A new concept of the mechanism of venous valve closure and role of valves in circulation
Robert L. KISTNER, MD,
Bo EKLOF, MD, PhD,
Darcy KESSLER, RVT.
John A. Burns School of Medicine,
University of Hawaii
One can not overestimate the importance of venous valves in the vascular system. They are relatively simple membranous structures, and yet, their malfunction causes almost all known venous disorders. In primary venous insufficiency malfunction of the valves leads to broad spectrum of pathological changes, from spider varicose veins to lypodermatosclerosis to venous ulcers. Venous thrombi originate from the valve sinus. As the thrombus resolves, additional damage to venous valves occurs, leading to secondary (post-thrombotic) chronic venous disorder.
The fascinating history of venous valve discovery and re-discovery shows that, even before Harvey, their role was seen as ensuring unidirectional flow in veins.1 Excercitarto Anatomicae de Motu Cordis et Sangunis in Animalibus laid the basis for modern understanding of the cardiovascular system by introducing the concept of circulation.2 Demonstration of unidirectional venous flow was an essential part of this concept. Since that time the venous valve has been viewed as a simple passive structure reacting to reversed flow by closing the vein’s lumen.
In 1926, E.B. Carrier first described an intricate blood flow pattern around the venous valve leaflets in his direct observation of red blood cell movement in the bat’s wing.3 This was exactly the same pattern as predicted by Leonardo da Vinci, and later confirmed by K.D. Kele for a geometrically similar aortic valve.4In vitro experimentation with saphenous valves confirmed that they do not open all the way out to touch the sinus wall.5 These findings have opened up new aspects of the physiology of venous valves.
They demonstrated the complexity of hemodynamics around the valve far exceeding the simple sequence of forward and backward flow, and challenged the simplicity of current concept of physics behind closing of the venous valve. In 1980s, E. Strandness’s group in Seattle developed modern ultrasound techniques for detecting valvular incompetence.6 At that time, ultrasound equipment did not allow reliable visualization of the valve itself. Instead, Dopplerbased registration of reversed blood flow in the venous segment in response to Valsalva or rapid compression-decompression, maneuvers were used to define valvular insufficiency. This approach advanced venous diagnosis by providing a reliable tool for reflux detection that is used to this day. Unfortunately, the indirect approach also created confusion between the presence of reversed flow in the vein and function of the valve itself. The terms “reflux time” and “valve closure time” were falsely used interchangeably. As a consequence, the view that reversed flow through the valve is necessary for valve closure was promulgated.7
A new generation of ultrasound equipment, particularly the introduction of ß-flow modality, has made it possible to observe venous valve and blood flow in the area of the valve in undisturbed physiologic conditions. Artificial maneuvers to force the blood backward to check the competency of the valve are no longer needed for normal valve observations. One can simultaneously observe the motions of valve leaflets, changes in venous sinus shape and size, and blood flow through the valve during normal respiratory cycle, in different positions of the body and during exercises such as dorsal and plantar flexion of the foot.
THE VALVE CYCLE
By observing valves in femoral and great saphenous veins of healthy volunteers, we identified a consistent pattern of flow events as the blood passes through a valve station during rhythmic opening and closing of the valve cusps. The flow events and the movements of the valve leaflets appear to be two parts of the same physiologic process of the “valve cycle” – the time period between two consecutive closures of the valve, which we arbitrarily divided into the four phases
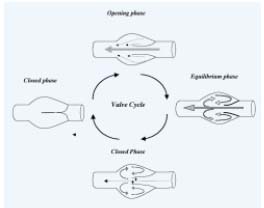
Figure 1. The valve cycle.
During the Opening phase the cusps move from the closed position
toward the sinus wall. After reaching a certain point, the valves
cease opening and enter the equilibrium phase. The valve is
maximally open during this phase. Still cusps maintain their
position at some distance from the wall, creating a funnel-like
narrowing of lumen. The flow accelerates in this stenotic area
resulting in a proximally directed flow jet. The smaller part
of the flow turns into the sinus pocket behind the valve cusp.
This part of the stream forms a vortex along the sinus wall and
the mural side of valve cusp before re-emerging in the main stream
in the vein. Rising pressures on the mural side and falling pressures
on the luminal side of the cusps initiate their movements toward
the center of the vein, ie the closing phase. The cusps of the valve
assume a symmetrical position at an equal distance from the
walls on both sides of the sinus remaining in this position during
the closed phase.
Opening phase: During this phase, the cusps move from the closed position toward the sinus wall. This phase lasts on average 0.27 +/-0.05 seconds when the patient is in the horizontal position. After reaching a certain point in this phase, the valves cease opening and enter the equilibrium phase. During this phase, the leading edges remain suspended in the flowing stream and undergo oscillations that resemble the flutter of flags in the wind. The valve is maximally open during this phase. Still cusps maintain their position at some distance from the wall, creating a funnel-like narrowing of lumen. The crosssectional area between the leaflets is about two thirds of the cross-sectional area of the vein distal to the valve. The flow accelerates in this stenotic area resulting in a proximally directed flow jet. Upon impact of the jet against a layer of much slower-moving blood proximal to the valve, reflection of flow occurs in the mural parts of the stream. While the larger stream located in the center of the vessel is directed proximally along the axis of the vein, the smaller part of the flow turns into the sinus pocket behind the valve cusp. This part of the stream forms a vortex along the sinus wall and the mural side of valve cusp before re-emerging in the main stream in the vein.
As vortical flow persists, it applies pressure upon the mural surface of the valve cusps. When the pressure on the mural side of the cusp and the pressure on the luminal side of the cusp are in equilibrium, the valve remains open and the cusps float in the stream. This dynamic equilibrium is sustained by equilibrium in velocities of the two streams – vortex on the mural side, and axial flow on the luminal side of the valve cusps. Changes in any of these streams can lead to the closure of the valve. Self-excited oscillations of the leading edges of the leaflets that occur during this equilibrium phase make this balance unstable and very sensitive to small changes in flow.
When the venous flow rate increases distal to the valve, as occurs during foot movements, the velocity of the flow between the valve cusps rapidly increases. This causes a fall in the pressure on the luminal side of the cusp, and the cusps start moving toward the axis of the vessel, further constricting the lumen (Figure 2). With rising pressures on the mural side and falling pressures on the luminal side of the cusps, valve closure is favored.
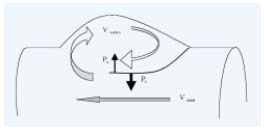
Figure 2. Mechanism of valve closure.
When the venous flow rate increases distal to the valve, as occurs
during foot movements, the velocity of the flow between the valve
cusps (Vaxial) rapidly increases. This causes a fall in the pressure on
the luminal side of the cusp (Po) while slower velocity of vortex
applies higher pressure on the mural side of cusp (Pi), and the
cusps start moving toward the axis of the vessel, further constricting
the lumen maintaining or even increasing the pressure gradient
between the two sides of the cusp.
The closing phase ensues. The leaflets move synchronously toward the center. The cusps of the valve assume a symmetrical position at an equal distance from the walls on both sides of the sinus. This phase lasts 0.41 +/- 0.07 sec when the patient is at rest and is much shorter when foot movements are performed. The last phase is the closed phase, during which the cusps remain closed.
The duration of the valve cycle and of each of its four phases depends upon the position of the body. In the standing position, the duration of the cycle is from 2.9 sec to 3.2 sec (95% confidence interval), which corresponds to frequency of 18.8 to 20.4 per minute (similar to respirations). In a horizontal position, the duration of the cycle was from 1.7 to 1.8 sec (95% CI). This rhythm (34.2 to 36.1 per minute) is most likely influenced by both respiratory and cardiac cycles. Muscle activity (dorsal and plantar flexions of the foot) causes shortening of the closing phase. As we observed, every single foot movement causes significant increase of velocities and closure of the valve.
Based on our observations, we proposed a new concept of the mechanism of venous valve closure and role of valve in circulation. In the absence of forced reversed flow, the valve cusps consistently undergo the four phases constituting the valve cycle. The local hemodynamic events such as vortical flow in the sinus pocket play important roles in the valve operation. These hemodynamic events are predetermined by the shape and mechanical properties of the sinus and the valve cusps, and they constitute a self-sustained mechanism for competent valve operation.
In addition to prevention of retrograde flow, the valve acts as a venous flow modulator. The vortical stream forms behind the valve cusp, and axial jet forms at the center of the vein. The vortex participates in the operation of the valve, and prevents stasis inside the valve pocket. The central jet possibly facilitates outflow.

REFERENCES
2. Harvey W. Exercitato Anatomica de Motu Cordis et Sanguinis in Animalibus. The Keynes English translation of 1928. Birmingham: The classics of Medicine library; 1978:86-87.
3. Carrier EB. Observation of living cells in the bat’s wing. In: Physiological papers dedicated to Prof August Krogh. W.M. Heinemann Ltd., London, 1926. Cited from: Franklin KJ. The valves in veins. A monograph on veins: Charles C Thomas; 1937:72.
4. Kele KD. Leonardo da Vinci on moment of the heart and blood. Harvey and Blythe, Ltd., London 1952. Cited from: Bellhouse BJ, Belhouse FH. Mechanism of closure of the aortic valve. Nature. 1968;217:86-87.
5. McCaughan JJ, Walsh DB, Edgcomb LP, Garrett HE. In vitro observations of greater saphenous vein valves during pulsatile and nonpulsatile flow and following lysis. J Vasc Surg. 1984;1:356-361.
6. Van Bemmelen PS, Bedford G, Beach K, Strandness DE. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425-431.
7. Van Bemmelen PS, Beach K, Bedford G, Strandness DE Jr. The mechanism of venous valve closure. Its relationship to the velocity of reverse flow. Arch Surg. 1990;125:617-619.
