A comprehensive review of the diagnosis and treatment of nutcracker syndrome
Mert Dumantepe, MD
Florence Nightingale Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey.
Cuneyd Öztürk, MD
Florence Nightingale Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey.
Vincenzo Ardita, MD, PhD
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Ferdinando B. A. Valente, MD
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Domenico Baccellieri, MD, PhD
Vein Center, Vascular Surgery Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
Alejandro Rodriguez Morata, MD
Department of Angiology and Vascular Surgery, Hospital Quirónsalud, Málaga, Spain
ABSTRACT
Nutcracker syndrome (NCS) describes the symptomatic compression of the left renal vein between the aorta and superior mesenteric artery. It causes a series of clinical symptoms including hematuria, proteinuria, flank pain, postprandial abdominal discomfort, and pelvic symptoms in women such as dysmenorrhea or dispareunia. Long-standing venous compression can encourage collateral drainage pathways through gonadal and pelvic veins, which may explain reported symptoms, and the syndrome may overlap with the pelvic congestion syndrome. Although it may be associated with substantial morbidity, the diagnosis can be challenging and variable, frequently involving a combination of Doppler ultrasound and cross-sectional imaging. For most centers, it appears that surgery remains the first-line treatment; however, endovascular alternatives are rapidly evolving in the field with favorable outcomes. This article reviews current concepts on NCS with a particular focus on diagnosis and contemporary surgical and endovascular treatment methods and their outcomes.
Introduction
Nutcracker syndrome (NCS) describes symptomatic compression of the left renal vein (LRV) between the aorta and superior mesenteric artery (SMA).1 The SMA typically arises from the aorta at a right angle, proceeding ventrally before curving caudad, usually preventing LRV entrapment. When the SMA arises at a more acute angle or the LRV follows a high course, compression may result. NCS refers to symptoms caused by venous hypertension in the left kidney due to mesoaortic compression of the LRV. The term “nutcracker phenomenon” indicates anatomical LRV compression without clinical symptoms. Asymptomatic compression is often seen on imaging, but NCS patients may present with hematuria, orthostatic proteinuria, flank or pelvic pain, dyspareunia, dysmenorrhea, and fatigue.2
The anatomical feature was first described by Grant in 1937: “the left renal vein, as it lies between aorta and superior mesenteric artery, resembles a nut between the jaws of a nutcracker.”3 In 1950, El Sadr and Mina described LRV compression by the aorta and SMA.4 Though “nutcracker” appeared in 1971, De Schepper coined “nutcracker syndrome” in 1972.5
NCS prevalence is unclear due to variable symptoms and lack of diagnostic consensus. However, unexplained hematuria is common, and NCS is diagnosed by Doppler ultrasonography (DUS) in 40% of such cases.6 It can affect all ages but peaks in the second and third decades, aligning with vertebral growth.7 Though once thought more prevalent in females, recent studies show equal gender distribution.8
Anatomy and pathophysiology
There are 2 main anatomical configurations of NCS. The most common, anterior NCS, refers to compression of the LRV between the abdominal aorta and SMA. In some cases, the third portion of the duodenum also lies between the aorta and the SMA, in front of the LRV. Therefore, anterior NCS may rarely occur with superior mesenteric artery syndrome (compression of the duodenum by the SMA, ie, Wilkie’s syndrome).9 The less common variant is posterior NCS, in which the LRV is compressed between the aorta and the vertebral body. NCS can also occur from other causes, such as malignancy, lymphadenopathy, severe lordosis, intestinal malrotation, pregnancy, and rapid weight loss.10
The normal aortomesenteric angle ranges from 38° to 65°.
According to Shin et al, to diagnose NCS, the angle between the SMA and the aorta needs to be less than 45° when measured in the sagittal plane specifically, an angle less than 35° is sufficient for a definitive diagnosis.7 In most of the symptomatic NCS patients, this angle is reduced to less than 22°, causing collapse of the LRV.
From a pathophysiological point of view, sustained elevation of pressure in the LRV causes venous hypertension with retrograde transmission toward perirenal and pelvic venous plexuses. This can lead to orthostatic proteinuria due to glomerular hyperfiltration secondary to venous congestion, hematuria due to rupture of submucosal calyceal venules, and flank pain due to renal capsule distension.11
Clinical manifestations
bsence of definitive diagnostic criteria and the variability in symptomatic presentation, and therefore probably underdiagnosed. Common symptoms include hematuria (micro or macroscopic), proteinuria, left flank pain, postprandial abdominal discomfort, and pelvic symptoms in women such as dysmenorrhea or dyspareunia. In a study of 112 patients, hematuria occurred in 79%, left flank pain in 38%, varicocele in 36%, proteinuria in 31%, and anemia in 13%.12 In men, it may appear as a left-sided varicocele unresponsive to conventional treatment. Elevated pressure in the stenosed LRV is thought to cause venous reflux and hypertension, leading to varices between the renal pelvis and ureter, manifesting as micro- or macrohematuria.13
Orthostatic proteinuria is another feature, believed to result from increased LRV pressure, subclinical immune injury, and altered renal hemodynamics on standing. These changes may trigger excessive angiotensin II and norepinephrine release.14 However, orthostatic proteinuria is relatively common in the pediatric population, affecting 2% to 5% of children and young adults, usually with a benign course.15 Whereas some patients experience persistent symptoms, others, especially children, may remain asymptomatic.
The LRV communicates with the left gonadal vein and lumbar plexus, which often dilate to compensate for venous outflow. In severe cases, ovarian vein dilatation and reflux may lead to PCS,16 characterized by noncyclic chronic pelvic pain or heaviness due to gonadal vein reflux and dilation.17
NCS should be distinguished from other causes of hematuria (eg, lithiasis, nephritis), abdominal pain (Wilkie’s syndrome, SMA syndrome, endometriosis), idiopathic varicocele, or isolated pelvic congestion. It may also coexist with other compression syndromes such as median arcuate ligament syndrome (MALS) and May-Thurner syndrome (MTS).
Diagnosis
The diagnostic approach for suspected NCS depends on clinical severity, comorbidities, and the need to exclude other conditions. Diagnosis is challenging due to symptom variability and lack of definitive criteria. Imaging—including DUS, computed tomography angiography (CTA), magnetic resonance imaging (MRI), venography, and intravascular ultrasound (IVUS)—is essential, but clinical correlation is crucial for management.
Doppler ultrasonography
DUS is the preferred initial imaging for NCS, with sensitivity of 69% to 90% and specificity of 89% to 100%.18 It assesses LRV collapse, blood flow velocity, and the aortomesenteric angle. Multiplanar imaging is essential, with patients hydrated and ideally fasted for 6 to 8 hours. Positional changes (supine, prone, upright) affect results.19 Ratios are often elevated in the upright position due to gravity worsening LRV compression.
DUS must exclude mass lesions and assess the SMA angle. The LRV diameter is measured at both proximal and distal sites of compression, with peak velocities recorded. Diagnostic DUS findings include a velocity >100 cm/s at the compressed site, >70% luminal narrowing, and a Vmax/Vmean ratio >4.5 between the narrowed and hilar segments.20
Computerized tomography angiography
CTA provides high-resolution images of renal vasculature and adjacent structures without being invasive,21 though it lacks flow data. Sensitivity and specificity are 92% and 89%, respectively.22 CTA identifies LRV compression by comparing the diameter at the renal hilum and narrowed segment, and by evaluating the SMA– aorta angle. Normally 45° to 90°, an angle ≤35° suggests NCS.23 On axial images, the “beak” sign—an abrupt narrowing of the LRV— has a sensitivity of 92% and specificity of 89% (Figure 1). 23 Since absolute diameters vary, the hilar-to-compressed diameter ratio is more reliable, with sensitivity of 67% and specificity of 100%.23,24
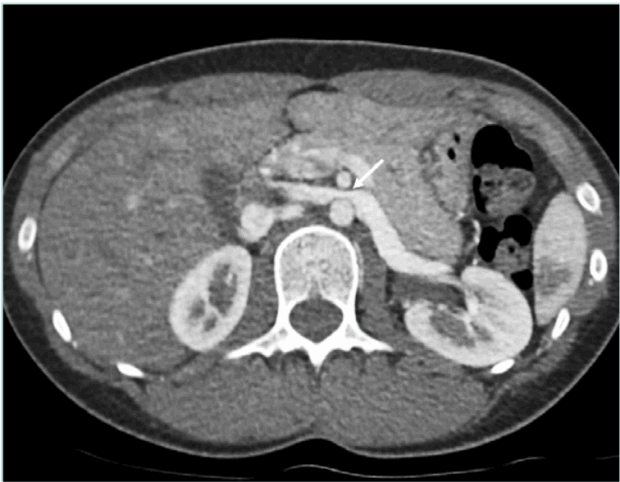
Figure 1. Axial CTA image shows compressed left renal vein between aorta and SMA and characteristic “beak” sign with abrupt narrowing of the LRV at the SMA level (white arrow). CTA, computed tomography angiography; LRV, left renal vein; SMA, superior mesenteric artery.
Magnetic resonance imaging
MRI avoids ionizing radiation and is thus preferable in children and pregnant individuals. It evaluates vein diameter ratios and the “beak sign” similarly to CTA. MRI offers superior soft-tissue contrast and comparable sensitivity and specificity.25
Venography and intravascular ultrasonography
If imaging remains inconclusive, venography with pressure gradient measurement or IVUS is considered. Though invasive, they remain the diagnostic gold standard.26 Direct measurement of the LRV to vena cava pressure gradient is considered the definitive diagnostic test for NCS, although it is usually unnecessary. A renocaval gradient >3 mm Hg confirms NCS. In healthy individuals, the gradient is usually 0 to 1 mm Hg.27
Venography is best reserved for preoperative planning. It demonstrates LRV narrowing, collaterals, and venous reflux (Figure 2). Pressure readings further aid diagnosis.27 However, some normal individuals may exhibit >3 mm Hg gradients, and patients with collaterals may show normal pressures.
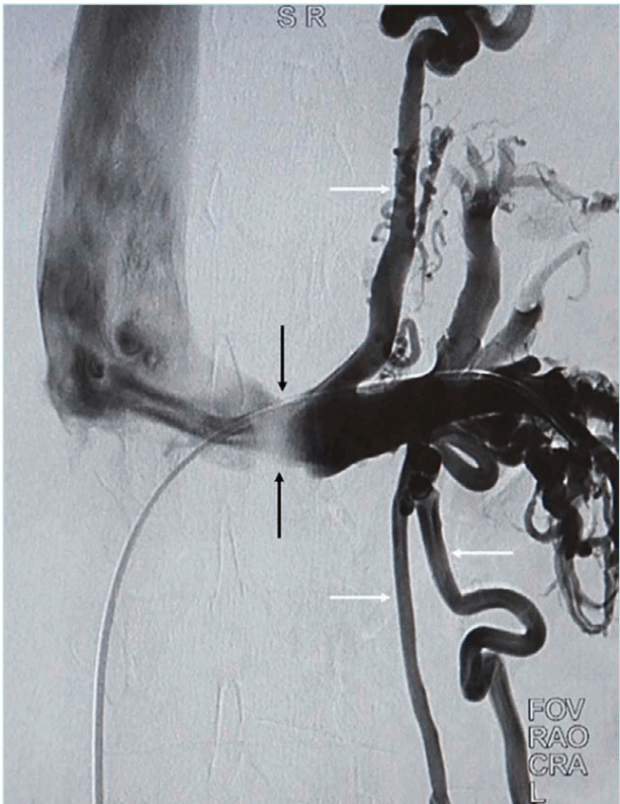
Figure 2. Venography demonstrates maximal compression and poor opacification of the aortomesenteric level of LRV (black arrows) with preferential collateral flow via the
splenic venous plexus and gonadal veins (white arrows). LRV, left renal vein.
IVUS assesses LRV diameter and can be done during venography.28 An ultrasonic catheter is introduced through femoral access and slowly withdrawn from the LRV through the SMA and into the inferior vena cava (IVC). IVUS is essential for sizing and confirming proper stent placement.28
Treatment
The treatment of NCS remains controversial due to inconsistent diagnostic criteria and the lack of consensus on the optimal treatment modality. Management is symptom driven. By consensus, the asymptomatic nutcracker phenomenon does not require treatment.1,2,29
Guidelines from the European Society for Vascular Surgery recommend 6 months of conservative therapy for adults with mild symptoms, and 24 months for children aged 18 or younger.2,30
Conservative management
Conservative treatment is often effective, especially in children. Most pediatric cases resolve spontaneously, likely due to increased intra-abdominal and retroperitoneal fat growth and fibrous tissue accumulation near the origin of the SMA, which relieves compression on the LRV. Collateral venous development also lowers LRV pressure.19 These mechanisms explain the complete symptom resolution in up to 75% of younger patients over 24 months.31
Angiotensin-converting enzyme inhibitors, especially alacepril, are widely used in pediatric patients to manage orthostatic proteinuria and hypertension. Aspirin may be added to improve renal perfusion.32
Elastic compression stockings can reduce pelvic or flank pain. Underweight or low-normal body mass index patients may benefit from weight gain, which resolves symptoms in up to 30% of such cases.33
Surgical treatment
Surgery is indicated to prevent chronic glomerulopathy, impaired renal function, permanent gonadal vein dilation, and renal vein thrombosis. Due to higher morbidity, open surgery is reserved for patients with persistent symptoms like hematuria, proteinuria, severe pain, autonomic dysfunction, renal failure, or varicocele. Common procedures include renal vein transposition, gonadal vein transposition, renocaval bypass, venous bypass, renal autotransplantation, and endovascular stenting.34
Open surgical treatment
Transposition of the LRV: Renal vein transposition is the standard of care for patients with non-remitting symptoms and is the most common invasive intervention for anterior NCS, with excellent immediate and long-term symptom control. This procedure was first described in 1982 and quickly became the standard surgical approach to NCS and between 80% and 100% of patients reported relief of flank pain and hematuria following renal vein transposition.2,34 Hartung et al reviewed 42 cases presenting with NCS managed surgically; 83.3% of patients reported successful resolution of symptoms, demonstrating the important role of open surgery in the management of this condition.35
LRV transposition entails excision of the vessels at the IVC junction with reimplantation distal to the SMA. It is carried out through a midline transperitoneal approach. For patients who have an LRV with a permanent distortion caused by long-term compression or those in whom the LRV is excessively tensioned after transposition, the great saphenous vein can be used as a patch or extension graft, respectively. Although renal vein transposition or bypass is a relatively low-risk procedure, reported complications include paralytic ileus, small bowel adhesion, retroperitoneal hematoma formation, and LRV restenosis.30,24
Renocaval bypass: A considerable number of patients undergo restenosis and occlusion of the transposed vein and require reintervention. The renocaval bypass technique employs the great saphenous vein to construct a bypass and does not require transposition of the LRV. The saphenous vein is anastomosed proximally to the IVC, below the LRV, and distally to the LRV. Both anastomoses are performed with partial clamping so that they have little effect on venous hemodynamics. The advantages of this operation are the short period of renal ischemia and few anastomoses and no need to ligate the lumbar veins, the gonadal vein, or the LRV if they are not refluxing, since they do not affect the anastomoses. However, owing to the risk of shortening, restenosis, or collapsing of the saphenous vein graft because of the aorta, the renocaval bypass with prosthetic graft is the preferred technique in adult patients with significant collaterals and unfavorable anatomy. For this purpose, ringed reinforced polytetrafluoroethylene (PTFE) grafts are used to connect the LRV to the IVC with a C-Shape (Figure 3). Deser et al report successful use of a PTFE prosthetic graft for renocaval bypass in a patient with posterior NCS.36 Furthermore, Morata renal failure, or varicocele. Common procedures include renal vein transposition, gonadal vein transposition, renocaval bypass, venous bypass, renal autotransplantation, and endovascular stenting.34 et al reported over 40 procedures and more than 6 years of follow-up with PTFE graft renocaval bypass.29 Severe thrombotic or stenotic complications occurred in only 11% of these patients. They also highlighted that it is essential to cover the bypass graft with an omental flap (epipoplasty) to prevent duodenal fistula risk. The advantages of this operation are the short period of renal ischemia and few anastomoses, with high rates of symptomatic resolution, especially for complaints of hematuria and flank pain, and it is considered the gold standard treatment for NCS.29,34,35
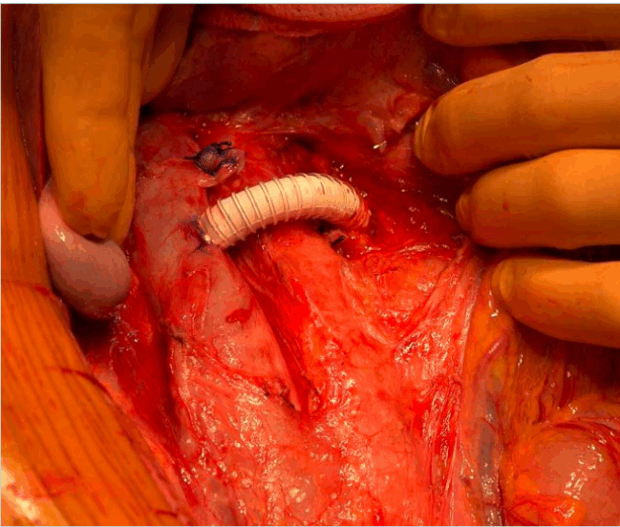
Figure 3. Renocaval bypass using ringed reinforced prosthetic polytetrafluoroethylene (PTFE) graft with transperitoneal approach.
Renal autotransplantation: This is also relatively common, though more invasive, as it involves relocating the kidney to the iliac fossa. The technique was first reported by Hardy et al following ureteral injury.37 It is considered a complete procedure because it effectively normalizes LRV pressure levels and corrects any possible posterior renal ptosis, offers excellent results, and is associated with low morbidity. Shokier et al reported cessation of hematuria in patients with CT-diagnosed NCS in all patients who underwent autotransplantation, with individuals remaining asymptomatic at 1-year follow-up.38 Despite being invasive, renal autotransplantation may be more efficacious at normalizing LRV pressures. However, as a more invasive procedure compared with LRV transposition, renal autotransplantation does carry additional risks including increased renal ischemia time, a more extensive surgical dissection, and a total of 3 anastomoses (artery, vein, and ureter) with consequent possible complications at any of these sites.
Transposition of the left ovarian vein: In well-selected patients, left ovarian vein transposition (LOVT) for NCS reliably relieves left renal venous hypertension by redirecting left renal venous outflow through the LOVT into the common iliac vein or IVC, reducing pelvic venous congestion and its attendant symptoms. It avoids a large open abdominal procedure, the use of prosthetic materials, and stents. The morbidity of the procedure is limited, patients recover quickly, and they experience sustained relief of symptoms.39 The retroperitoneal approach for LRV decompression requires minimal manipulation of the ovarian vein, provides access to treatment of the symptomatic pelvic varicose veins, and avoids the subsequent risks of intra-abdominal problems (Figure 4). It provides an alternative for failed renal vein transposition or renal vein stents.40 Cosmetically, the incision is smaller and less apparent, which is of importance to the many young women affected by NCS.
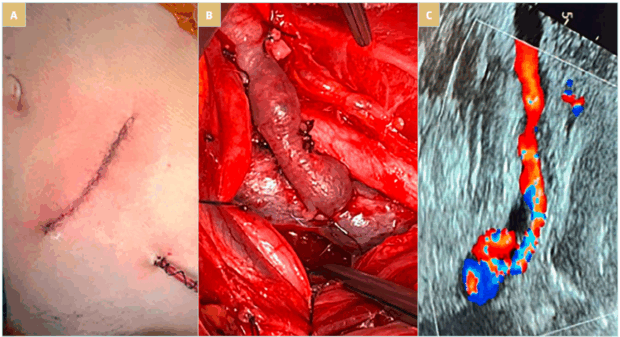
Figure 4. (A)The oblique incision for retroperitoneal approach to the ovarian vein for transposition. (B) Left ovarian vein to common iliac vein anastomosis. The proximity of the veins permits the construction of a well configured anastomosis. (C) Follow-up duplex ultrasonography shows sufficient left ovarian vein flow at 1 year.
Laparoscopic surgery: Minimally invasive approaches with laparoscopic or robot assisted–laparoscopic techniques reduced recovery times and are increasingly becoming standard practice.41 Nevertheless, reintervention rates have been reported to be up to 68%, mostly for restenosis of the LRV.42 The literature on laparoscopic surgery in NCS is largely limited to case reports or small series. However, the outcomes of laparoscopic procedures reported in the literature are comparable with those of open procedures; these include laparoscopic spleno-renal venous bypass and laparoscopic LRV-IVC transposition, with the latter avoiding complications to the spleen from ischemia and tears, as it bypasses the portal venous circulation where pressure is higher than central venous pressure, hence maintaining LRV hypertension. Hartung et al reported successful outcomes at 12-month follow-up following laparoscopic transposition of the LRV into the IVC for a 40-year-old lady where disabling pain in the left lumbar region and hematuria resolved, and on duplex ultrasound the reconstruction remained patent.43 Experience with robotically assisted laparoscopic LRV transposition has also been limited to case reports. Wang et al report a case with no postoperative complications and hematuria resolved in 1 month.44 Thaveau et al report the use of robotically assisted LRV transposition followed by left ovarian vein embolization with DUS performed at 6-month follow-up revealing a competent LRV.45 Further studies are required to assess the long-term outcomes and cost-effectiveness of this procedure with robotically assisted LRV transposition.
Endovascular treatments
Endovascular stenting: This approach is gaining popularity due to its minimally invasive nature, despite the lack of stents specifically designed for the renal vein. Currently available venous stents with high radial force and flexibility have proven effective. A retrospective analysis reported 96.7% symptom improvement in 59 of 61 patients at 6 months, with no significant restenosis at 66 months.46 Wang et al found pressure gradient reduction and symptom regression in 29 of 30 patients within 6 months.47 In a comparative study, all 15 patients treated with stents became asymptomatic, though one required surgery for stent migration.48 Over 150 successful endovascular cases have been documented; however, long-term data remain limited, especially in younger patients.49
The most used stents are self-expanding and should be 60 to 80 mm in length, placed near the LRV’s first division. Oversizing by 20% is advised to prevent migration. Complications include incorrect placement, migration (7.3% rate), partial or complete displacement into the IVC or renal hilum, and rare cases of embolization, thrombosis, restenosis, or fracture.47-49 Migration is linked to cardiac motion, early activity, mismatched sizing, or poor positioning.30,48 A study with 75 patients and 55-month follow-up showed a 6.7% migration rate, without clear correlations to stent size or placement.50 In a review of 18 cases, 2-year patency was 85.2%, but stenting after LRV transposition showed limited benefit, with 3 of 5 patients still symptomatic.50,51
Hybrid treatment: To mitigate stent migration at the aortomesenteric level, hybrid treatment combines conventional endovenous stenting with transfixing polypropylene stitches (Figure 5). 52 This laparoscopic or open surgical fixation method stabilizes the stent and reduces migration risk. Hybrid procedures are emerging as promising, especially for young patients with high-risk anatomy.
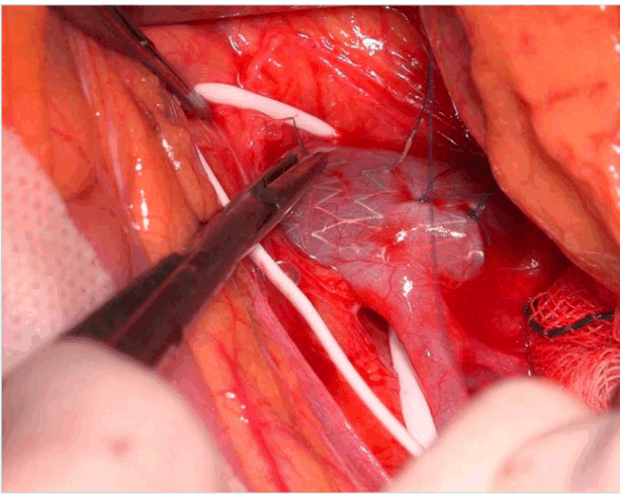
Figure 5. Peroperative view of hybrid treatment shows transfixing polypropylene stitch through the renal vein wall and the stent meshes after endovascular venous stenting.
Embolization of the LGV: Patients with or without hematuria may experience gonadal vein insufficiency, resulting in varicocele in men and pelvic congestion in women. The ovarian vein connects to the gonadal, uterine, gluteal, and vulvoperineal venous plexuses, which may contribute to pelvic symptoms. Embolization of the LGV is the gold standard for treating pelvic congestion due to ovarian or pelvic vein insufficiency. Treating LRV compression may not sufficiently address gonadal reflux. Embolic agents include foam, glue, plugs, liquid sclerosants, and coils. The decision to treat should be based on the presence of renal or pelvic symptoms, reflux severity, and varicose anatomy. Clinical expertise should guide embolization in conjunction with anatomical and functional assessments.53,54
Conclusions
Patients with NCS may experience a wide spectrum of symptoms. Early recognition and treatment of those with significant pain and hematuria are essential. Clinical judgment and a high index of suspicion are critical, particularly in patients without prior imaging. Multiple surgical options exist, and only solid theoretical and technical expertise ensures proper selection. LRV transposition and autotransplantation
remain the mainstay treatment. However, laparoscopic techniques are gaining ground due to lower postoperative morbidity. Further research is needed to refine diagnostic protocols, assess long-term outcomes of treatments, and create consensus guidelines.
CORRESPONDING AUTHOR
Mert Dumantepe
Florence Nightingale Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey
e-mail: mdumantepe@gmail.com
Dr Mert Dumantepe received an honorarium from Servier for the writing of this article.
References
1. Dieleman F, Hamming JF, Erben Y, van der Vorst JR. Nutcracker syndrome: challenges in diagnosis and surgical treatment. Ann Vasc Surg. 2023;94:178-185.
2. Ananthan K, Onida S, Davies AH. Nutcracker syndrome: an update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53:886-894.
3. Grant J. Anonymous method of anatomy. Williams and Wilkins; 1937:137.
4. El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev. 1950;54:257-262.
5. De Schepper A. “Nutcracker” phenomenon of the renal vein and venous pathology of the left kidney. J Belge Radiol. 1972;55:507-511.
6. Ahmed K, Sampath R, Khan MS. Current trends in the diagnosis and management of renal nutcracker syndrome: a review. Eur J Vasc Endovasc Surg. 2006;31:410-416.
7. Shin JI, Lee JS, Kim MJ. The prevalence, physical characteristics and diagnosis of nutcracker syndrome. Eur J Vasc Endovasc Surg. 2006;32:335-336.
8. He Y, Wu Z, Chen S, et al. Nutcracker syndrome – how well do we know it? Urology. 2014;83:12-17.
9. D’Oria M, Zlatanovic P, Anthony A, et al. International Union of Angiology consensus document on vascular compression syndromes. Int Angiol. 2023;42(4):282-309.
10. Muraoka N, Sakai T, Kimura H, et al. Rare causes of hematuria associated with various vascular diseases involving the upper urinary tract. Radiographics. 2008;28:855-867.
11. Pascarella L, Penn A, Schmid-Schonbein GW. Venous hypertension and the inflammatory cascade: major manifestations and trigger mechanisms. Angiology. 2005;56(Suppl 1):S3-S10.
12. Orczyk K, Łabetowicz P, Lodziński S, Stefańczyk L, Topol M, Polguj M. The nutcracker syndrome. Morphology and clinical aspects of the important vascular variations: a systematic study of 112 cases. Int Angiol. 2016;35(1):71-77.
13. Stewart BH, Reiman G. Left renal venous hypertension “nutcracker” syndrome. Managed by direct renocaval reimplantation. Urology. 1982;20:365- 369.
14. Mazzoni MB, Kottanatu L, Simonetti GD, et al. Renal vein obstruction and orthostatic proteinuria: a review. Nephrol Dial Transplant. 2011;26:562-565.
15. Mahan JD, Turman MA, Mentser MI. Evaluation of hematuria, proteinuria, and hypertension in adolescents. Pediatr Clin North Am. 1997;44:1573-1589.
16. Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34:812-819.
17. Durham JD, Machan L. Pelvic congestion syndrome. Semin Intervent Radiol. 2013;30:372-380.
18. Nastasi DR, Fraser AR, Williams AB, Bhamidi V. A systematic review on nutcracker syndrome and proposed diagnostic algorithm. J Vasc Surg Venous Lymphat Disord. 2022;10(6):1410-1416.
19. Kim SH, Cho SW, Kim HD, et al. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996;198:93-97.
20. Englund KM, Rayment M. Nutcracker syndrome: a proposed ultrasound protocol. Australas J Ultrasound Med. 2018;21(2):75-78.
21. Duncan AA. How I treat nutcracker syndrome. J Vasc Surg Cases Innov Tech. 2023;9(4):101344.
22. Galanakis N, Kontopodis N, Matthaiou N, Tsetis D, Kehagias E. Multimodality assessment of nutcracker syndrome in a young patient. Vasc Specialist Int. 2023;39:11.
23. Hangge PT, Gupta N, Khurana A, et al. Degree of left renal vein compression predicts nutcracker syndrome. J Clin Med. 2018;7(5):107.
24. Kim KW, Cho JY, Kim SH, et al. Diagnostic value of computed tomographic findings of nutcracker syndrome: correlation with renal venography and renocaval pressure gradients. Eur J Radiol. 2011;80(3):648- 654.
25. Er A, Uzunlulu N, Guzelbey T, Yavuz S, Kiyak A, Kayhan A. The nutcracker syndrome: the usefulness of different MRI sequences for diagnosis and follow-up. Clin Imaging. 2019;55:144-147.
26. Avgerinos ED, McEnaney R, Chaer RA. Surgical and endovascular interventions for nutcracker syndrome. Semin Vasc Surg. 2013;26:170-177.
27. Beinart C, Sniderman KW, Saddekni S, Weiner M, Vaughan Jr ED, Sos TA. Left renal vein hypertension: a cause of occult hematuria. Radiology. 1982;145:647-650.
28. Williams B, Keefe NA. Utilization of intravascular ultrasound in the management of venous disease. Tech Vasc Interv Radiol. 2023;26(2):100898.
29. Rodriguez-Morata A, González-Fajardo JA. Controversias en el síndrome de congestion pélvica. Una perspectiva hemodinámica. Angiologia. 2020;72(5):215-218.
30. De Macedo GL, Dos Santos MA, Sarris AB, Gomes RZ. Diagnosis and treatment of the nutcracker syndrome: a review of the last 10 years. J Vasc Bras. 2018;17(3):220- 228.
31. Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552-559.
32. Miró I, Serrano A, Pérez-Ardavín J, et al. Eighteen years of experience with pediatric nutcracker syndrome: the importance of the conservative approach. J Pediatr Urol. 2020;16(2):218:e1-218.e6.
33. Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Surg. 2001;34(5):812-819.
34. Erben Y, Gloviczki P, Kalra M, et al. Treatment of nutcracker syndrome with open and endovascular interventions. J Vasc Surg Venous Lymphat Disord. 2015;3(4):389-396.
35. Hartung O, Grisoli D, Boufi M, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42:275-280.
36. Deser SB, Onem K, Demirag MK, Buyukalpelli R. Surgical treatment of posterior nutcracker syndrome presented with hyperaldosteronism. Interact Cardiovasc Thorac Surg. 2016;22(5):682- 684.
37. Hardy JD. High ureteral injuries. Management by autotransplantation of the kidney. JAMA. 1963;184:97-101.
38. Shokeir AA, el-Diasty TA, Ghoneim MA. The nutcracker syndrome: new methods of diagnosis and treatment. Br J Urol. 1994;74:139-143.
39. White JV, Ryjewski C, Messersmith RN, Sbrana F, Schwartz LB. Left ovarian to left external iliac vein transposition for the treatment of nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4(1):114-118.
40. Diab K, Auyang PL, Bavare C. Left ovarian vein transposition for the treatment of nutcracker syndrome: a surrogate for failed left renal vein transposition. J Vasc Surg. 2020;72(1):e170-e171.
41. Chau AH, Abdul-Muhsin H, Peng X, et al. Robotic-assisted left renal vein transposition as a novel surgical technique for the treatment of renal nutcracker syndrome. J Vasc Surg Cases Innov Tech. 2018;4:31-34.
42. Reed NR, Kalra M, Bower TC, Vrtiska TJ, Ricotta JJ, Gloviczki P. Left renal vein transposition for nutcracker syndrome. J Vasc Surg. 2009;49(2):386-393; discussion 393-394.
43. Hartung O, Azghari A, Barthelemy P, Boufi M, Alimi YS. Laparoscopic transposition of the left renal vein into the inferior vena cava for nutcracker syndrome. J Vasc Surg. 2010;52:738-741.
44. Wang P, Jing T, Qin J, Xia D, Wang S. Robotic-assisted laparoscopic transposition of the left renal vein for treatment of the nutcracker syndrome. Urology. 2015;86(6):e27-e28.
45. Thaveau F, Nicolini P, Lucereau B, Georg Y, Lejay A, Chakfe N. Associated Da Vinci and Magellan robotic systems for successful treatment of nutcracker syndrome. J Laparoendosc Adv Surg Tech A. 2015;25(1):60-63.
46. Chen S, Zhang H, Shi H, Tian L, Jin W, Li M. Endovascular stenting for treatment of nutcracker syndrome: report of 61 cases with long-term follow-up. J Urol. 2011;186:570-575. 68 Nutcracker syndrome
47. Wang X, Zhang Y, Li C, Zhang H. Results of endovascular treatment for patients with nutcracker syndrome. J Vasc Surg. 2012;56(1):142-148.
48. Quevedo HC, Arain SA, Abi Rafeh N. Systematic review of endovascular therapy for nutcracker syndrome and case presentation. Cardiovasc Revasc Med. 2014;15(5):305-307.
49. Rodríguez-Morata A, Robles-Martín ML, Reyes-Ortega JP. Endovascular treatment of posterior nutcracker syndrome with a new autoexpandable stent. J Vasc Surg Venous Lymphat Disord. 2019;7(1):118- 121.
50. Wu Z, Zheng X, He Y, et al. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4:193-199.
51. Avgerinos ED, Saadeddin Z, Humar R, et al. Outcomes of left renal vein stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2019;7:853- 859.
52. Mirandola M, Migliara B, Grosso G, Bertoloni R, Griso A, Bissacco D. Hybrid approach for recurrence nutcracker syndrome involving left renal vein stenting with laparoscopic stent external fixation. J Vasc Surg Cases Innov Tech. 2025;11(2):101739.
53. Steffen DA, Najafi A, Festas G, Binkert CA. Treatment rationale in nutcracker syndrome with concurrent pelvic congestion syndrome. CVIR Endovasc. 2025;8(1):13.
54. O’Brien MT, Gillespie DL. Diagnosis and treatment of the pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2015;3(1):96-106.

