Venous thoracic outlet syndrome: a comprehensive clinical and therapeutic review
Ahmed S. Gaweesh, MD, MSc, PhD
Department of Vascular Surgery, Faculty of Medicine, University of Alexandria, Egypt
iVein Clinics, Egypt
Mohammed A. Elsabbagh, MD, PhD, FRCS
Department of Vascular Surgery, Faculty of Medicine, University of Alexandria, Egypt
University Hospitals Birmingham, United Kingdom
ABSTRACT
Venous thoracic outlet syndrome (VTOS) is a vascular disorder caused by extrinsic compression of the axillary-subclavian vein, leading to impaired venous drainage of the upper extremity. Compression most commonly occurs at the costoclavicular junction (CCJ) and, less frequently, at the pectoralis minor space, either independently or as part of a combined “double crush” mechanism. This venous compression may be initially asymptomatic, discovered only incidentally in high-risk populations, but it can evolve into symptomatic disease—manifesting as intermittent swelling and venous congestion, or progressing to acute thrombosis with significant morbidity. VTOS is clinically categorized into 4 subtypes: asymptomatic compression, nonthrombotic VTOS (McCleery syndrome), thrombotic VTOS (Paget-Schroetter syndrome), and postthrombotic VTOS. The diagnostic process requires a high index of suspicion and dynamic imaging under provocative conditions. Advances in duplex ultrasound, magnetic resonance venography, and intravascular ultrasound (IVUS) have improved the detection and characterization of both positional and chronic venous obstruction. Contemporary management emphasizes early thrombus removal, timely surgical decompression, and venous reconstruction in selected cases. Prompt diagnosis and intervention are critical to prevent progression to postthrombotic syndrome and irreversible functional impairment. This review offers a comprehensive overview of VTOS, including its pathophysiology, classifications, diagnostic pathways, and management algorithms, supported by current literature.
Introduction
Thoracic outlet syndrome (TOS) refers to a group of disorders caused by compression of neurovascular structures as they pass through the thoracic outlet—the anatomical space bounded by the first rib, clavicle, and scalene muscles. Among the subtypes—neurogenic, arterial, and venous—venous thoracic outlet syndrome (VTOS) is the second most common, less frequent than neurogenic TOS but more prevalent than arterial TOS. VTOS, comprising approximately 3% to 5% of all TOS cases, represents a complex vascular condition with potential for serious morbidity if not managed promptly and comprehensively.1
VTOS most frequently affects young, otherwise healthy individuals—typically athletes or those engaged in repetitive overhead arm activity. When thrombosis occurs, it is often sudden and can be dramatically symptomatic, characterized by upper-limb swelling, cyanosis, pain, and venous distention.2
The underlying pathophysiology stems from repeated extrinsic compression of the axillary-subclavian vein, leading to endothelial trauma, inflammation, and, in some cases, spontaneous thrombosis. If inadequately treated, VTOS may result in chronic venous hypertension, postthrombotic syndrome (PTS), and significant long-term functional impairment, especially in young patients whose livelihoods depend on physical performance.3,4
This review aims to offer a structured and evidence-based framework for understanding, diagnosing, and managing VTOS. Emphasis is placed on contemporary debates in treatment sequencing—including the roles of anticoagulation, thrombolysis, decompression, and venous revascularization—and the clinical decision-making paradigms that underpin these interventions.
Anatomic zones of compression and pathophysiology
Venous compression in VTOS typically occurs at 2 critical anatomical junctions within the thoracic outlet: the costoclavicular junction (CCJ) and the pectoralis minor space (PMS).5 Understanding these sites and the forces acting upon them is fundamental for accurate diagnosis and effective treatment planning.
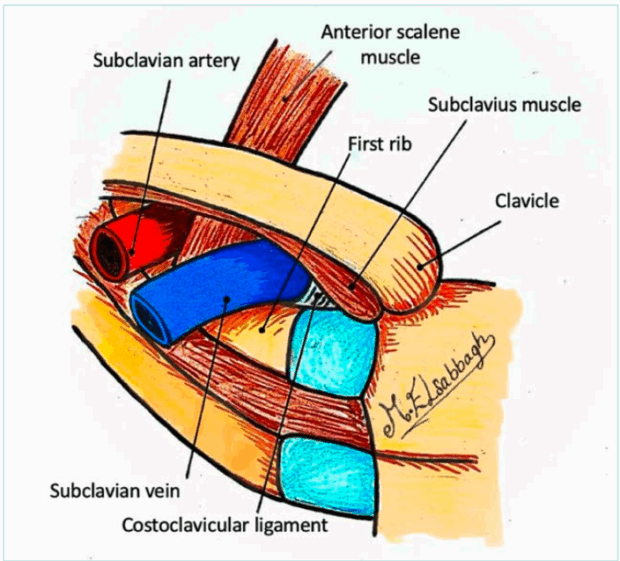
Costoclavicular junction (CCJ)
The costoclavicular junction is the most common site of venous obstruction in VTOS. The subclavian vein passes anterior to the anterior scalene muscle and posterior to the clavicle with its attached muscle (subclavius muscle), traversing a confined space above the first rib lateral to the costoclavicular ligament (Figure 1). 6 Arm abduction and retroversion, particularly in muscular individuals or overhead athletes, significantly narrow this space. Contributing anatomical features that further narrow the space include: i) congenital anomalies such as cervical ribs, anomalous fibrous bands, and elongated transverse processes of the cervical vertebrae; ii) muscular hypertrophy, especially of the scalene or subclavius muscles; and iii) posttraumatic remodeling or callus formation from the clavicle or the rib fractures.5
Pectoralis minor space (PMS)
Compression of the axillary vein can also occur underneath the pectoralis minor tendon, particularly during forward elevation and abduction of the arm. While less commonly isolated, PMS compression may coexist with CCJ compression—a phenomenon known as “venous double crush syndrome.”7 This dual pathology is increasingly recognized in overhead athletes and must be considered during surgical planning to avoid incomplete decompression.5
The repetitive nature of mechanical stress during overhead or hyperabduction arm movements may contribute to subtle but progressive venous damage over time that results in chronic endothelial irritation (Figure 2). This microtrauma leads to mechanical endothelial injury, which initiates intimal hyperplasia, inflammatory responses, and venous scarring. If unchecked, progression to thrombosis is the expected outcome. Over time, even nonthrombotic compression can evolve into a thrombotic phenotype. Importantly, this transition may occur silently, without overt clinical warning, underscoring the need for vigilance in high-risk populations.
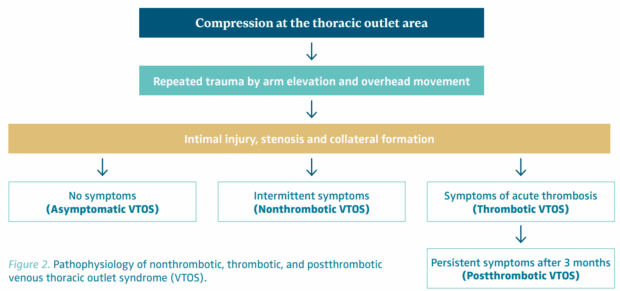
Figure 2. Pathophysiology of nonthrombotic, thrombotic, and postthrombotic venous thoracic outlet syndrome (VTOS).
Clinical spectrum and classification
VTOS presents along a clinical continuum that reflects both the degree of venous compromise and the presence or absence of thrombosis. A practical 4-tiered classification facilitates appropriate triage and management (Figure 2).
Asymptomatic VTOS
The initial phase of VTOS may be asymptomatic due to the development of collateral veins that facilitate drainage of the upper extremity.8 This type of VTOS is usually discovered incidentally in irrelevant investigations conducted as a part of the screening of high-risk populations (eg, athletes) or discovered in relevant investigations conducted to investigate other types of symptomatic TOS, ie, patients with arterial or neurogenic symptoms. Despite that dynamic tests possibly show positional venous narrowing, this group of population does not present with symptoms or signs of VTOS. The natural history remains unclear—some remain stable, whereas others may progress to become symptomatic. The true prevalence of asymptomatic VTOS remains unknown. It is likely underdiagnosed due to the lack of symptoms and reliance on provocative imaging.
Nonthrombotic VTOS (McCleery syndrome)
In this (nonthrombotic) subtype of VTOS, patients present with intermittent positional symptoms of swelling, heaviness, and bluish discoloration during overhead activity. These symptoms resolve with rest. The absence of objective persistent physical signs often leads to repeated medical evaluations without conclusive findings. Patients are frequently misdiagnosed with musculoskeletal or neurologic conditions. A high index of clinical suspicion is critical. The diagnosis is challenging and can often be missed without dynamic imaging using duplex ultrasound (DUS) and/or venography in both neutral and hyperabducted arm positions.
Thrombotic VTOS (Paget-Schroetter syndrome)
Thrombotic VTOS is the most commonly diagnosed subtype of VTOS due the acute nature of its presentation related to the acute thrombosis of the axillary-subclavian vein. Typically, it occurs in young, active individuals, particularly in the dominant arm hence it is called “effort thrombosis.” Patients present with sudden onset of swelling, cyanosis, pain, and prominent superficial veins. Offering urgent interventions in a timely manner to restore the venous patency and prevent chronic sequelae is of paramount importance, particularly in those with severe symptoms who do not respond to medical treatment. In the absence of timely treatment, it can potentially progress to phlegmasia cerulea dolens, pulmonary embolism, or irreversible postthrombotic changes. Early thrombus removal by thrombolysis or percutaneous thrombectomy followed by surgical decompression is increasingly being advised to prevent long-term sequelae.
Postthrombotic VTOS
Postthrombotic VTOS represents the chronic phase, more than 3 months, following an axillary-subclavian vein thrombosis episode. In addition to the preexisting extrinsic compression impeding the venous outflow, it can also be associated with venous scarring that causes luminal narrowing related to the postthrombotic changes leaving residual synechiae on the inner venous wall. Patients usually present with a clear history of prior upper-limb deep vein thrombosis (DVT) for which they were treated with anticoagulation, or sometimes with no history of previous DVT and they present for the first time with venous insufficiency symptoms and signs (chronic limb swelling, heaviness, fatigue, bluish discoloration, prominent venous collaterals on the shoulder and the chest wall and reduced functional capacity). It should be noted that in some cases recanalization can mask the presence of significant luminal narrowing in resting position using DUS or even venography (Figure 3). However, with arm hyperabduction, it becomes clearly visible (Figure 4). Also doing a balloon test in these cases can help detect a masked venous stenosis (Figure 5).9
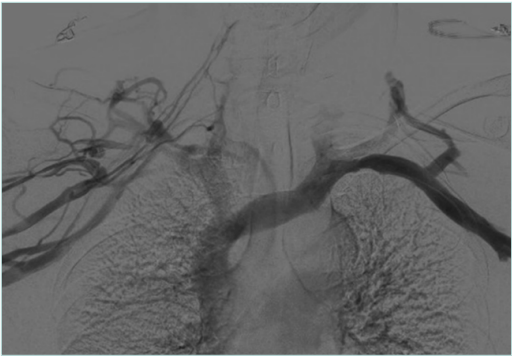
Figure 3. Venogram of arms in neutral position with postthrombotic syndrome (PTS) on the right side and no venographic signs of venous obstruction on the left side.
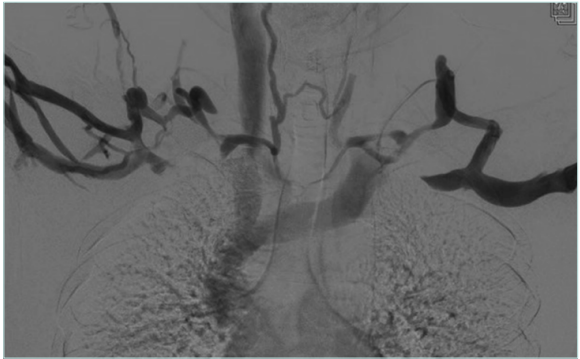
Figure 4. Venogram for arms in abduction position showing subclavian vein compression on both sides.
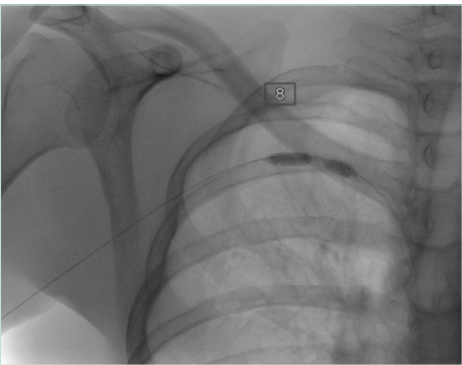
Figure 5. Balloon test for the right side with postthrombotic syndrome (PTS) with visible waist of the balloon denoting significant venous stenosis.
Postthrombotic VTOS can significantly impair quality of life particularly if the dominant arm is affected; in a systematic review, the rate of PTS can be as high as 46% in patients treated with anticoagulation only.10 Advanced imaging and multidisciplinary evaluation are essential to guide appropriate intervention.
Diagnostic approach
A successful diagnostic strategy for VTOS requires a high index of suspicion and integrating clinical evaluation with dynamic imaging. The diagnostic process must account for the episodic nature of symptoms and the need to evaluate venous function under provocative conditions. Provocative testing is crucial—static imaging may miss dynamic compression.
Clinical evaluation
The clinical assessment begins with a detailed history that explores occupation, sports participation, prior trauma, and the timing of symptom onset. Patients with VTOS often report upper extremity swelling, heaviness, fatigue, or discoloration, which may be intermittent or persistent. Intermittent symptoms that occur during overhead activity and resolve at rest are more suggestive of nonthrombotic VTOS (McCleery syndrome). Constant or persistent symptoms raise suspicion for acute thrombosis (Paget-Schroetter syndrome) or postthrombotic sequelae. Physical examination should document visible arm swelling, cyanosis, or prominent venous collaterals over the shoulder and chest wall. Provocative maneuvers (eg, arm abduction or hyperabduction) may elicit visible venous congestion or reproduce symptoms. Palpation and percussion over the thoracic outlet may reveal tenderness or fullness due to venous engorgement.
Imaging modalities
Duplex ultrasound
DUS is a primary, noninvasive modality and the initial imaging test of choice in suspected VTOS. A standard venous DUS may miss the diagnosis if performed only in the neutral position. Therefore, a comprehensive dynamic DUS is essential. This entails examining the axillary-subclavian vein in both neutral and provocative positions, such as with the arm abducted, elevated, or placed in military brace posture. The dynamic scan assesses for the following: i) positional compression with loss or reduction in venous waveform phasicity (Figure 6); ii) retrograde flow or cessation of flow in abduction; iii) visualization of thrombus, echogenic webs, or wall thickening; and iv) presence of prominent collaterals.
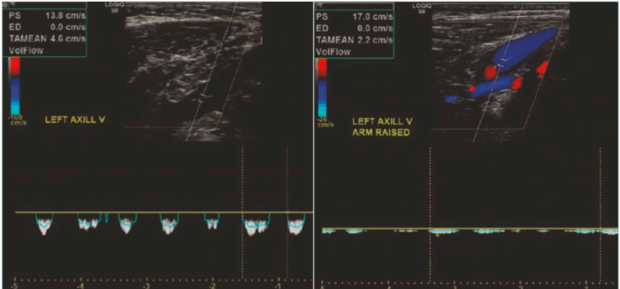
Figure 6. Presence of respiratory phasicity in the spectral wave of the axillary vein in the neutral position (left image) and loss of the respiratory phasicity in the abduction position (right image).
Dynamic DUS is operator-dependent and must be interpreted with care. Its sensitivity for detecting acute thrombosis is high, but its ability to assess external compression hinges on precise positioning, patient cooperation, and real-time visualization. High-resolution probes and color Doppler enhancement improve diagnostic yield.
Computed tomography (CT) and magnetic resonance (MR) venography
These modalities offer a comprehensive view of the thoracic outlet and associated musculoskeletal structures. CT venography is particularly useful for identifying bony anomalies, whereas MR venography provides better soft tissue resolution. Both are vital for preoperative planning. MR venography has a sensitivity for detecting stenoses and obstructions of 100% with a specificity of 97%.11
Catheter-based venography
Catheter-based venography is considered the gold standard for dynamic assessment. Performed with the arm in neutral and abducted positions, it can reveal positional stenosis, intraluminal webs, and collaterals (Figures 3 and 4). However, it may underestimate intimal abnormalities or early fibrosis.
Intravascular ultrasound
Intravascular ultrasound (IVUS) is increasingly used for detailed intraluminal evaluation. It detects subtle wall thickening, fibrosis, and residual thrombus better than venography.12 IVUS also guides interventions such as patch venoplasty and stenting.
Plain radiography
Plain radiography is used adjunctively to detect cervical ribs, elongated C7 transverse processes, or posttraumatic bone healing that may alter thoracic outlet anatomy. It is not definitive but is often a useful first step (Figure 7).
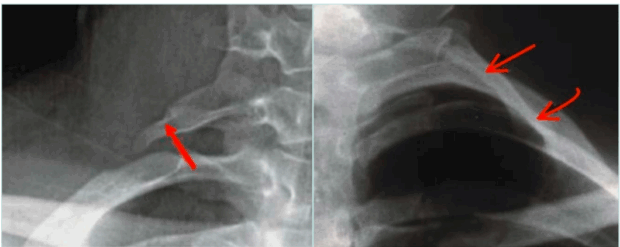
Figure 7. X-ray with elongated transverse process (right image) and complete cervical rib (left image).
Management strategies
The management of VTOS must be individualized based on the patient’s clinical presentation, symptoms’ severity and duration, underlying anatomy, and degree of thrombotic involvement. Four interdependent pillars form the foundation of VTOS treatment, as follows: i) anticoagulation; ii) thrombus removal (thrombolytic, pharmacomechanical, or mechanical); iii) surgical decompression; and iv) venous revascularization (as needed).
Anticoagulation
Anticoagulation13 is the standard of care in all patients with thrombotic VTOS. It aims to prevent thrombus extension and embolization, stabilizes the acute event, and serves as a bridge to the definitive intervention. Anticoagulation needs to be initiated immediately upon diagnosis of axillary-subclavian DVT (Paget-Schroetter syndrome).
Patients offered early thrombus removal usually need to continue therapeutic anticoagulation even if the thrombus removal was successful. Prescribing prophylactic anticoagulation to protect against developing axillary-subclavian DVT also plays an important role in the nonthrombotic and postthrombotic cases after decompression surgery and revascularization. Anticoagulation is not indicated in nonthrombotic VTOS (McCleery syndrome).
Historically, low-molecular-weight heparin (LMWH) and vitamin K antagonists (VKAs) were the only approved anticoagulants for venous thrombosis. Now, there is established evidence supporting the use of direct oral anticoagulants (DOACs) as an alternative anticoagulation due to their fixed dosing and safety profile where routine monitoring is not required. Furthermore, in a recently published meta-analysis it was shown that treatment of patients with upper extremity DVT with DOACs might be associated with lower PTS rates than treatment with other anticoagulants.14
The principal treatment phase of Paget-Schroetter syndrome is 3 to 6 months; however, an extended phase of anticoagulation can be considered in patients with non-recanalized thrombus, venous stents, and those known with thrombophilia. The duration of the extended phase is variable and can be lifelong in confirmed thrombophilia.
Thrombus removal: thrombolysis vs mechanical options
Early thrombus clearance is critical in acute Paget-Schroetter syndrome. Successful intervention restores venous patency, reduces inflammatory damage, and potentially reduces the risk of progression to PTS. For optimum outcome, a thrombus removal strategy needs to be offered in the first 14 days of DVT onset for patients with confirmed axillary-subclavian DVT and a threatened limb (painful, swollen, and cyanotic limb) particularly for those who show poor response to the initial anticoagulation. In a previous retrospective study, patients with the worst outcomes, when offered decompression and revascularization at a later stage, were those patients who had persistent symptoms despite anticoagulation.9
Methods of thrombus removal
Catheter-directed thrombolysis (CDT) : This technique involves infusion of tissue plasminogen activator (tPA) via multi-side-hole catheter inserted within the thrombus over 12 to 24 hours. During this time, the patients require one-on-one care in a high dependency unit (HDU) or intensive treatment unit (ITU) bed (Figures 8 and 9).
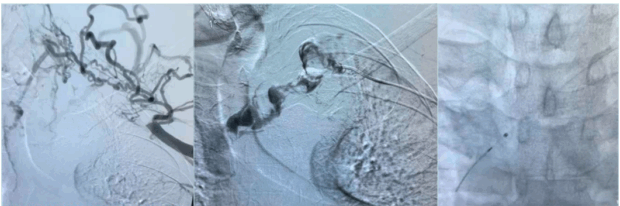
Figure 8. Initial venogram shows thrombosed axillary-subclavian vein (left image), selective venogram beyond the thrombus (middle image), and fluoroscopic picture confirming location of the tip of the infusion catheter distal to the thrombus (right image).
Pharmacomechanical thrombectomy (PMT) : This technique combines the local thrombolytic infusion with mechanical clot fragmentation and aspiration (eg, AngioJet catheter) using lower doses of r-tPA over shorter duration (short procedure time) to reduce the bleeding risk.
Mechanical thrombectomy (MT) : This technique offers pure mechanical clot retrieval without infusion of any thrombolytic material, it is particularly useful in patients with contraindication to thrombolytics, eg, allergy, recent surgery, bleeding disorder, etc.
After thrombus removal, immediate completion venography with or without IVUS is recommended to confirm the flow restoration; detect any residual webs, stenosis, or intimal hyperplasia; and to guide decisions about the need for additional decompression or revascularization procedure (Figure 10).
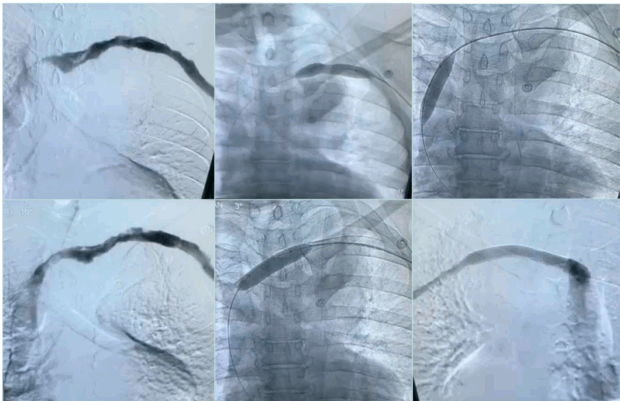
Figure 10. Post-lysis venous stenosis was persistent after balloon venoplasty, treated with venous stent.
Studies show that thrombus removal should be followed by surgical decompression for definitive treatment of VTOS.
The primary 5-year patency of the axillary-subclavian vein in patients who undergo thrombolysis followed by surgical decompression is 84%.15 Thus, there is a growing consensus that early diagnosis and early thrombus removal followed by operative first rib resection (FRR) produces the most desirable long-term outcome. Therefore, an early thrombus removal strategy should always be considered as a temporizing measure that enables definitive decompression.
Surgical decompression
Surgical decompression is the cornerstone of definitive VTOS therapy. It is indicated in all symptomatic types, the thrombotic (Paget-Schroetter syndrome) after thrombus removal, the nonthrombotic (McCleery syndrome) with severe disabling symptoms, and the postthrombotic type with persistent disabling symptoms. Resection of the first rib eliminates the anatomical cause of venous compression, reduces recurrence risk, and improves long-term functional outcomes. In a systematic review and meta-analysis, the clinical improvement after surgical decompression of TOS was 90%.16 Decompression is not indicated in asymptomatic VTOS or if the symptoms are mild.
Surgical techniques for first rib resection (FRR)
Transaxillary approach: This is the most widely performed approach, cosmetically appealing due to the hidden scar in the axilla. It facilitates complete resection of the first rib; however, it provides limited vascular exposure and control, therefore open surgical revascularization cannot be performed from this approach.
Infraclavicular approach: This approach has been gaining popularity in recent years due to its versatility. It allows both the decompression facilitating partial FRR with its associated costoclavicular ligament and subclavius muscles and the revascularization by venous patching or endovenectomy. Furthermore, it gives the advantage of avoiding dissection near the brachial plexus and thoracic duct, reducing the risk of injuring these structures; on the other hand, it may not allow complete rib resection.
Paraclavicular approach: This approach combines the supra-and infraclavicular incisions; it allows complete resection of the first rib but with more risk of nerve or thoracic duct injury. It is usually reserved for complex cases requiring extensive neurovascular decompression.
There is lack in the literature of the direct comparison between the infraclavicular approach and the other approaches; most studies compare the transaxillary approach to the paraclavicular one (supra- +/- infraclavicular) or to the minimally invasive video-assisted thoracic surgery (VATS).
In a prospective study, the FRR using the infraclavicular approach showed similar technical success and clinical improvement when compared with the paraclavicular approach, however, with a trend toward less morbidity.9
Rib-sparing decompression
This novel strategy advocates decompressing the subclavian vein by excising the soft tissue elements contributing to the compression without excising the first rib, such as division of hypertrophied subclavius muscle, resection of the fibrous band and the scalene muscle (scalenectomy). This strategy is an emerging alternative for nonthrombotic VTOS; however, its long-term durability remains uncertain.17
Paget-Schroetter syndrome patients who undergo a thrombus removal procedure can be offered decompression surgery early (within 2 weeks from the thrombus removal procedure) or delayed (4-6 weeks after the thrombus removal procedure). Early decompression offers the advantage of dissection in a field free of fibrosis or scarring, and it enables patients to restore their limb function sooner. Delayed decompression allows the inflammation to resolve, potentially reducing the bleeding and the technical difficulty; however, it carries a risk of recurrent thrombosis while awaiting the decompression surgery.
Retrospective studies favor earlier decompression, showing better symptom resolution and long-term patency. However, optimal timing remains debated and must consider patient-specific factors.
Venous revascularization: when decompression alone is not enough
Despite effective decompression, some patients—particularly those with chronic postthrombotic VTOS—may continue to exhibit residual venous narrowing or functional obstruction. This can result from long-standing intimal fibrosis, wall thickening, or anatomical webs. In these cases, adjunctive venous revascularization becomes necessary to restore adequate flow and optimize long-term symptom control.
The most common indication of revascularization after decompression is the persistent significant venous stenosis (due to incomplete lumen restoration or intimal damage and scarring) confirmed intraoperatively by IVUS or completion venography. If neither venography nor IVUS are employed in the same sitting of the decompression, the persistence of significant disabling symptoms after the decompression can be indicative of flow limitation despite anatomic release and hence an indication for revascularization.
Revascularization modalities
Open surgical revascularization: This can involve patch venoplasty and/or endovenectomy.
Patch venoplasty can be performed either through the infraclavicular or the paraclavicular approach. It involves longitudinal venotomy and patch augmentation with autologous, biologic, or synthetic material and is suitable for segmental strictures and severely fibrotic veins.
The endovenectomy involves surgical removal of intimal webs, the adherent thrombus, and/or the fibrotic tissue. It is often combined with patch closure, and it is technically demanding, requiring high-level surgical expertise.
Percutaneous revascularization: This can involve balloon venoplasty and/or venous stenting.
Balloon venoplasty can be performed in the same sitting of the decompression surgery if venography is employed or be performed in a later stage after the decompression surgery. It is usually effective; however, recoil is common in chronic cases.
Venous stenting is reserved for cases of recurrent or refractory stenosis where open repair is not feasible. The long-term durability is uncertain, especially in young, active individuals. The risks associated with venous stent include stent fracture, compression, migration, and in-stent restenosis due to thoracic outlet mobility.
In retrospective studies, the venous reconstruction in Paget-Schroetter syndrome was done by either venous patch through the infraclavicular approach or by balloon venoplasty with or without venous stent and showed significant symptoms improvement (98.9%) with overall secondary venous patency rate of 98.5% and freedom from reintervention of 89.9% over an average follow-up of 23 months.18
Integrated treatment algorithm
The algorithm in Figure 11 is a clinical type-specific framework that allows individualized, stepwise escalation of therapy while minimizing unnecessary interventions.
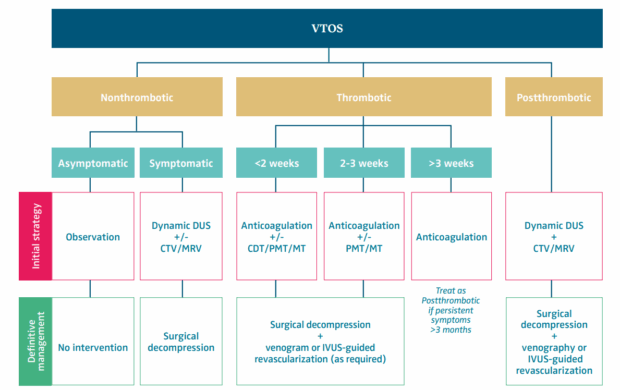
Figure 11. Treatment algorithm for different forms of venous thoracic outlet syndrome (VTOS). AC, anticoagulation; CDT, catheter-directed thrombolysis; CTV, computed tomography venography; DUS, duplex ultrasound; FRR, first rib resection; IVUS, intravascular ultrasound; MRV, magnetic resonance venography; MT, mechanical thrombectomy; PMT, pharmacomechanical thrombectomy; PTS, postthrombotic syndrome.
Conclusion
VTOS represents a complex, under-recognized vascular disorder with considerable functional consequences if left untreated. Although historically less emphasized than neurogenic forms, VTOS is increasingly identified in young, active individuals and demands a proactive, multidisciplinary treatment strategy.
Timely diagnosis is crucial, relying on a combination of clinical suspicion and provocative imaging. Dynamic DUS, complemented by advanced modalities such as MR venography and IVUS, plays a pivotal role in determining both the presence and nature of the venous lesion.
Management requires more than anticoagulation. In acute cases, thrombolysis or thrombectomy restores venous patency and enables decompression. Surgical decompression, most commonly via transaxillary or infraclavicular FRR, addresses the mechanical cause. When decompression alone is insufficient, venous revascularization—particularly patch venoplasty and endovenectomy guided by IVUS—provides a path toward functional recovery. Stenting remains a controversial but occasionally necessary adjunct.
As treatment paradigms evolve, areas ripe for future research include the following: comparative outcomes of rib-sparing versus rib-resecting approaches, the optimal timing of decompression, durability of venous reconstruction, and the long-term safety of thoracic outlet stenting.
VTOS, when approached systematically, is highly treatable. Structured evaluation, tailored intervention, and vigilant follow-up can restore limb function, prevent recurrence, and dramatically improve patient outcomes. This shift from underdiagnosis to protocol-driven care marks the next frontier in modern venous practice.
CORRESPONDING AUTHOR
Ahmed S. Gaweesh
Department of Vascular Surgery, Faculty of Medicine, University of Alexandria, iVein Clinic, 8 Koleyet el Teb Street, Alexandria, Egypt
e-mail: agaweesh@gmail.com
Dr Ahmed Gaweesh received an honorarium from Servier for the writing of this article.
References
1. Illig KA, Rodriguez-Zoppi E, Bland T, Muftah M, Jospitre E. The incidence of thoracic outlet syndrome. Ann Vasc Surg. 2021;70:263-272.
2. Audu CO, Vemuri C, Urschel HC, Pool JM, Patel AN. Anatomy and pathophysiology of venous thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, Donahue DM, Jordan SE, Lum YW, Gelabert HA, eds. Thoracic Outlet Syndrome. 2nd ed. Springer; 2021:487-494.
3. Thiyagarajah K, Ellingwood L, Endres K, et al. Post-thrombotic syndrome and recurrent thromboembolism in patients with upper extremity deep vein thrombosis: a systematic review and meta-analysis. Thromb Res. 2019;174:34- 39.
4. Kahn SR, Elman EA, Bornais C, Blostein M, Wells PS. Post-thrombotic syndrome, functional disability and quality of life after upper extremity deep venous thrombosis in adults. Thromb Haemost. 2005;93(03):499-502.
5. Illig KA. Diagnosis of vTOS: 2016 consensus guidelines. In: Illig KA, Thompson RW, Freischlag JA, Donahue DM, Jordan SE, Lum YW, Gelabert HA, eds. Thoracic Outlet Syndrome. 2nd ed. Springer; 2021:495-499.
6. Illig KA, Donahue D, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg. 2016;64(3):e23-e35.
7. Pesser N, van den Houten MM, van Sambeek MR, Teijink JA. Venous thoracic outlet syndrome caused by double compression of the axillosubclavian vein: a case report. EJVES Vascular Forum. 2020;47:38-41.
8. Tsekouras N, Comerota AJ. Current trends in the treatment of venous thoracic outlet syndrome: a comprehensive review. Interv Cardiol. 2014;6(1):103-115.
9. Elsabbagh MA, Salem ME, Mabrouk MK, El-Emam AA, Gaweesh A, Tiwari A. Early results of interventions in patients with venous thoracic outlet syndrome. Ann Vasc Surg. 2025;110:323-328.
10. Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609- 614.
11. Chang YC, Su CT, Yang PC, Wang TC, Chiu LC, Hsu JC. Magnetic resonance angiography in the diagnosis of thoracic venous obstruction. J Formos Med Assoc. 1998;97(1):38-43.
12. Kim TI, Sarac TP, Orion KC. Intravascular ultrasound in venous thoracic outlet syndrome. Ann Vasc Surg. 2019;54:118- 122.
13. Gelabert MC, Gelabert HA. Controversies in VTOS: how long should anticoagulation be used in VTOS? In: Illig KA, Thompson RW, Freischlag JA, Donahue DM, Jordan SE, Lum YW, Gelabert HA, eds. Thoracic Outlet Syndrome. 2nd ed. Springer; 2021:699- 708.
14. Espitia O, Raimbeau A, Planquette B, et al. A systematic review and meta-analysis of the incidence of post-thrombotic syndrome, recurrent thromboembolism, and bleeding after upper extremity vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2024;12(1):101688.
15. Doyle A, Wolford HY, Davies MG, et al. Management of effort thrombosis of the subclavian vein: today’s treatment. Ann Vasc Surg. 2007;21(6):723-729.
16. Peek J, Vos CG, Ünlü Ç, van de Pavoordt HD, van den Akker PJ, de Vries JP. Outcome of surgical treatment for thoracic outlet syndrome: systematic review and meta-analysis. Ann Vasc Surg. 2017;40:303- 326.
17. Jaklin FJ, Platzgummer H, Reissig L, et al. Rib-sparing subclavian vein decompression in venous thoracic outlet syndrome. J Plast Reconstr Aesthet Surg. 2025;100:24-31.
18. Samoila G, Twine CP, Williams IM. The infraclavicular approach for Paget– Schroetter syndrome. Ann R Coll Surg Engl. 2018;100(2):83-91.