amplitude, and limited response to compressive maneuvers or Valsalva maneuvers. Iliac and caval vessels need to be investigated with a 2-3–MHz probe in B mode to directly evaluate diameters and morphologic compression. Peak vein velocity (PVV) is measured in the poststenotic segment and compared with the prestenotic segment often dilated. If the PVV ratio is more than 2.5, the finding is significant. Reflux in the ipsilateral internal iliac vein is associated with proximal compression due to compensatory reversal flow.8,9
Plethysmography
Air plethysmography measures the global change in volume in mL/s of the part of the calf enclosed by the cuff in response to gravitational filling on dependency (venous filling index) and drainage on leg elevation (venous drainage index). It is a volumetric tool used several years ago as a method to evaluate venous refluxes and proximal obstructions. Rapid filling and low elevation drainage are indicative of global venous incompetence and obstruction, respectively. This technique can be used to assess severity of venous symptoms, particularly in severe reflux, but is not currently recognized as a diagnostic tool for MTS.10
Computed tomography/magnetic resonance venography
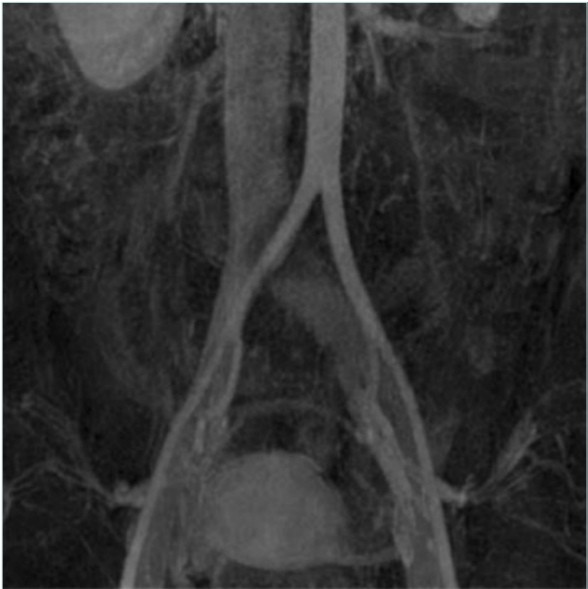
CTV and MRV are second-level diagnostic tools in case of suspected MTS and offer complete examination of the venous system that can also be enhanced by 3D reconstruction. CTV has some disadvantages related to radiation exposure and iodinated contrast, but it’s well recognized that it provides high-quality studies and a complete evaluation of the venous anatomy. MRV is the most versatile imaging, with dynamic sequences that can provide information regarding velocity and volume while providing high-quality morphologic images of the compressed vein such as with fibrotic scarring, postthrombotic fibrosis, as well as collaterals and varicose veins (Figure 1).11,12
Venography
Historically the gold standard for diagnosis of venous diseases, venography has recently become a useful procedure combined with IVUS and possibly with simultaneous endovascular procedures. Imaging in two or three projections is mandatory before advancing catheters or endovascular devices after any guidewire advancement in occlusive disease. Regarding MTS, venography can provide information about patency of vessels, anatomical variations, and associated reflux. It’s often possible to observe the classical arterial “shadow” associated with the “pancake effect” typically present in the antero-posterior view and secondary to vein enlargement below the compression. Furthermore, venography can show hypertrophic collateral circulation, internal iliac vein branches, and ascending lumbar vein.11,13
Intravascular ultrasound
IVUS has become the cornerstone imaging modality for the diagnosis and endovascular management of iliac vein compression syndromes. Offering real-time, 360-degree, high-resolution cross-sectional views from within the venous lumen, IVUS provides superior anatomical detail compared with traditional venography or static cross-sectional imaging modalities like CTV or MRV.
IVUS excels in detecting subtle intraluminal abnormalities such as fibrotic spurs, endoluminal webs, and residual thrombus that may not be apparent on other imaging. Its diagnostic accuracy exceeds 95% in experienced hands and is especially useful for evaluating dynamic changes in vein caliber that may occur with respiration or body position.14,15
During interventions, IVUS allows precise assessment of the compressive lesion, measurement of luminal diameters, and identification of healthy vein segments both proximal and distal to the lesion—critical for determining optimal stent length and placement. This helps avoid common complications such as undersizing, which may result in stent migration or restenosis, and oversizing, which can lead to excessive radial force, vessel wall damage, and uncontrollable back pain. After stent deployment, IVUS is used again to verify that the stent is fully expanded, well-apposed to the vein wall, and that no residual stenosis or mechanical obstruction remains. Its integration into standard practice has been shown to significantly improve patency rates and reduce the risk of reintervention.14-16 (Figure 2).
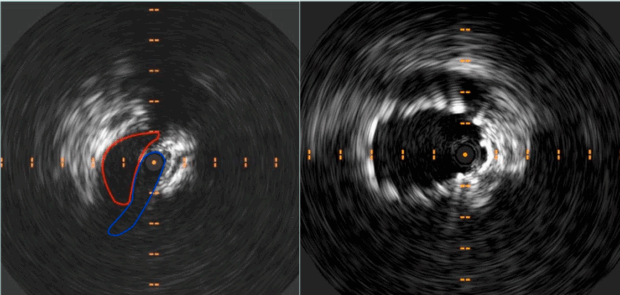
Figure 2. Intravascular ultrasound (IVUS) showing preoperative compression at May-Thurner’s point and subsequent restored patency post stenting.
Treatment
Treatment of asymptomatic patients, even with high-grade stenosis or occlusion, is not supported by any evidence that suggests this reduces the risk of subsequent DVT.
In case of C3-C6 CEAP (clinical-etiological-anatomical-pathophysiological) classification, interventions need to be considered. Furthermore, venous claudication (heaviness and pain during exercise) with severe swelling and persistent edema is a possible indication in young patients if associated with a debilitating quality of life.17
Open surgery
Prior to the advent of endovascular techniques, management of symptomatic iliac vein compression relied on open surgical interventions, which were often technically challenging and associated with substantial morbidity. These procedures included venous bypass grafting, transposition of the iliac vein, venolysis (surgical release of the compressed vein), and patch angioplasty. In bypass procedures, the obstructed segment of the vein was circumvented using autologous vein grafts or synthetic conduits, aiming to restore venous outflow. Transposition, on the other hand, attempted to physically relocate the compressed vein segment to a position less prone to arterial impingement.18,19
Despite their theoretical effectiveness, open surgical approaches were limited by the deep pelvic location of the iliac vessels, which complicated access and increased the risk of injury to adjacent structures such as the ureter, bowel, and arteries. Postoperative recovery was lengthy, and the potential for thrombosis or graft failure remained significant. Moreover, because many cases of iliac vein compression were not diagnosed until after thrombosis had occurred, surgery often addressed chronic sequelae rather than providing prophylactic benefit.20
In select cases, such as when endovascular options are unavailable, contraindicated, or have failed, open surgery may still be considered. However, it is now largely reserved for exceptional circumstances due to the superior safety, efficacy, and durability of modern endovascular solutions. One of these exceptions is represented by extravascular stenting.
The evolution of IVUS and dedicated venous stents has made minimally invasive management the preferred first-line approach in most vascular centers.17
Endovascular treatment
An endovascular approach is focused on restoring patency of compressed/occluded vessels by endoluminal stent implantation. The goal of stenting MTS is to solve compression, achieving correct venous return. Multiple devices are available on the market with different features. An optimal venous stent must possess a well-balanced combination of mechanical strength, conformability, and precise deployment characteristics to address the complex anatomical and pathophysiological features of venous disease. Among the critical determinants of stent performance are radial resistive force, chronic outward force, crash resistance, and flexibility—all of which vary according to stent design.21-23
Radial resistive force, the ability of a stent to withstand radial compression, is typically higher in closed-cell configurations than in open-cell designs. This is due to the smaller free-cell area within the stent mesh, which enhances structural integrity and helps maintain luminal patency in the face of compressive forces. Chronic outward force, the centrifugal pressure exerted by the stent after deployment, is another essential property. This force increases as stent diameter decreases, underscoring the importance of selecting an appropriately sized stent. Significant oversizing should be avoided, as a minimally oversized stent delivers optimal circumferential stress to the venous wall, promoting endothelial integration while reducing the risk of restenosis.24
Crash resistance, defined as the stent’s capacity to ensure unidirectional external compression—particularly from overlying arterial structures or musculoskeletal motion—is a pivotal factor in long-term patency. Stents with larger nominal diameters typically exhibit superior crash resistance, retaining shape and function even under substantial mechanical load (Figure 3).24
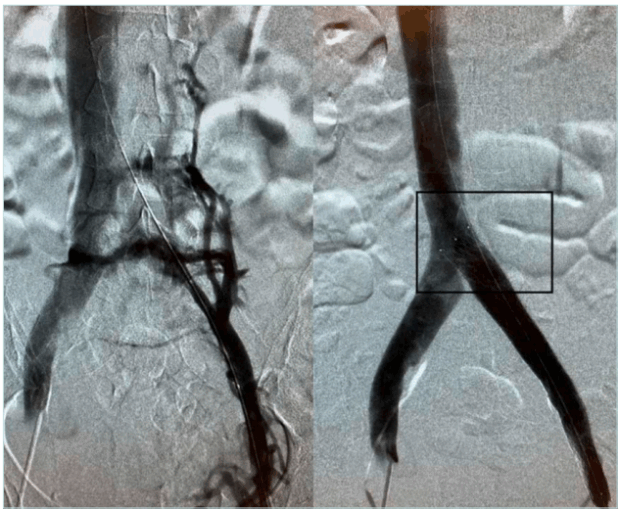
Figure 3. Venography showing pre (left panel) and post (right panel) recanalization of a May Thurner syndrome patient with a long stent anchored to the origin of the common iliac vein.
Equally important is the stent’s flexibility. In contrast to arterial stents, venous stents must navigate the more variable and dynamic architecture of the pelvic venous system. High flexibility allows the device to adapt to the curvatures and shifts in vessel geometry without kinking or altering its cross-sectional shape. This requirement further emphasizes the need to avoid overlapping multiple stent segments, which can lead to increased rigidity and compromise physiological motion.24
Taken together, these mechanical properties define the ideal stent profile for treating venous obstructions, where both durability and adaptability are essential for long-term clinical success.
According to common practice, venous stents need to be positioned using IVUS guidance based on the size of the LEIV anchoring point rather than the LCIV, as the latter may be dilated due to the compression, resulting in an increased risk of contralateral iliac DVT.14,15
Referral centers have different stent configurations available, so the physician can decide which stent is appropriate for implantation according to the IVC confluence anatomy. Several trials are ongoing with satisfactory results for MTS treatment and long-term results with secondary patency near 100%.25,26
One of the most crucial and debated issues is stent migration; this event may occur upon choosing a shorter or smaller stent. Undersizing a venous stent, particularly within the iliac vein, poses a greater clinical risk than modest oversizing. Inadequate stent diameter can lead to a fixed iatrogenic stenosis, a complication that is often difficult to reverse once established. This issue frequently arises from the incorrect assumption—borrowed from arterial stenting practice— that restoring flow and achieving patency are sufficient therapeutic end points. However, in the venous system, these goals must be accompanied by restoration of full luminal caliber. Ensuring an adequately sized stent is crucial for effective decompression and sustained relief of venous hypertension. IVUS guidance leads to the right choice of sizing and length and allows correct identification of landing zones in the external iliac vein and in the IVC confluence.25,26
After treatment, immediate anticoagulation with low-molecular-weight heparin is started and intermittent pneumatic compression is administered for the first 12 hours, then active mobilization is allowed. There is no evidence on postprocedural drug treatment, according to the Delphi Consensus proposed by The Imperial College of London. Direct anticoagulants seem to be the treatment of choice for MTS patients after implantation, at least for 1 month, which can be extended to 6 months; in case of previous DVT, treatment needs to be personalized according to hematological indications.17
Preventive strategies and screening
Although there are no formal screening guidelines for MTS in the general population, clinicians should consider proactive assessment in high-risk individuals. This includes young women with unprovoked left-sided DVT, recurrent thrombosis, or severe chronic pelvic symptoms. An early diagnosis can prevent long-term complications such as postthrombotic syndrome or venous ulcers, which significantly affect quality of life.
Preventive strategies include prompt investigation of unilateral leg swelling or unexplained pelvic discomfort, routine DUS in patients with known hypercoagulable states and chronic venous symptoms, and multimodal imaging (CT angiography/MRV) in recurrent DVT without obvious provocation, incorporating MTS suspicion into the diagnostic workflow of vascular medicine.2
In institutional settings, development of a risk-stratified protocol or checklist for evaluating patients with idiopathic left-sided DVT can help guide early investigation for iliac vein compression. Raising awareness among primary care and emergency physicians can also reduce delays in referral to vascular specialists. Further research may eventually support formal screening criteria, especially as noninvasive imaging becomes more accessible and affordable.
Research and future directions
Ongoing research in MTS is focused on improving diagnostic precision, treatment durability, and understanding the natural history of asymptomatic compression. Advanced imaging technologies—including dynamic MRV and IVUS with AI-assisted interpretation—are under investigation for their potential to detect subclinical disease and guide more individualized treatment decisions. Novel stent materials and designs, such as bioresorbable or drug-eluting venous stents, are also being evaluated to reduce restenosis and improve long-term patency.27
In parallel, there is growing interest in identifying biomarkers of venous injury and inflammation that could predict symptom progression or stent response. Randomized controlled trials are increasingly being designed to compare outcomes between different stent types, anticoagulation protocols, and follow-up strategies, such as in the European Venous Registry. Furthermore, development of comprehensive registries, and long-term follow-up cohorts is essential to better understand the impact of MTS treatment on quality of life, recurrence rates, and cost-effectiveness.
Ultimately, the integration of precision imaging, personalized medicine, and advanced device engineering may pave the way for earlier detection, safer interventions, and more durable outcomes in patients with iliac vein compression syndromes.
Conclusions
Iliac vein entrapment syndrome, though historically underdiagnosed, is increasingly recognized as a significant contributor to venous pathology in young patients, particularly women. The spectrum of its clinical presentation—ranging from asymptomatic compression to debilitating venous insufficiency—underscores the importance of clinical vigilance. Advancements in diagnostic imaging, particularly IVUS, have made early detection more feasible, and the evolution of endovascular techniques has provided a reliable, minimally invasive solution for symptomatic cases.
Timely intervention can prevent severe complications, including DVT and postthrombotic syndrome, thereby improving quality of life and reducing the burden on health care systems. Clinicians should maintain a high index of suspicion in patients with unilateral leg swelling, unexplained DVT, or pelvic venous symptoms, and ensure appropriate imaging is pursued. A multidisciplinary approach involving vascular surgeons, interventional radiologists, hematologists, and primary care providers is key to successful management.
CORRESPONDING AUTHOR
Professor Domenico Baccellieri
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Hospital,
Vita-Salute San Raffaele University, Via Olgettina, 60, 21300, Milan, Italy
e-mail: Baccellieri.domenico@hsr.it
Dr Domenico Baccellieri received an honorarium from Servier for the writing of this article.
References
1. Heller T, Teichert C, Hafer J, Weber MA, Kröger JC, Meinel FG. Prevalence of May-Thurner syndrome in patients with deep vein thrombosis at a large medical referral center. Rofo. 2019;191(12):1107-1117.
2. Harbin MM, Lutsey PL. May-Thurner syndrome: history of understanding and need for defining population prevalence. J Thromb Haemost. 2020;18(3):534-542.
3. Poyyamoli S, Mehta P, Cherian M, et al. May-Thurner syndrome. Cardiovasc Diagn Ther. 2021;11(5):1104-1111.
4. Galanakis N, Kontopodis N, Kehagias E, et al. Direct Iliac vein stenting in phlegmasia cerulea dolens caused by May-Thurner Syndrome. Vasc Specialist Int. 2021;37:37.
5. Raju S, Neglén P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143.
6. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937-943.
7. Mangla A, Hamad H. May-Thurner syndrome. In: StatPearls [Internet]. StatPearls Publishing; 2025 Jan–. Updated March 11, 2024. PMID:32119264.
8. Barry A. Sonography’s role in the diagnosis of May–Thurner Syndrome. J Diagn Med Sonography. 2017;34(1):65-70.
9. Malgor RD, Labropoulos N. Diagnosis of venous disease with duplex ultrasound. Phlebology. 2013;28(Suppl 1):158-161.
10. Criado E, Farber MA, Marston WA, Daniel PF, Burnham CB, Keagy BA. The role of air plethysmography in the diagnosis of chronic venous insufficiency. J Vasc Surg. 1998;27(4):660-670.
11. Knuttinen MG, Naidu S, Oklu R, et al. May-Thurner: diagnosis and endovascular management. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S159-S164.
12. Lan YQ, Chen YM, Lan KS, Feng N, Xi ZF. Demographic characteristics, clinical manifestations, and treatment outcomes of May-Thurner syndrome: a five-year retrospective analysis using computed tomography venography in a Chinese population. Curr Med Res Opin. 2024;40(11):2013-2019.
13. Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10):e002772.
14. Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord. 2019;7(6):801- 807.
15. Dwivedi A, Singh SN, Sharma A, Sharma R, Mishra T. A systematic review of radiological diagnosis and management of May-Thurner syndrome. J Pharm Bioallied Sci. 2024;16(Suppl 2):S1012-S1016.
16. Melian CM, Giannopoulos S, Volteas P, Virvilis D. Intravascular ultrasound in treating iliac vein compression with endovascular stenting: a necessary tool for optimal outcomes. Vasc Endovascular Surg. 2023;57(3):299-305.
17. De Maeseneer MG, Kakkos SK, Aherne, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 Clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267. Erratum in: Eur J Vasc Endovasc Surg. 2022;64(2-3):284- 285.
18. Rutherford RB. Role of surgery in iliofemoral venous thrombosis. Chest. 1986;89(5 Suppl):434S-437S.
19. Swedenborg J, Hägglöf R, Jacobsson H, et al. Results of surgical treatment for iliofemoral venous thrombosis. Br J Surg. 1986;73(11):871-874.
20. Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979-990.
21. Shamimi-Noori SM, Clark TWI. Venous stents: current status and future directions. Tech Vasc Interv Radiol. 2018;21(2):113-116.
22. Razavi MK, Black S, Gagne P, Chiacchierini R, Nicolini P, Marston W; VIRTUS Investigators. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12(12):e008268.
23. Lichtenberg M, Stahlhoff S, Özkapi A, de Graaf R. Braided nitinol stent for chronic iliofemoral venous disease – the real-world BLUEFLOW registry. Vasa. 2021;50(5):372-377.
24. Kim DB, Choi H, Joo SM, et al. A comparative reliability and performance study of different stent designs in terms of mechanical properties: foreshortening, recoil, radial force, and flexibility. Artif Organs. 2013;37(4):368-379. Erratum in: Artif Organs. 2013;37(6):585.
25. Ardita V, Galati N, Miglioranza E, Lembo R, Chiesa R, Baccellieri D. Endovascular treatment of chronic ilio-femoral vein obstruction with extension below the inguinal ligament in patients with post-thrombotic syndrome. J Vasc Surg Venous Lymphat Disord. 2024;12(3):101816.
26. Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology. 2018;33(7):451-457.
27. Matei SC, Olariu S, Ungureanu AM, Malita D, Petrașcu FM. Does artificial intelligence bring new insights in diagnosing phlebological diseases? A systematic review. Biomedicines. 2025;13(4):776.