Deep venous reconstructive surgery to treat reflux in the lower extremities
Chassieu, France
INTRODUCTION
The development of deep venous reconstructive surgery (DVRS) for reflux in the lower extremities has been far more limited than arterial reconstructive surgery. This discrepancy may be explained by a variety of factors:
– The importance of deep venous reflux (DVR) has been identified as a major pathophysiological factor only in the last 20 years.
– Indications for surgery are uncommon.
– The efficacy of surgical repair of the deep veins remains controversial.
Furthermore, many questions remain partially answered or unanswered. Is there a correlation between clinical and hemodynamic results? When superficial venous insufficiency (SVI) and/or perforator insufficiency (Pe I) are associated with DVR, should they be treated as a first step or in combination with correction of DVR?
ETIOLOGY
It must be kept in mind that DVR has different etiologies. The most common is secondary DVR, ie, post-thrombotic syndrome (PTS), followed by primary DVR. Congenital venous malformation may be identified in some cases.
Pathology and pathophysiology
PTS is the end result of thrombosis and the subsequent inflammation of the valve cusps and vein wall during the process of recanalization. This leads to scarring and shortening of the cusps.1 Failure of the cusps to achieve normal coaptation results in reflux. Reflux secondary to the PTS is usually not amenable to direct surgical repair of the now-destroyed valve.
Primary reflux is the result of structural abnormalities in the vein wall and valve itself. Although the precise etiology has not been characterized, it is presumed to be congenital in origin.2 Redundant, malopposed cusps and venous dilation permit valve prolapse and reflux. Unlike the PTS, there is no evidence of previous thrombosis or inflammation near the valve.3
A rare cause of congenital reflux is the complete absence of valves secondary to agenesis.
TECHNIQUES
Surgical techniques for treating DVR can be classified into two groups: those that do involve phlebotomy and those that do not involve phlebotomy.
Techniques with phlebotomy
– Internal valvuloplasty: since the first procedure using a longitudinal phlebotomy (Figure 1) described by Kistner in 19684 various procedures have been proposed.
– Venous segment transfer: (Figure 2) The purpose of venous segment transfer is to transpose the incompetent axial deep venous system, ie, the femoropopliteal axis into a competent valve-bearing system, namely the great saphenous vein or the deep femoral vein at the groin level. Devised again first by Kistner5 several surgical variations of venous segment transfer have been employed. Unfortunately this technique can be used in only approximately 20% of the PTS, as there is no competent valve into the great saphenous vein or the deep femoral vein valve in their proximal segment in 80%.
– Vein valve transplantation: Taheri6 deserves the credit for introducing the use of vein valve transplant (Figure 3).
A 2 cm to 3 cm segment of axillary vein is inserted as an interposition graft at the termination of the (superficial) femoral vein just below the junction of the deep femoral vein with the (superficial) femoral vein. O’Donnel7 initiated transplantation to the above-knee popliteal vein as the gatekeeper role of the popliteal vein had been underlined.8
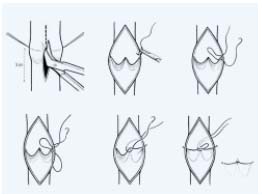
Figure 1.
Internal valvuloplasty according to Kistner. Using a longitudinal
phlebotomy each valve is repaired by interrupting a series
of sutures placed at the commissures. Each suture progressively
shortens the leading edge of the cusp.
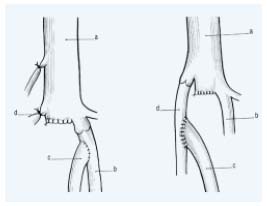
Figure 2. Venous segment transfer:
The refluxive (superficial) femoral vein (FV) may be transposed
onto the great saphenous vein (GSV) or the profunda femoral vein
(PFV) provided they have a competent proximal valve above
the transposition.
a, common femoral vein; b, profunda femoral vein; c, (superficial)
femoral vein; d, great saphenous vein.
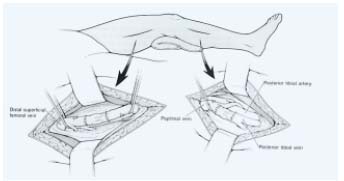
Figure 3. Above-knee or below-knee axillary
vein-to-popliteal vein transplantation
end-to-end anastomosis.
– Neovalve: Various techniques for creating a neovalve have been developed. Plagnol9 used the termination of the great saphenous vein to construct a neobicuspid valve by invagination. Maleti10 created a valvular cusp by dissection of the femoral venous wall to obtain a single or a bicuspid valve (Figure 4). Both these techniques have been used in PTS.
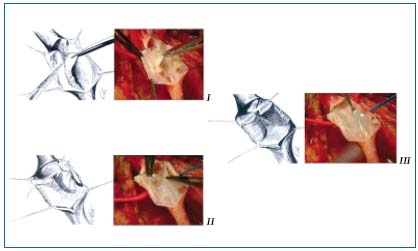
Figure 4. Neovalve according to Maleti. I After a longitudinal phlebotomy a blade is used
to perform a parietal dissection to create a neovalve which is derived from the union of the two
dissection lines, transversal and longitudinal. II The second valve created is then fixed to the vein wall
(bicuspid valve). III Bicuspid neovalve.
Techniques without phlebotomy
– Wrapping, banding, cuffing, and external stenting (Figure 5) were developed initially by Jessup and Lane11 for saphenous vein incompetence, and later for primary deep vein incompetence.12
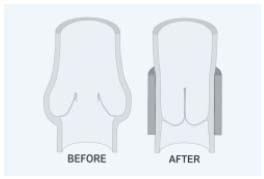
Figure 5. External stenting.
Schematics of incompetent venous valve demonstrating “floppy”
free edge border (BEFORE) and competent valve following external
stenting (AFTER).
– External valvuloplasty: The first step consists in advential dissection until the valve insertion lines are clearly identified as an inverted V. The commissural angle is normally acute and widened in the refluxive valve.
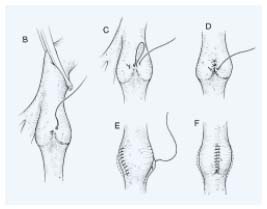
Figure 6. External transmural valvuloplasty.
The placating interrupted sutures are placed from outside the
lumen to tighten the commissural angle until the valve becomes
competent.
Transmural valvuloplasty: Kistner introduced external valvuloplasty (Figure 6) in 1990.13 He placed an external row of sutures along the diverging margins of the valve cusp in the vein wall. The interrupted sutures are carried inferiorly along both commisures until the valve becomes competent by strip-testing.
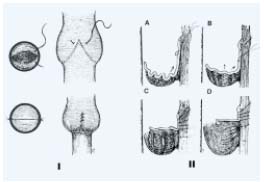
Figure 7. External transcommisural valvuloplasty.
I Correct suture placement narrows the angle between valve attachment
lines and tightens cusps, resulting in good apposition.
II Transluminal sutures with each successive suture biting deeper
and less obliquely than the suture above to pull up the valve,
tighten the cusp edge, deepen the sinus and appose valve
attachment lines. Each suture is tied up before the next is placed.
Transcommissural valvuloplasty was promoted by Raju.14<./sup> Transcommissural valvuloplasty (Figure 7) differs from the transmural valvuloplasty described previously, as a transluminal suture is carried out. “A through-and-through transluminal resuspension suture (7-0 Prolene) is placed obliquely across the inserted commissural V, traversing the valve cusps blindly near their wall attachment to pull them up.”
– Angioscopy-assisted external valve repair:15,16 An angioscope is introduced through a saphenous side branch and advanced into the proximal (superficial) femoral vein and positioned directly above the valve. Prolene sutures are passed from outside to inside the lumen directed by video-enhanced, magnified angioscopic visualization allowing for precise approximation of the valve cusps.
– Percutaneously placed device: the Portland valve17 consisting of a square stent and porcine small intestinal submucosa covering has initiated a clinical phase I study.
CLINICAL ASPECTS
Though there exist some clinical features that enable to distinguish superficial venous insufficiency from deep insufficiency, they are not reliable, particularly considering the fact that deep venous insufficiency is frequently combined with superficial insufficiency. In addition, primary deep vein reflux is difficult to distinguish from secondary deep vein reflux.
It is generally admitted that when deep venous reflux exists the chronic venous disease is more severe.
INVESTIGATIONS
– Duplex scanning (DS) provides both hemodynamic and anatomic information. Assessment of reflux is carried out using manual calf compression or inflating-deflating distal cuff and duration of reflux (normal less than 0.5 seconds) is measured at the femoral, popliteal, and tibial levels for the deep system and at similar levels for the superficial system.18,19 Perforators are also investigated. DS allows discrimination of primary reflux from PTS and is also helpful for planning DVRS. Presence or absence of competent proximal valves at the termination of the GVS or the profunda femoris vein may allow consideration of venous segment transfer. DS of the axillary vein and brachial veins segments determines whether there is a segment containing a functioning valve, which can be used for transplantation.
– Photoplethysmography, with and without inflatable cuff compression of the superficial venous system, can help when superficial and deep venous refluxes are combined to identify the predominant pathophysiological components by measuring venous return time with and without compression. VRT is considered normal above 20 s and frankly severe when less than 12 s. However, the value of this investigation is debated.
It would seem logical to go beyond the two investigations described above only in those patients in whom DVRS may be considered. That means that continuing investigations are dominated by the clinical context and absence of contraindication to DVRS:
– uncorrectable coagulation disorder: antithrombin III or C and S deficiency, etc.
– ineffective calf pump: frozen or stiff ankle after physiotherapy, permanent muscular deficit of the triceps surae.
– Volume plethysmography. Air plethysmography20 is one of the most useful currently available methods, identifying the various pathophysiological mechanisms involved. It enables the measurement of venous filling time, ejection fraction, and residual volume after dynamic test.
– Pressure measurements. The ambulatory venous pressure (AVP) measurement is supposed to be the “gold standard” for evaluating the global hemodynamic situation, providing pressure figures both before and after exercise and VRT. Improvement in VRT using a tourniquet was assumed to indicate isolated great or small saphenous vein incompetence21 but this point has been questioned.22
– Venography remains essential when DVRS is considered, as it provides the necessary information accurately.
Ascending phlebography provides both anatomic and etiologic information but very few hemodynamics.
Descending phlebography is performed by puncturing the ipsilateral common femoral vein, or better, the contralateral one or the brachial vein. It is strongly recommended to videotape the procedure and obtain spot films during the course of the examination. The Valsalva maneuver is essential to the performance of accurate descending phlebography, and it can be standardized. Reflux grading is according to the system previously reported and scored from 0 to 423 (Figure 8). Descending phlebography also gives precise information on the morphology of the valve and allows to determine whether a valve is reparable or not by valvuloplasty (
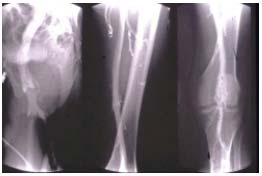
Figure 8. Descending phlebography.
Primary deep vein insufficiency. Reflux grade 4. Cascading reflux
to the calf.
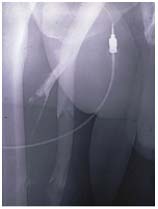
Figure 9. Descending phlebography.
Primary deep vein insufficiency.
The highest valve of the (superficial) vein
is refluxive but obviously reparable by valvuloplasty.
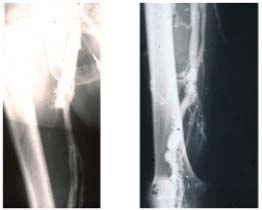
Figure 10. Descending phlebography.
Post-thrombotic syndrome. Reflux grade 4 (Calf not seen). No valve
is reparable.
The objective of surgery for treating deep venous reflux
The goal of DVRS is to correct the reflux related to deep venous insufficiency at a subinguinal level. This reflux leads to a permanent increase in venous pressure, unaffected by the activity of the calf venomuscular pump. However, it must be kept in mind that DVR is frequently combined with superficial and perforator reflux; consequently all these mechanisms have to be corrected in order to reduce the permanent increased venous pressure.
RESULTS
DVRS results are somewhat difficult to assess as superficial venous surgery and/or perforator surgery have often been performed in combination with DVRS.
In primary deep reflux the most frequent procedure used is valvuloplasty. Results are summarized in Table I.14,24,26-28 On the whole, valvuloplasty is credited with achieving a good result in 70% of cases in terms of clinical outcome, defined as freedom, ulcer recurrence from the reduction of pain, valve competence, and hemodynamic improvement over a follow-up period of more than 5 years. In all series, a good correlation was observed between these three criteria. External transmural valvuloplasty does not seem to be as reliable as internal valvuloplasty in providing long-term valve competence or ulcer-free survival.25 Other procedures used in primary deep vein reflux including angioscopic repair15,16 and wrapping are more difficult to assess as they have a shorter follow-up, except in the series reported by Lane12 (Table II).12,25,29,30
In PTS long-term results are available for transposition (Table III)24,26,28,31,32 and transplantation (Table IV).6,26,28,33-36 In terms of clinical result and valve competence, a meta-analysis demonstrates that a good result is achieved in 50% of cases over a follow-up period of more than 5 years, with a poor correlation between clinical and hemodynamic outcome.
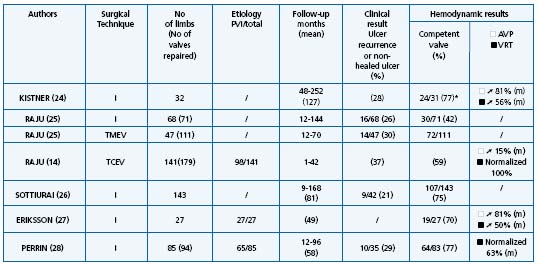
TMEV = Transmural external valvuloplasty; ➚ = Improved; TCEV = Transcommissural external valvuloplasty; m = Mean; * = No reflux or moderate reflux (less 1 s)
Table I. Valvuloplasty results.
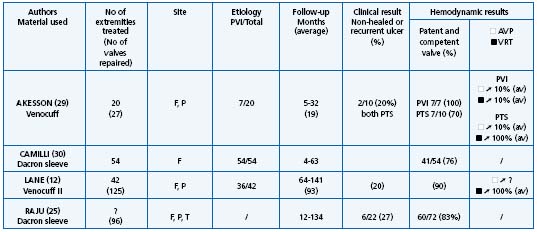
Table II. Banding, cuffing, external stent, wrapping results.
Transposition as isolated surgical procedure.
Table III. Transposition results.
Axillary vein transfer in trabeculated (poorly recanalized) vein.
Table IV. Transplantation results.
Neovalve according to Plagnol9 and to Maleti10 had given interesting results but their follow-up is short. Cryopreserved valve results were poor.37,38
INDICATIONS
► Hemodynamics and imaging
Only reflux graded 3-4 according to Kistner is usually treated with DVRS.23 It is generally recognized that, to be significantly abnormal, the values for VRT must be less than 12 s, and the difference between pressures at rest and after standardized exercise in the standing position must be less than 40%. The decision to operate should be based on the clinical status of the patient, not the noninvasive data, since the patient’s symptoms and signs may no correlate with the laboratory findings.39 In addition to meeting the clinical criteria, patients selected for surgery should be highly motivated to participate in their recovery since ultimate success is dependent on their compliance with postoperative management.
► Indications according to etiology
The indications for surgery can be simplified according to the clinical, hemodynamic, and imaging criteria described above.
In primary reflux reconstructive surgery is recommended after failure of conservative treatment and in young and active patients reluctant to wear permanent compression. Valvuloplasty is the most suitable technique, with Kistner, Perrin, and Sottiurai favoring internal valvuloplasty24,26,28 and Raju transcommissural external valvuloplasty.14
Secondary deep venous reflux (PTS) may be treated only after failure of conservative treatment. As the results achieved by subfascial endoscopic perforator surgery associated or not with superficial venous surgery are not convincing,40 it is recommended that this procedure might be carried out in combination with deep reconstructive surgery. The techniques to be used, given that valvuloplasty is rarely feasible (Figure 11), in order of recommendation, are: transposition, transplantation, neovalve, and cryopreserved allograft.
In PTS obstruction may be associated with reflux; most of the authors agree that when significant obstruction is localized above the inguinal ligament, obstruction must be treated first. Patients must be informed that in PTS surgery for reflux has a relatively high failure rate. Contraindications to reconstructive surgery that must be taken into account include inactivity, stiff ankle, and severe coagulopathy disorder.
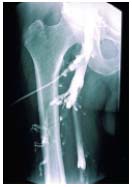
Figure 11. Descending phlebography.
The common femoral vein and
the upper half of the
(superficial) femoral vein are
refluxive and their appearance
is in favor of primary reflux.
Valvuloplasty seems feasible.
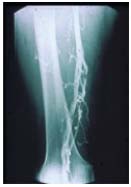
However, the lower half of the
(superficial) femoral
and popliteal veins are typically
post-thrombotic.
CONCLUSIONS
As large randomized control trials comparing conservative treatment and DVRS for DVR would be difficult to conduct, we must rely on the outcome of present series treated by DVRS. Their analysis provides grade C recommendation. Better results are obtained in the treatment of primary reflux compared with secondary reflux. Such surgery is not however, often indicated, and the procedure must be performed in specialized and centers with highly trained staff.

REFERENCES
2. Raju S. Operative management of chronic venous insufficiency. In Rutherford RB, ed. Vascular Surgery. Philadelphia: WB Saunders. 1995:1851-1862.
3. Kistner RL. Surgical repair of the incompetent femoral vein valve. Arch Surg. 1975;110:1336-1342.
4. Kistner RL. Surgical repair of a venous valve. Straub Clin Proc. 1968;24:41-43.
5. Kistner RL, Sparkuhl MD. Surgery in acute and chronic venous disease. Surgery. 1979;85:31-43.
6. Taheri SA, Lazar L, Elias S. Status of vein valve transplant after 12 months. Arch Surg. 1982;117:1313-1317.
7. O’Donnell TF, Mackey WC, Shepard AD, Callow AD. Clinical, hemodynamic and anatomic follow-up of direct venous reconstruction. Arch Surg. 1987;122: 474-482.
8. O’Donnell TF. The surgical management of deep venous valvular incompetence. In: Rutherford RB, ed. Vascular Surgery. Philadelphia: WB Saunders; 1989: Chapter 14.
9. Plagnol P, Ciostek P, Grimaud JP, Prokopowicz SC. Autogenous valve reconstruction technique for post-thrombotic reflux. Ann Vasc Surg. 1999;13:339-342.
10. Maleti O. Venous valvular reconstruction in post-thrombotic syndrome. A new technique. J Mal Vasc. 2002;27:218-221.
11. Jessup G, Lane R. Repair of incompetent venous valves: a new technique. J Vasc Surg. 1988;8:569-575.
12. Lane RJ, Cuzilla ML, McMahon CG. Intermediate to long-term results of repairing incompetent multiple deep venous valves using external stenting. ANZ J Surg. 2003;73;267-274.
13. Kistner RL. Surgical technique of external venous valve repair. Straub Found Proc. 1990;55:15.
14. Raju S, Berry MA, Neglen P. Transcommissural valvuloplasty: technique and results. J Vasc Surg. 2000;32:969-976.
15. Gloviczki P, Merrel SW, Bower TC. Femoral vein valve repair under direct vision without venotomy: a modified technique with use of angioscopy. J Vasc Surg. 1991;14:645-648.
16. Welsh HJ, McLaughlin RL, O’Donnel TF Jr. Femoral vein valvuloplasty: intra operative angioscopic evaluation and hemodynamic improvement. J Vasc Surg. 1992;16: 697-670.
17. Pavcnik D, Uchida BT, Timmermans HA, et al. Percutaneous bioprosthetic venous valve: a long term study in sheep. J Vasc Surg. 2002;35:598-602.
18. Van Bemmelen PS, Bedford G, Beach K, et al. Quantitative segmental evaluation of venous valvular reflux with ultrasonic duplex scanning. J Vasc Surg. 1989; 10:425-431.
19. Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lowerextremity veins. J Vasc Surg. 2003; 38:793-798.
20. Christopoulos DC, Nicolaides AN, Cook A, et al. Pathogenesis of venous ulceration in relation with the calf pump function. Surgery. 1989;106:829-835.
21. Nicolaides AN, Zukowsky AJ. The value of dynamic pressure measurements. World Surg. 1986;17:919-924.
22. MacMullin GM, Coleridge-Smith PD, Scurr JH. A study of tourniquets in the investigation of chronic venous insufficiency. Phlebology. 1991;6: 133-139.
23. Kistner RL, Ferris RG, Randhawa G, Kamida CB. A method of performing descending venography. J Vasc Surg. 1986:464-468.
24. Masuda EM, Kistner RL. Long term results of venous valve reconstruction: a four to twenty-one year follow-up. J Vasc Surg. 1994;19:391-403.
25. Raju S, Fredericks R, Neglen P, Bass JD. Durability of venous valve reconstruction for primary and post-thrombotic reflux. J Vasc Surg. 1996;23:357-367.
26. Sottiurai VS, Current surgical approaches to venous hypertension and valvular reflux. Int J Angiology. 1996;5:49-54.
27. Eriksson I, Almgren B. Surgical reconstruction of incompetent deep vein valves. Up J Med Sci. 1988;93:139-143.
28. Perrin M. Reconstructive surgery for deep venous reflux. Cardiovasc Surg. 2000;8: 246-255.
29. Akesson A, Risberg B, Bjôrgell O. External support valvuloplasty in the treatment of chronic deep vein incompetence of the legs. Int Angiol. 1999;18:233-238.
30. Camilli S, Guarnera G. External banding valvuloplasty of the superficial femoral vein in the treatment of primary deep valvular incompetence. Int Angiol. 1994;13:218-222.
31. Cardon JM, Cardon A, Joyeux A, et al. La veine saphène interne comme transplant valvulé dans l’insuffisance veineuse post-thrombotique : résultats à long terme. Ann Chir Vasc. 1999;13:284-289.
32. Johnson HD, Queral LA, Finn WR, et al. Late objective assessment of venous valve surgery. Arch Surg. 1981;116:1461-1466.
33. Mackiewicz Z, Molski S, Jundzill W, Stankiewicz W. Treatment of postphlebitic syndrome with valve transplantation: 5-year experience. Eurosurgery 95. Bologna Monduzzi. 1995:305-310.
34. Nash T. Long-term results of vein valve transplants placed in the popliteal vein for intractable post-phlebitic venous ulcers and pre-ulcer skin changes. J Cardiovasc Surg. 1988;29:712-716.
35. Bry JD, Muto PA, O’Donnel TF, Isaacson LA. The clinical and hemodynamic results after axillary-to-popliteal valve transplantation. J Vasc Surg. 1995;21; 110-119.
36. Raju S, Neglen P, Meydrech J, Doolittle EF. Axillary vein transfer in trabeculated post-thrombotic veins. J Vasc Surg. 1999;29:1050-1064.
37. Dalsing MC, Raju S, Wakefield TW, Taheri S. A multicenter, phase 1 evaluation of cryopreserved venous valve allografts for the treatment of chronic deep venous insufficiency. J Vasc Surg. 1999;30: 854-856.
38. Neglen P, Raju S. Venous reflux repair with cryo-preserved vein valves. J Vasc Surg. 2003;37:552-557.
39. Iafrati MD, Welch H, O’Donnel TF, Belkin M, Umphrey S, MacLaughlin R. Correlation of venous non-invasive tests with the Society for Vascular Surgery clinical classification of chronic venous insufficiency. J Vasc Surg. 1994;19: 1001-1007.
40. Gloviczki P, Bergan JJ, Menawat SS, et al. Safety, feasibility, and early efficacy of subfascial endoscopic perforator surgery. A preliminary report from the North American registry. J Vasc Surg. 1997; 25:94-105.
