Popliteal compression syndrome
Ionel Droc, MD, PhD
Cardiovascular Surgery Department, Central Military Hospital, Bucharest, Romania
Raluca Dantis, MD
Cardiovascular Surgery Department, Central Military Hospital, Bucharest, Romania
Rene Milleret, MD, PhD
Vascular Surgery Department, Vein Center, Vichy, France
ABSTRACT
Popliteal entrapment (compression) syndrome is a rare disease affecting principally young people, without atherosclerotic involvement. It is due to extrinsic compression of the neurovascular bundle at the popliteal fossa. There is no consensus or guidelines for the diagnosis or management, especially for functional popliteal entrapment. Compression of the popliteal vein manifests itself symptomatically in dynamic situations, such as prolonged standing or physical exercises. These symptoms are not specific, and clinical examination does not identify specific signs either. The ultrasound (US), in supine position, is usually nondiagnostic. Actually, a normal US in patients who complain of symptoms, related to venous insufficiency, should evoke venous compression as a possible diagnostic. Dynamic imaging by US is the key to visualizing the conflict between veins and anatomical structures. If a surgical treatment is considered, magnetic resonance imaging and/or dynamic phlebography are necessary. Nonsurgical procedures for light or mild cases include stretching exercises and botulinic toxin, under US-assisted injection. In more symptomatic patients, and depending on the etiology of compression, several surgical options can be offered: direct decompression through popliteal fossa dissection, aponeurectomy, isolated or combined with lengthening of gastrocnemius and soleus muscles. An often-overlooked syndrome is the chronic venous insufficiency described by Dijkstra and colleagues in patients with morbid obesity: weight loss is able to stabilize or improve venous return without direct vascular interventions and even allows healing of chronic ulcers.
Introduction
Popliteal entrapment syndrome is an uncommon disease of the lower limbs, mainly affecting athletes or military personnel (generally young patients without any atherosclerotic risk factors). It is due to compression of the popliteal artery, popliteal vein, or tibial nerve in the popliteal fossa by surrounding musculoskeletal structures.1,2
Popliteal artery entrapment syndrome (PAES) is a rare pathology, but by far the most common entrapment that could lead to arterial insufficiency in young and physically active persons.3,4
Compression of the popliteal vein was considered less frequent than popliteal artery entrapment, until ultrasound (US) became widely available.5 Most authors considered venographic findings of venous compression as a benign situation without pathologic consequences.
The implication of vein compression in symptomatic venous insufficiency was pointed out in 2000, when Rajuand Neglen6 published a study of 30 patients who underwent operation to free the popliteal vein, where 82% were significantly improved.
Attention to this problem was awakened, new diagnostic and therapeutic solutions were devised, but popliteal vein compression remains underdiagnosed and undertreated.
Anatomy
Different anatomical variations may be involved. A first condition is compression by an aberrant insertion of the lateral head of gastrocnemius; this is the most frequent situation, often associated with fibrous bands and/or fibrotic perivascular tissue; but also, a more lateral insertion of the medial head of gastrocnemius muscle to the femoral condyle can compress the vein. Another etiology is hypertrophy of the upper part in gastrocnemius and soleus muscles, with shortening of the sural triceps: type 6 in the Whelan and Rich classification.7 Obese people often present with this pattern. Figure 1 illustrates the anatomy of popliteal fossa and the classification of popliteal entrapment syndrome (into 4 types), showing common variants responsible for arterial entrapment8 A new classification into 6 types (by Levien) added type V where both popliteal artery and vein are compressed and type VI, functional entrapment: no visible anatomical anomaly, but symptoms appear during exercise due to muscle hypertrophy.9
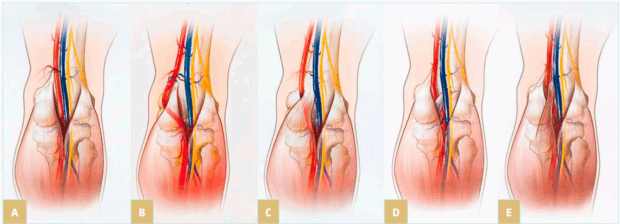
Figure 1. Illustration of the anatomy of popliteal fossa and the classification of popliteal entrapment syndrome. Graphic illustrations show normal anatomy of popliteal fossa and common variants responsible for arterial entrapment. A) Normal popliteal artery is adjacent to and lateral to medial head of gastrocnemius muscle, which is normally attached just superior to medial femoral condyle. B) Type I. Popliteal artery takes abnormal course medial to normally attached medial head of
gastrocnemius muscle. C) Type II. Abnormal embryologic development results in medial head of gastrocnemius attached more laterally than is normal. D) Type III. Popliteal artery and gastrocnemius are normally positioned, but fibrous band is responsible for entrapment. E) Type IV. Popliteal artery courses beneath popliteus muscle. Reproduced from reference 8: Bradshaw et al. Cardiovasc Diagn Ther. 2021;11(5):1159-1167. doi:10.21037/cdt-20-186. Copyright 2021,
Cardiovascular Diagnosis and Therapy. Material under Creative Commons License CC BY-NC-ND 4.0. [The figure in reference 8 is an adaptation from Macedo TA, Johnson CM, Hallett JW, et al. Popliteal artery entrapment syndrome: role of imaging in the diagnosis. Am J Roentgenol.
2003;181:1259-1265 by permission from the Mayo Foundation for Medical Education and Research, all rights reserved.]
Dynamic popliteal vein compression
Pathophysiology
The compression can be limited to upper, medial, or lower popliteal vein. In aberrant insertions of the medial gastrocnemius and in hypertrophy of the muscle it is usually medial. Upper compression is related to a stenosis at femoral canal level, whereas low compression is related to the soleus ring.
Dijkstra and colleagues10 measured popliteal compartment pressure in obese patients and found a significant increase in pressure when body mass index (BMI) was more than 35.
In athletes, a compartment syndrome may be an association, or it can be the main problem.
Intrafascial pressure measurements help in diagnosis, but the procedure is invasive and not available in many labs.
Symptoms and signs
Symptoms reported by the patients are nonspecific: swelling at ankle level, heavy legs, cramps after standing for a long time or keeping the knee extended. Pain when exercising is mainly experienced by athletes. Inspection may show dilatation of calf muscles. Visible varicose veins are seen mainly in patients who experience recurrence after surgery. Popliteal fossa perforators feeding nonsaphenous varices should raise a flag. Though popliteal fossa perforators are a frequently encountered finding, they would be considered a warning sign mainly in patients who show early recurrence of varicose veins after small saphena ablation.
At a later stage, lipodermatosclerosis appears, often misdiagnosed as superficial venous thrombosis. On the other hand, dynamic compression has been identified as a possible cause of deep vein thrombosis (DVT); for instance, in long distance flights due to prolonged sitting.2
Ultrasounds
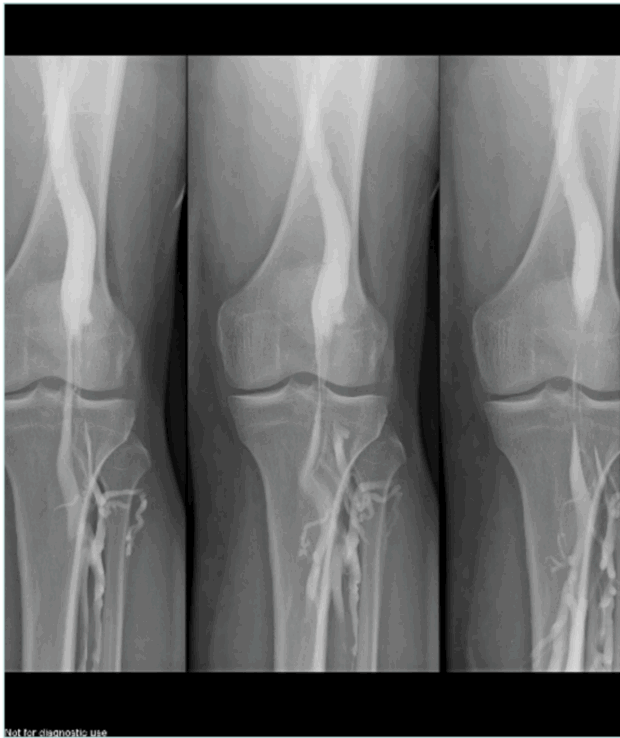
If there is no superficial venous insufficiency associated, in a supine patient, US can appear as normal (Figure 2).
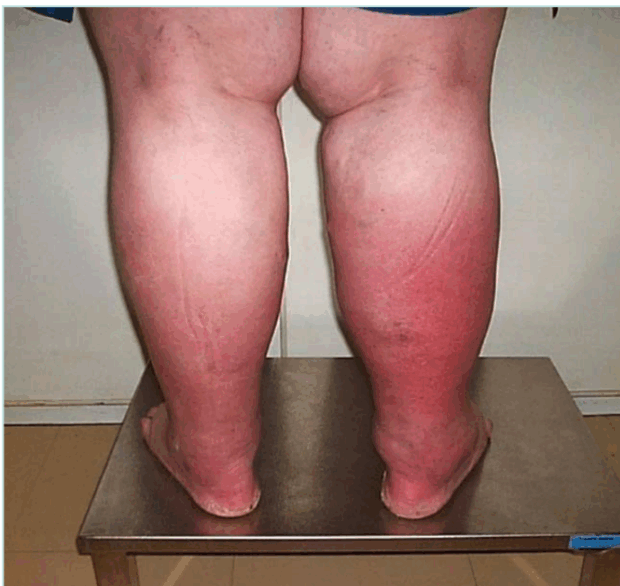
Figure 2. Obese patient with normal non-dynamic venous duplex ultrasound and popliteal venous compression.
When symptoms of venous insufficiency are documented, dynamic tests must be added to confirm or eliminate the diagnosis of popliteal vein compression.
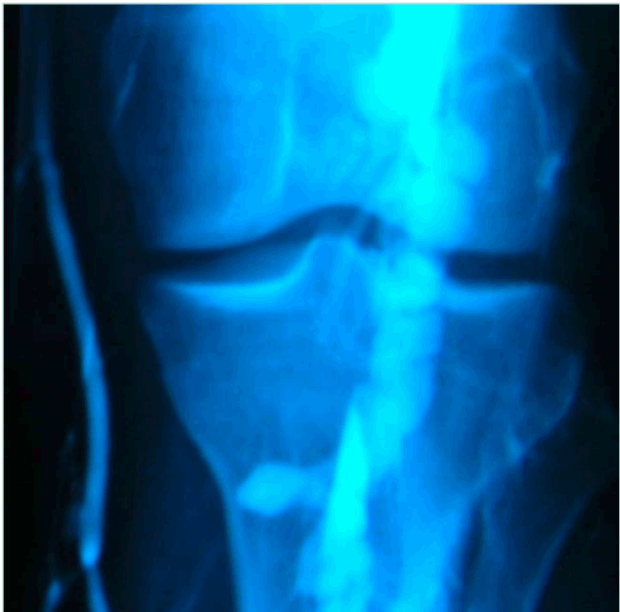
In the tiptoe test, the patient is standing, preferably on a phlebologic stool, with only the anterior part of his feet on the edge. The US probe is placed longitudinally on the popliteal fossa to identify the popliteal vein. The patient is asked to slowly move up and down: compression of the vein by muscular structures of the sural triceps is confirmed by flow restriction in color or power Doppler mode. We consider that clinically significant compression implies a positive test in both extension and flexion of the foot (Figure 3).
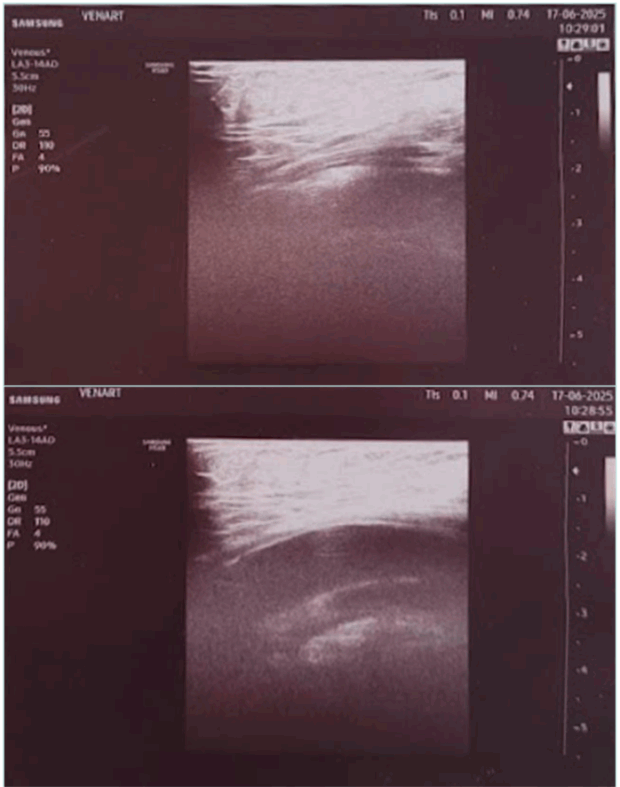
Figure 3. Ultrasound image from the tiptoe test. (Upper image) foot extended. (Lower image) foot in neutral position.
Other imaging studies
Other imaging studies are performed if an intervention is planned.
Angio-magnetic resonance imaging (MRI) and computed tomography (CT) scans are not very informative because dynamic testing takes a lot of time and can only be done on a supine patient.11 Standing MRI and CT scan are rarely available.
Our reference test remains dynamic phlebography.2 The examination is performed on a tilting table at 60° angle, with 3 manual injections of contrast agent: in neutral, foot extension, and foot flexion positions.
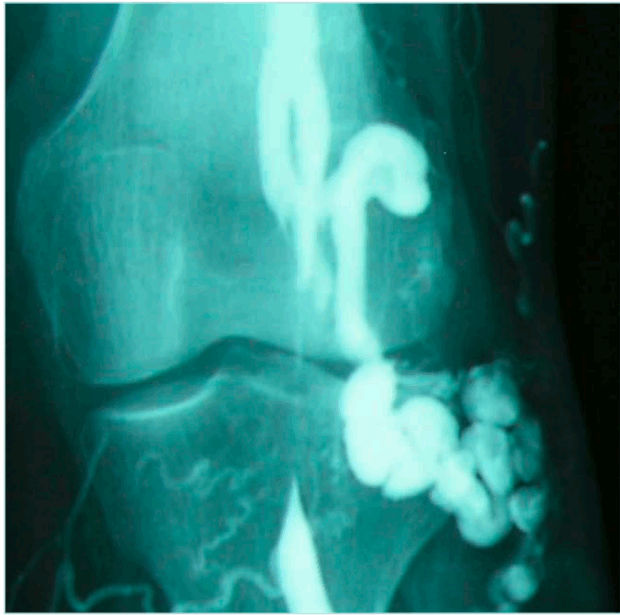
The surgeon should be in the room during the phlebography, for better evaluation of the anatomical pattern of the compression and to ask for more incidences as needed. In recurring varicose veins after small saphenous ablation, the surgeon can map the tributaries and perforators, which act as a spontaneous bypass over the compression (Figure 4 shows ascending phlebography: vein compression in the 3 positions of the foot; Figures 5 and 6 show ascending phlebography: spontaneous bypass of a venous popliteal compression).
Hall and colleagues12 used intravascular US (IVUS) in patients diagnosed with popliteal artery entrapment. The investigators found a popliteal vein compression in 100% of patients with arterial entrapment and consider IVUS to be superior to angiograms. But the cost is a limiting factor.
Popliteal venous compression and compartment syndrome are related entities: they can provoke similar symptoms and be associated in the same patient.
Intrafascial pressure measurements are helpful to rule out the compartment issue but are not available in many vascular laboratories.
Sport medicine specialists should be consulted in case of doubt before deciding for a surgical procedure.
Differential diagnosis
As dynamic compression of the popliteal vein is a common situation (27% of a “normal” population according to Raju and Neglen6), and often coexists with other pathologies, the problem is mainly to evaluate its relevance in the symptoms related by the patient.
In young active patients, leg pain can be related to tendinomuscular pathologies.
Obesity impairs venous return not only at popliteal level; retroperitoneal hyperpressure caused by fat deposits and respiratory insufficiency are cofactors.10
Some patients have varicose veins; others, postthrombotic syndrome. Air plethysmography can help but is rarely available. Spáčil13 performed US and photoplethysmography in 61 patients (116 limbs). He found reduction in maximum venous outflow when the limb was fully extended in 50 patients.
Treatment
Conservative options are proposed in most cases.
Kinetotherapy
Kinetotherapy consists of elongation exercises every day with a tilting board or against a wall.
When symptoms appear during sport activities, in cooperation with the coach, the training can be adapted to limit the shortening and bulging of the sural triceps.
Wearing high heels is discouraged, and the patient is asked to progressively reduce their height while continuing the elongation program.
A 6-month medical treatment with good compliance is necessary before any invasive therapy is considered.
Botulin toxin
Botulin toxin US-assisted injections are efficient and minimally invasive. Gandor and colleagues described the first series in 201414 and obtained good results in 80%. Isner-Horobeti and colleagues15 published a case report in 2015 with 3 years of follow-up and normalization of the US tests, with no more symptoms. We observed similarly good results for our own patients.
Unfortunately, this technique is not widely used by vascular surgeons because in most countries use of botulinic toxin is restricted to some specialties only.
We refer our patients to a physical medicine specialist who is an expert in treating spasticity in neurological patients. Eighty percent of them report an improvement of their symptoms after 2 sessions with 6-month interval, which is in line with published data. If the result is not good enough, we inform the patient about surgical options.
Surgery
Surgery can be direct or indirect.
Direct surgery implies dissection of the vein in the popliteal fossa section of any abnormal muscular fiber and fibrous tracts compressing the vein. Superficial aponeurosis is not sutured in order to relieve pressure in the popliteal fossa.
Indirect surgery is indicated mainly when patients present with a hypertrophic and/or shortened gastrocnemius muscle bundle.
A Silfverskiold positive test is mandatory to select a surgical option. This test measures the insufficiency of passive dorsal ankle flexion and can be performed without specific equipment. Lengthening of the medial gastrocnemius bundle by section of the white aponeurotic fibers is minimally invasive surgery, and there is no risk of nerve damage. It is ambulatory, performed under regional and local anesthesia.
Recently, an incisionless gastrocnemius release technique performed with a needle under echoguidance has been described16; we have not tested it yet.
After gastrocnemius recession, a specific rehabilitation program must be followed during 4 to 8 weeks.
In summary, dynamic compression of the popliteal vein is multifactorial, with nonspecific symptoms. The knowledge of sport medicine and physical therapy specialists is very useful to evaluate the significance of venous compression in the clinical presentation: we often rely on multidisciplinary consultations before proposing a treatment plan, which should always begin by noninvasive options.
Randomized studies are missing and needed now because efficient and minimally invasive treatments are available.
The influence of dynamic popliteal compression in the development of leg varicose veins deserves to be studied; this hemodynamic disturbance should be suspected in cases of recurring varices in the popliteal fossa.
Permanent popliteal vein entrapment
Permanent popliteal vein entrapment is less common than arterial compression. It is often part of the type V popliteal compression classification. It may also be present as recurrent DVT in young patients without risk factors.
DVT is rarely associated with popliteal artery entrapment syndrome (PAES). Nowadays, 10% to 15% of PAES are involved in popliteal vein thrombosis.17 These patients should be treated with anticoagulants. Surgical intervention with myotomy with or without venous thrombectomy should be performed.
Popliteal venous compression may be associated with popliteal artery aneurysm. The aneurysm size is usually moderate (between 2 and 3 cm); a bigger aneurysm can produce popliteal vein thrombosis.18 The single clinical symptom may be calf swelling, which can vary with the patient’s leg position.18 This cause of extrinsic compression of the ipsilateral popliteal vein should be included in the differential diagnosis of the unilateral leg swelling, without DVT. The treatment of the popliteal aneurysm is surgical or endovascular.
The technique in surgical repair is aneurysmal exclusion or resection and arterial bypass with interposition of a reversed saphenous vein graft or a synthetic polytetrafluoroethylene (PTFE) graft, via a medial approach (most used) or posterior approach.
In the endovascular repair of this region with a lot of mobility, questions about durability arise.
We should have landing zones 20 mm proximal and distal and at least 2 distal vessels that are patent.
Figure 7 shows the type of grafts used for proximal to distal popliteal bypass.
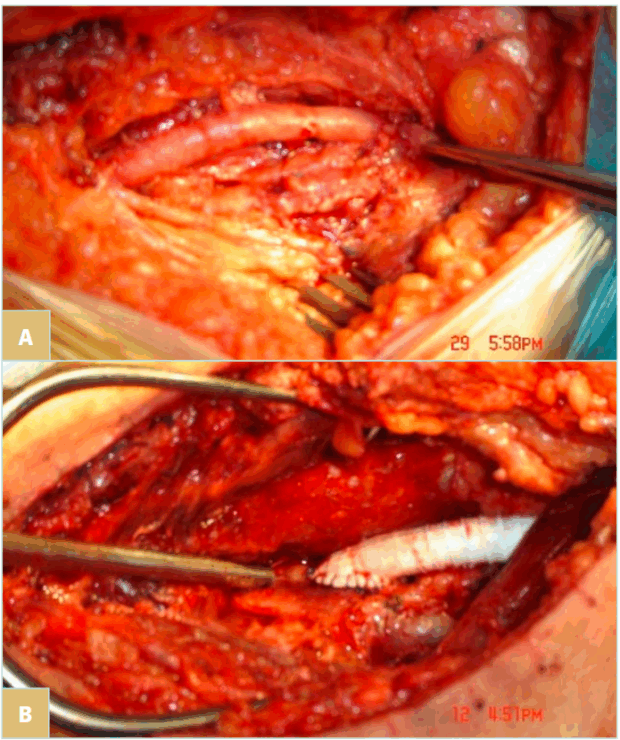
Figure 7. The type of grafts used for proximal to distal popliteal bypass. A) Vein graft (recommended whenever available). B) Polytetrafluoroethylene (PTFE) graft.
Another rare cause of venous compression is proximal tibial artery aneurysms or pseudoaneurysms, especially when the first clinical sign is leg swelling in young patients without atherosclerotic involvement. A similar case, a popliteal artery pseudoaneurysm was reported by Kim and colleagues19 in 2023. The treatment was surgical, a posterior approach with pseudoaneurysm resection and interposition of a reversed saphenous vein graft.
Here, we present a case of a 42-year-old man presenting with swelling of the right calf and pain at this level on exercise, with normal peripheral pulses. Duplex ultrasonography revealed vein compression by a pulsatile mass, filled with blood during the systole. The angio-CT suggests the diagnosis of pseudoaneurysm, which originates at the origin of the tibial posterior artery. Figure 8 shows the surgical repair by medial approach with pseudoaneurysm excision and vessel repair with autologous vein patch.
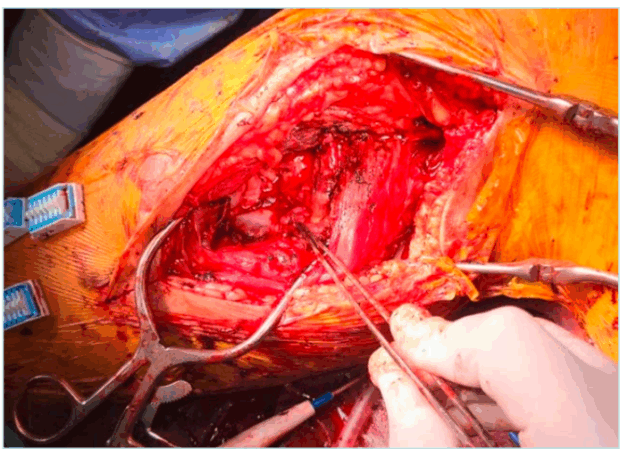
Figure 8. Intraoperative view of surgical repair of proximal tibial artery pseudoaneurysm treatment with a saphenous vein patch.
Popliteal artery entrapment syndrome (PAES)
PAES was first described by Stuart in 1879. Its incidence is 0.17% to 3.5% of the general population in the United States.20 This embryological development anomaly reveals an aberrant relationship of the popliteal artery with the surrounding myofascial structures.
This is considered a progressive disease because of the repetitive compression that can determine progressive arterial damage ending with arterial occlusion.
Clinical signs of PAES are intermittent claudication in young patients, apparent without signs of atherosclerotic involvement. The median delay between symptoms and diagnosis of PAES is 12 months. Arterial occlusion represents 24% of cases; poststenotic dilatation or aneurysm formation, 13.5%. The median age is 32 years old with 83% of cases being male.
It is important to keep in mind that distal pulses (tibial anterior and posterior) are normal in the absence of severe popliteal artery stenosis. Investigation should continue if PAES is suspected.
Diagnostic
We have at our disposal multiple imaging modalities. The risk of delay in making a diagnosis can raise the risk of arterial injury.
First line is US, which reveals arterial stenosis or occlusion.21 Angio-CT confirms the diagnostic and shows the arterial bed proximal and distal from the popliteal stenosis. Subtraction angiography should be performed only in special situations, but it remains the gold standard, especially associated with provocative maneuvers (eg, ankle plantar flexion).22 MRI of the knee reveals the anatomical cause of the compression.
Early diagnosis should be desired in order to detect the popliteal artery stenosis before complete occlusion of the artery, the treatment strategy being different in these two situations.
Treatment
The goal of the treatment is surgical decompression of the vessel with or without thrombectomy or reconstruction. There are a few cases reported in the literature from different parts of the world (Korea, USA, Japan, Turkey, etc).17,23-25
We can begin by medical treatment: vasodilators and antiplatelet drugs should be started from the onset of symptoms.
Surgical treatment remains the gold standard. Usually, the posterior approach should be performed, with resection of the head of the gastrocnemius muscle that is responsible for the extrinsic compression. After the dissection of the popliteal artery, if the artery is occluded, we can perform a short bypass or interposition using an ipsilateral saphenous vein graft.26 If there is only a stenosis, we can make a longitudinal incision of the artery and suture a venous enlargement patch. The postoperative treatment should be an association of an antiplatelet drug and a vasodilator (pentoxifilinum, for example).
Another option is endovascular treatment but because of the mobility of the region and the spiral forces of the popliteal artery, the outcomes are not favorable (stent fracture, thrombosis).
Functional PAES
Functional PAES (FPAES) was not described until 1985 when noninvasive diagnostic modalities like US were largely used.27 In FPAES, the popliteal artery is compressed by the calf muscles during exercise, leading to leg pain and atypical claudication usually in active young adults. These patients have normal anatomy at rest.
It is difficult to diagnose; in many cases, angiography and even MRI do not identify the entrapment. It would be useful to perform diagnostic tests with provocative maneuvers (like active plantar flexion).
The “gold standard” in the identification of abnormal popliteal fossa myofascial anatomy related to PAES is MRI because of its great sensitivity.3
A new protocol for diagnosis has been proposed by the University of Wisconsin School of Medicine, consisting of the following: i) ankle brachial index (ABI) with exercise; ii) arterial Doppler US with plantar flexion and standing; and iii) magnetic resonance angiography with plantar flexion.27
Also in the United States, the team of Boniakowski28 has proposed the use of IVUS as an adjunct to angiography. The advantage is that it can provide not only the exact location of the compression but also the quality of the vessel wall, indicating whether or not arterial repair is needed. It is a new tool, but the procedure has adjunctive costs.
A new treatment is US-guided botox injection—a nonsurgical treatment option.29 Botulinum toxin A (BTX A) could reduce the volume and tonus of gastrocnemian muscles in patients with FPAES. Isner-Horobeti and colleagues reported a case of bilateral FPAES treated with success after a failed surgical intervention, with maintenance of the result during a medium-term follow-up.15
Hislop and colleagues reported on a larger cohort (27 patients), of which 59% of patients had good results (improvement of symptoms maintained at 1 year), 22% a mixed response (the improvement reduced over the 1-year period of the survey), and 19% a poor response.29
Conclusions
PAES is a rare vascular pathology affecting young patients. The diagnostic is difficult and often requires an interdisciplinary team. Symptomatic patients require treatment, often surgical. Until now, there are no guidelines for this entity.
CORRESPONDING AUTHORS

Dr Ionel Droc
4 Democratiei Street, 077190 Voluntari,
Ilfov, Romania
e-mail: ionel.droc@gmail.com
Dr Rene Milleret
32 rue Alphonse Daudet, 03700
Bellererive sur Allier, France
e-mail: Rmilleret@gmail.com
Dr Ionel Droc received an honorarium from Servier for the writing of this article.
References
1. Debus ES, Grundmann RT. Popliteal entrapment syndrome. In: Evidence-based Therapy in Vascular Surgery, 2nd edition. Springer; 2023:327-340.
2. Chen CK, Kolber M. Venous popliteal entrapment syndrome. Cardiovasc Diagn Ther. 2021;11(5):1168-1171.
3. Mansour W, Miceli F, Di Girolamo A, et al. Long-term results of surgical treatment for popliteal artery entrapment syndrome. Diagnostics. 2024;14:1302.
4. Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg. 2012;55(1):252-262.
5. Levien LJ, Veller MG. Popliteal artery entrapment syndrome: more common than previously recognized. J Vasc Surg. 1999;30:587-598.
6. Raju S, Neglen P. Popliteal vein entrapment: a benign venographic feature or a pathologic entity? J Vasc Surg. 2000;31(4):631-641.
7. Rich NM, Collins GJ, MAC Donald PT, et al. Popliteal vascular entrapment. Its increasing interest. Arch Surg. 1979;114:1377-1384.
8. Bradshaw S, Habibollahi P, Soni J, Kolber M, Pillai AK. Popliteal artery entrapment syndrome. Cardiovasc Diagn Ther. 2021;11(5):1159-1167.
9. Jayaraj A, Gloviczki P, Duncan AA, et al. Popliteal entrapment syndrome – the case for a new classification. Vascular. 2022;30(2):285-291.
10. Dijkstra ML, Khin NY, Thomas SD, Lane RJ. Popliteal vein compression syndrome pathophysiology and correlation with popliteal compartment pressures. J Vasc Surg Venous Lymphat Disord. 2013;1(2):181-188.
11. Yamamoto Y, Tsukuda K, Kazama A, Kagayama T, Kudo T. Popliteal vein entrapment syndrome associated with an accessory slip of the lateral head of the gastrocnemius muscle. J Vasc Surg Cases Innov Tech. 2024;10(4):101502.
12. Hall MR, Vyas Y, Kang J, Nagarsheth K, Sarkar R. Intravascular ultrasound imaging for diagnosis and characterization of the popliteal compression syndrome. J Vasc Surg. 2022;9(1):101076.
13. Spáčil J. Popliteal vein compression with the limb extended. Vasa. 2012;42(5):357- 362.
14. Gandor F, Tish S, Grabs A, et al. Botulinum toxin A in functional popliteal entrapment syndrome: a new approach to a difficult diagnostic. J Neural Transm (Vienna). 2014;121(10):1297-1301.
15. Isner-Horoberti ME, Muff G, Masat J, et al. Botulin toxin as a treatment for functional popliteal artery entrapment syndrome. Med Sci Sports Exerc. 2015;47(6):1124- 1127.
16. Marcos AJ, Villanueva Martinez M, Saddi Diaz HF. Needle-based gastrocnemius lengthening: a novel ultrasound-guided noninvasive technique. J Orthop Surg Res. 2022;17(1):435.
17. Lee S, Hwang D, Yun WS, Huh S, Kim HK. Case report: popliteal artery entrapment syndrome as a cause of deep vein thrombosis and subsequent popliteal artery occlusion. Front Surg. 2024:11:1384331.
18. Kotval PS, Shah PM, Babu SC, Charalel J, Reiter B. Popliteal vein compression due to popliteal artery aneurysm: effects of aneurysm size. J Ultrasound Med. 1995;14(11):805-811.
19. Kim HJ, Huh S, Kim HK. Popliteal artery entrapment syndrome presented with popliteal artery pseudoaneurysm: a case report. Vasc Specialist Int. 2023;39(1). doi:10.5758/vsi.230077.
20. Davis DD, Shaw PM. Popliteal artery entrapment syndrome. StatPearls [Internet]. StatPearls Publishing; 2025 Jan. 2023 Aug 28.
21. Obara H, Cupta PC, Levien L, Mistry P, Bollineni V, Shaw PM. A short review of popliteal artery entrapment syndrome. JVS Vasc Insights. 2025;3:100186.
22. Pillai J, Levien LJ, Haagensen M, Candy G, Cluver MD, Veller MG. Assessment of the medial head of the gastrocnemius muscle in functional compression of the popliteal artery. J Vasc Surg. 2008;48:1189-1196.
23. Gaunder C, McKinney B, Rivera J. Popliteal artery entrapment or chronic exertional compartment syndrome? Case Rep Med. 2017:2017:6981047.
24. Tanaka H, Higashi M, Fukumoto Y, Ogino H. Entrapment of the popliteal artery. J Vasc Surg. 2010;52(2):479.
25. Gokkus K, Satgas E, Bakalim T, Tsakaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J. 2014;4(2):141-148.
26. Kwon YJ, Kwon TW, Um EH, et al. Anatomical popliteal artery entrapment syndrome caused by an aberrant plantaris muscle. Vasc Specialist Int. 2015;31(3):95-99.
27. Morgan C, Huang A, Turnipseed W. Optimizing the diagnostic approach of functional popliteal artery entrapment syndrome. J Vasc Surg. 2022;77(2):580- 589.
28. Boniakowski AE, Davis F, Campbell D, Khaja M, Gallagher KA. Intravascular ultrasound as a novel tool for the diagnosis and targeted treatment of functional popliteal artery entrapment syndrome. J Vasc Surg Cases Innov Tech. 2017;3(2):74-76.
29. Hislop M, Brideaux A, Dhupelia S. Functional popliteal artery entrapment syndrome: use of ultrasound guided Botox injection as a non-surgical treatment option. Skeletal Radiol. 2017;46:1241- 1248.