Endovascular treatment of chronic lliofemoral venous obstruction – A review
MD, PhD
Mississippi, USA
SUMMARY
The importance of venous outflow obstruction is increasingly recognized as an important contributing factor in chronic venous disease. Obstruction of the iliofemoral venous outflow appears particularly important, and results in more severe symptoms than does lower segmental blockage. Selection of patients for treatment is hampered by the lack of accurate noninvasive or invasive tests for evaluation of obstruction. In fact, it is not known precisely what degree of venous stenosis should be considered hemodynamically critical. It is presently impossible to detect potentially hemodynamically important borderline obstructions. The diagnosis must be made on clinical signs and symptoms having a high index of suspicion, and treatment must be based on morphologic investigations, eg, transfemoral phlebography or, preferably, intravascular ultrasound (IVUS). Percutaneous iliac venous balloon venoplasty and stenting is a safe, minimally invasive method with minimal complication rate, no mortality, a 4-year acceptable patency, and substantial sustained clinical improvement. It is a less invasive alternative, and relatively safer than open surgery, and can therefore be offered to a larger group of patients. In case of immediate or late failure venous stenting does not preclude subsequent bypass surgery or surgical correction of reflux when necessary. Although venous stenting is presently the preferred treatment for iliofemoral obstruction, more research is necessary to define accurate hemodynamic criteria for assessment and treatment and to study the long-term effects of stents in the venous system.
INTRODUCTION
Following successful arterial endovascular surgery, balloon venoplasty and stenting of chronic venous obstructions were introduced in the late 1980s and early 1990s. Most studies of venous stents have reported results of stent treatment of residual obstruction following removal of acute iliac or subclavian vein thrombosis,1,2 or in the venous outflow tract of arteriovenous fistulae used for hemodialysis.3,4
The behavior of venous stents varies greatly depending on indication and anatomic placement of the stents. Assessment of the results of 707 throm bolyzed and stented iliofemoral veins in 24 reports shows primary and assisted primary patency rates varying from 50% to 100% and 63% to 100%, respectively, during a follow-up time of 1 to 46 months. Few of these patients had late follow-up with imaging and no actuarial curves were constructed.5 The primary patency rate following balloon angioplasty without stent and decompression surgery in the subclavian vein after thrombolysis is very poor, as low as 6% at 2 years.6 Owing to its anatomic location, the subclavian vein is prone to flexion during movement, and the vessel may be compressed by external structures, most often between the first rib and clavicle. Stents have been reported to be deformed or even fractured by the forceful compression in the thoracic outlet, resulting in secondary thrombosis.7,8 Although no larger long-term study exists, probably balloon angioplasty and stenting of the subclavian vein should always be combined with decompression surgery.9 The result following angioplasty and stenting of the catheter-induced central vein stenoses and the dialysis access outflow tract of the upper or lower extremities is dismal. The neointimal hyperplasia at the venous anastomosis is very resistant to balloon angioplasty; the primary patency rates at 24 months after angioplasty and stenting for central veins and peripheral veins are only 9% and 17%, respectively.3 The best results have been obtained with treatment of the iliofemoral vein with chronic nonmalignant obstruction. This review will focus mainly upon the symptoms, diagnostic dilemma, technique, and clinical and morphological results (patency and in-stent restenosis rates) after iliofemoral stenting of chronic obstructions.
SYMPTOMS
Symptoms of chronic venous disease may vary greatly, ranging from moderate swelling and pain to discoloration and stasis ulcers. The main emphasis has been on treatment of severe skin changes and stasis ulcer, chiefly by controlling reflux. It is our experience that a substantial number of patients with CVI, however, complain of disabling limb pain and swelling without skin changes.10 These symptoms are not always improved by wearing compression stockings or performing venous valve repair. The dominant pathophysiologic component in these patients may be obstruction rather than reflux, and it is possible that these symptoms are mainly attributable to the outflow blockage. “Venous claudication” is described as an exercise induced “tense” pain, which requires several minutes of rest and leg elevation to subside. Certainly patients with significant outflow obstruction may have less dramatic symptoms, with less distinct lower extremity pain and discomfort, with decreased quality of life and moderate disability. Previous estimations that obstruction is a major contributor in only 10% to 20% of patients with severe chronic venous disease are probably markedly low.
DIAGNOSIS
Often when algorithms are constructed for workup of patients with chronic venous insufficiency, investigations for reflux are emphasized, and testing for outflow obstruction is completely omitted. This is probably owing in part to a lack of accurate objective noninvasive or invasive tests for evaluation of hemodynamically significant chronic venous obstruction, and in part to the lack of practical treatment alternatives prior to the introduction of venous stenting. There are many tests for delineating focal and global reflux but this is not so for outflow obstruction. Ultrasound investigation and outflow fraction determinations by plethysmographic methods have been shown to be unreliable and play only a limited role. Although abnormal plethysmography findings may indicate obstruction to the venous outflow, significant blockage may be present with normal findings.11-13 Even the invasive pressures, ie, hand/foot pressure differential and reactive hyperemia pressure increase, and indirect resistance calculations appear insensitive and do not define the level of obstruction.11 Only a small pressure gradient over a venous stenosis or pressure increase below an obstruction on exercise or hyperemia may indicate significant obstruction. These pressure differences are certainly much lower than in the arterial system, often as low as 2 to 3 mm Hg, which may be difficult to measure accurately.14-16 Thus, although a positive hemodynamic test may indicate hemodynamic significance, a normal test does not exclude it. Unfortunately, it is presently impossible to detect borderline obstructions, which may potentially be of hemodynamic importance. Since accurate hemodynamic tests are unavailable, diagnosis and treatment must be based on morphological findings. Single-plane transfemoral phlebogram is the standard investigation, and may show obstruction and development of collaterals. Increased accuracy may be achieved with multiple angled projections (Figures 1a-c).17
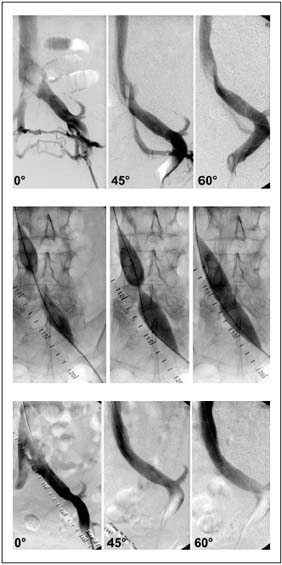
Figure. 1. Transfemoral phlebogram showing a May-Thurner
syndrome in multiple projections: a) before venous stenting,
showing absence of stenosis but presence of collaterals in the AP
view. The stenosis is detected by rotation; b) waisting of balloon
during inflation by the stenosis; and c) post-stent phlebogram
revealing no stenosis.
Although the degree of stenosis may be obtained, the hemodynamic impact of this stenosis is not known from morphologic studies. In fact, it is not known what degree of through this meandering vessel may replace that through the straighter main vein. The collaterals observed prestent often disappear promptly following stenting of a significant stenosis (Figure 2).
Figure 2. Chronic iliofemoral thrombotic stenosis before and
after stenting. Note the disappearance of collaterals after
disobliteration.
The flow through the stent is obviously favored. It may be that the development of collaterals should be considered an indicator of obstruction and a failed compensatory feature if the patient is symptomatic. Intravascular ultrasound (IVUS) can detect only axial collaterals running close to the original vessel. Transpelvic collaterals will escape detection. Several studies have shown, however, that IVUS is superior in detection of the extent and morphologic degree of stenosis as compared to the single-plane phlebography.19-21 IVUS shows intraluminal details, eg, trabeculations and webs, which may be hidden in the contrast dye (Figure 3).
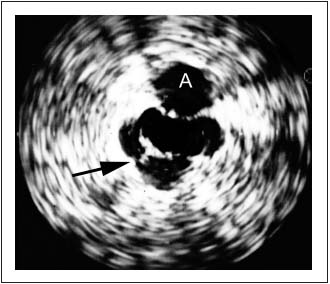
Figure 3. Intravascular ultrasound (IVUS) showing trabeculation
(arrow) not visualized by phlebogram. The adjacent artery is
marked with an A.
An external compression with the resulting deformity of the venous lumen can be directly visualized, and wall thickness and movement can be seen. Most importantly, IVUS appears superior to standard single-plane venography for estimating the morphological degree of iliac vein stenosis. On average the transfemoral venogram significantly underestimated the degree of stenosis by 30%. The phlebogram was actually considered “normal” in one fourth of limbs despite the fact that IVUS showed >50% obstruction.22 IVUS is clearly superior to single-plane venography in providing adequate morphological information, and is presently the best available method for diagnosing clinically significant chronic iliac vein obstruction. Owing to the lack of hemodynamic tests, the diagnosis and treatment must presently be based on invasive morphological investigations of the iliac venous outflow (preferably IVUS). Limiting workup of patients with chronic venous disease to only duplex ultrasound will not suffice. In order to identify patients with potentially important venous outflow obstruction, transfemoral phlebography (multiplane) or IVUS should be utilized more generously in patients with significant signs and symptoms of CVI.
We consider the following as indicators of obstruction warranting further investigations: 1) phlebographic stenosis >30%; 2) presence of pelvic collaterals; and 3) positive invasive pressure test.
The patient is taken to the OR, and IVUS is performed when one or several of these indicators are present and the patient is symptomatic. The symptoms may range from painful swelling, pain in excess of clinical findings to severe stages with lipodermatosclerosis or ulcer. We have arbitrarily chosen to stent only venous stenosis of >50% as seen on IVUS and clinical results have been good. With this policy 10 to 15% of iliofemoral veins are found to be normal.
TECHNICAL HINTS
Venous stenting is a minimally invasive procedure and may appear to be simple. To achieve good results, however, attention to detail is important. Venous balloon angioplasty and stenting is a different procedure from that employed in the arterial system. Some important aspects are emphasized:19,23
1. Balloon angioplasty is insufficient in the venous system. Stent insertion is mandatory. Severe recoil of the vein is already observed intraoperatively in the majority of limbs and simple balloon dilation leads to early restenosis.
2. Always use ultrasound to guide cannulation of the femoral vein, especially when low thigh cannulation is necessary to enter below iliofemoral occlusions. Ultrasound guidance has largely eliminated access complications.
3. IVUS is invaluable, both as a diagnostic tool and as an intraoperative aid in direct placement of the stent.20,21
4. Stenting of a stenosis adjacent to the confluence of the common iliac veins requires that the stent be placed well into the IVC to avoid early restenosis.19 This is especially important when a Wallstent® is used. Owing to its inherent property this stent may be “squeezed” distally and a proximal restenosis may develop. This placement of the stent into the IVC does not appear to significantly impair the flow from the contralateral limb resulting in thrombosis. A few cases of contralateral limb thrombosis have been observed and raised concern. These clots, however, appear to be caused by recurrent attacks of thrombosis rather than stent occlusion.
5. The “kissing” balloon technique utilized at the aortic bifurcation is unnecessary at the confluence of the common iliac veins and bilateral stents are not inserted at this location.
6. Insertion of a large-diameter stent (14 to 16 mm wide) is recommended. Unlike the artery, the vein accepts extensive dilation without clinical rupture. No such rupture has been reported, even when a total occlusion is recanalized and dilated up to a width of 14 to 16 mm.
7. It is important to redilate the stent after insertion to avoid its possible movement. A good wall apposition should be achieved as evaluated by IVUS.
8. The diseased vein segment is more extensive in reality than seen on phlebography. To avoid early restenosis and occlusion it is vital to cover the entire obstruction as outlined by the IVUS investigation. Short (<5 cm) skip areas in between two stents should be avoided. The occlusion rate does not appear to be related to the length of stent or metal load per se. Probably the most common cause of early restenosis is inadequate stenting of the entire lesion.
COMPLICATIONS
The nonthrombotic complication rate related to the endovascular intervention is minimal and comprises mostly cannulation site hematoma, although a few cases of retroperitoneal hematoma requiring blood transfusions have been described.24,25 The utilization of ultrasoundguided cannulation and closure with collagen plugs has largely abolished these problems, reducing the nonthrombotic complication rate from 3% to <1%. After introduc- tion of these modifications, we had no procedure-related complications. The mortality has been zero.
Data regarding early rethrombosis rate (<30 days) after iliofemoral stenting are sparse. The rate was found to be 11% in the Creighton University experience following stenting after thrombolysis of an acute DVT26 and 15% in the National Registry study.27 The early thrombosis rate was found to be lower (4%) when stenting was performed for chronic iliofemoral venous obstruction without earlier thrombolysis.22 All early occlusion occurred in patients with thrombotic disease (8% thrombosis rate), while none occurred in nonthrombotic limbs. Early failures appear more common with long stents extending below the inguinal ligament, stents placed across complete occlusions rather than across stenoses, and in patients with thrombophilia.28 (Neglen P, Raju S, 2003, unpublished data) The occlusions appeared to be related to limbs with incomplete dilation owing to nonyielding obstruction leaving residual stenosis after stenting. Thrombolysis of the newly formed clot may be attempted in initially technically successful limbs to reveal and treat unknown additional obstructions.
PATENCY RATES
There are many small case reports in the literature, but only a few larger series with acceptable follow-up. The majority of these mix obstructions of different etiologies. O’Sullivan et al have reported a 1-year patency of 79% in a retrospective analysis of 39 patients.29 Only half of the patients presented with chronic symptoms. When initial technical failures are removed, the stented patients had a 1-year patency of 94%. The clinical results were excellent in the stented limbs.
A similar group of 18 patients were reported by Hurst et al.13 Six limbs were treated after disobliteration of an acute deep vein thrombosis. The primary patency rates at 12 and 18 months were 79% and 79%, respectively. Most patients (13/18) had resolution or substantial improvement of leg swelling and pain. However, five patients (28%) continued to have pain despite resolved swelling and widely patent stents on venogram. Similarly, Binkert et al reported a 100% patency at an average follow-up time of 3 years in 8 patients (in 4 limbs following surgical thrombectomy) with resolution or substantial improvement of symptoms in most patients.30 Nazarian et al reported a 1-year primary assisted patency rate of 66% of 29 iliac obstructions.28 The lower patency rate may be explained by the selection of patients (13/29 had complete occlusion and 16/19 were caused by malignancy). Interestingly, few occlusions occurred after 6 months and the patency rate remained the same at 1- and 4 -year follow-up. The same group has also reported overall 1-year primary and secondary patency rates of 50% and 81%, respectively, in a mixed population including 56 patients with iliac obstruction caused by malignancy, trauma, pregnancy, and postoperative stenosis.31 Lamont et al32 have reported an 87% patency rate at median follow-up of 16 months in 15 patients stented for common iliac vein compression following clearance of acute thrombus by thrombolysis.
We have followed 455 limbs which underwent iliac vein stenting between 1997 and 2001.25 Transfemoral venogram was performed in 324 of these limbs. The obstructive lesion was considered thrombotic if the patient had a known occurrence of deep vein thrombosis diagnosed with duplex ultrasound or ascending venogram and subsequently treated by anticoagulation; or findings on venogram (occlusion, stenosis, or collaterals) and/or duplex ultrasound indicating previous deep vein thrombosis below the inguinal ligament (direct visualization of thrombus or indirect indication by partial or total inability to compress the vein [54%]). The remaining limbs were considered nonthrombotic. Cumulative primary, assisted-primary, and secondary patency rates at 4 years were 57%, 92%, and 93%, respectively (Figure 4).
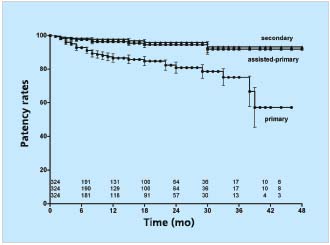
Figure 4. Cumulative primary, assisted-primary, and secondary
patency rates of 324 limbs after iliofemoral stenting.
The lower numbers represent limbs at risk for each time interval
(all SEM <10%).
The stented limbs with non-thrombotic disease appeared to fare significantly better than did those with thrombotic disease (primary, assisted-primary and secondary cumulative patency rates of 89%, 100% and 100%, and 65%, 85%, and 88% at 36 months, respectively) (Figure 5). Thus, it appears that balloon venoplasty and stenting of an iliac vein obstruction for a chronic obstruction is a safe, minimally invasive method with minimal complication rate, no mortality and a 4-year acceptable patency. A smaller number of patients have been followed for 5 years or more without precipitous deterioration of clinical efficacy and stent patency.
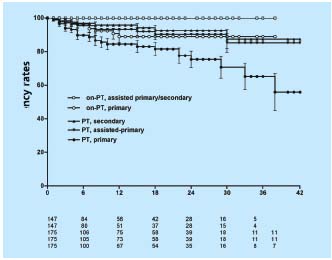
Figure 5. Cumulative primary, assisted-primary and secondary
patency rates for stented limbs with thrombotic (PT) and nonthrombotic
(non-PT) obstruction. The lower numbers represent
total limbs at risk for each time interval (all SEM <10%).
IN-STENT RESTENOSIS RATE
To assess the development of in-stent restenosis (IRS), any narrowing of the stent on transfemoral phlebography was measured in the same group of patients described above using a caliper and expressed as percentage diameter reduction of patent lumen. (Neglen P, Raju S, 2003, unpublished data) At 42 months only 23% of limbs remained with no in-stent restenosis at all, but only the minority of limbs had severe (>50%) obstruction. Most had only minimal narrowing. The cumulative IRS-free rates of limbs with >20% and >50% in-stent restenosis were 39% and 85%, respectively (Figure 6). The gender of the patient or sidedness of treated extremity did not affect the outcome. At 36 months, limbs with thrombotic disease had lower IRS-free rates than did nonthrombotic (37% and 59% of >20% narrowing, and 77% and 96% of >50% narrowing, respectively (P<0.01)(Figure 7). Similarly, IRS-free rates were found in patients with thrombophilia and long stents extending below the inguinal ligament. The three major risk factors appear to be presence of thrombotic disease, positive thrombophilia test, and stent placement below the inguinal ligament (long stents). Although the cumulative patency rate and in-stent restenosis-free rate curves have a similar course in both nonthrombotic and thrombotic limbs, the hyperplasia-free curve drops more rapidly than do the patency curves. The development of in-stent restenosis appears to precede the development of obstruction (Figure 7). These findings may suggest a cause-effect relationship. Also, the stented limbs that eventually occluded during this study had similar risk factors. However, no conclusion regarding cause-effect relation- ship between in-stent restenosis and occlusion can be drawn from the data presented. Whether the late occlusions occur owing to acute thrombosis or to gradual development of true intimal hyperplasia needs further study.
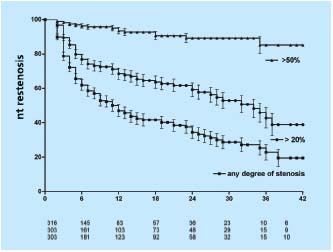
Figure 6. Cumulative in-stent restenosis-free rates for all limbs
with any degree of stenosis, >20% and severe (>50%) narrowing.
The lower numbers represent total limbs at risk for each time
interval (all SEM <10%).
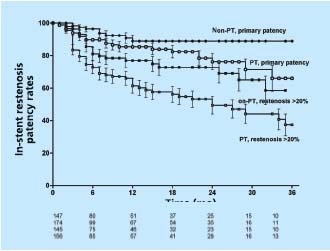
Figure 7. Cumulative in-stent restenosis-free rates (>20% instent
restenosis) and cumulative primary rates for stented limbs
with thrombotic (PT) and nonthrombotic (non-PT) obstruction.
The lower numbers represent total limbs at risk for each time
interval (all SEM <10%).
CLINICAL RESULTS
As alluded to above, the reports describing patency rates indicate clinical improvement in most patients (>90%).29,30 Hurst et al showed resolution or substantial improvement in 72% of limbs.13 In addition to ulcer healing and ulcer recurrence rate, we have followed the patient’s clinical result by quality-of-life questionnaires,33 degree of swelling assessed by physical examination (Grade 0: none; Grade 1: pitting, not obvious; Grade 2: ankle edema; and Grade 3: obvious swelling involving the limb), and level of pain measured by the visual analogue scale method.19, 22, 25, 34 The incidence of ulcer healing after iliac vein balloon dilation and stent placement in 41 limbs with active ulcer was 68% and the cumulative ulcer recurrence-free rate at 2 years was 62%.25 Frequently these limbs had remaining reflux, which was untreated during the observation period. Despite the presence of reflux the stasis ulcers stayed healed. Median swelling and pain severity scores decreased significantly (grade 2 to 1 and 4 to 0, respectively). The rates of limbs with any objective swelling or pain decreased significantly by approximately 55% (from 88 % to 33% and from 83 % to 29 %, respectively). Using a quality-oflife questionnaire assessing subjective pain, sleep disturbance, morale, and social activities, and routine and strenuous physical activities, the patients indicated significant improvement in all major categories after venoplasty and stenting.
The presence of ulcer may influence the degree of pain and swelling apart from that caused by iliac obstruction. However, the reduction in pain and swelling was significant in both limbs with and without stasis ulcer. The prestenting level of pain was similar in both groups, suggesting the important contribution by iliac vein obstruction to pain and swelling in these patients. Interestingly, patients with recurrence of obstruction also had recurrence of symptoms after a symptom-free period. These results clearly indicate a significant symptom relief after balloon venoplasty and stenting of iliofemoral vein obstruction, even in the presence of remaining reflux. The results also suggest that outflow obstruction is a more important and frequent component of chronic venous disease than previously realized.
CONCLUSIONS
Patients have now been followed for more than 6 years after stenting of the iliofemoral venous outflow. Ongoing evaluation indicates that venous balloon angioplasty and stenting is a safe, relatively simple, and efficient method for the treatment of iliofemoro-caval vein obstruction. It is now the preferred treatment over open surgery for this condition and may be offered to a larger group of patients. An immediate or late failure of the procedure does not preclude later open surgery to correct the obstruction. Additional interventions to correct any associated reflux may be performed subsequently when necessary.
Although a promising technique, some caveats must be stated. Selection of patients for treatment based on morphologic investigations is not satisfactory. Hemodynamic criteria are preferred. Further research should be encouraged to achieve better understanding of the nature of venous obstruction and to develop reliable methods to test hemodynamic significance. Monitoring of these patients should continue to acquire knowledge of the longterm effects of stents in the venous system and potential development of threatening in-stent restenosis.

REFERENCES
2. Kreienberg PB, Chang BB, Darling RC 3rd, et al. Long-term results in patients treated with thrombolysis, thoracic inlet decompression, and subclavian vein stenting for Paget-Schroetter syndrome. J Vasc Surg. 2001;33(2 Suppl):S100-105.
3. Oderich GS, Treiman GS, Schneider P, Bhirangi K. Stent placement for treatment of central and peripheral venous obstruction: A long-term multiinstitutional experience. J Vasc Surg. 2000;34:760-769.
4. Funaki B, Szymski GX, Leef JA, Funaki AN, Lorenz J, Farrell T, et al. Treatment of venous outflow stenoses in thigh grafts with Wallstents. Am J Roentgenol. 1999;172:1591-1596.
5. Thorpe PE, Osse FJ, Dang HP. Endovascular reconstruction for chronic iliac vein and inferior vena cava obstruction. In Gloviczki P, Yao SJT, eds. The Handbook of Venous Disorders, Guideline of The American Venous Forum. 2nd ed. London: Arnold; 2001:347-361.
6. Glanz S, Gordon DH, Lipkowitz GS, Butt KMH, Hong J, Sclafani SJ. Axillary and subclavian vein stenosis: Percutaneous angioplasty. Radiology. 1988;168:371-373.
7. Phipp LH, Scott DJ, Kessel D, Robertson I. Subclavian stents and stentgrafts: Cause of concern? J Endovasc Surg. 1999;6:223-226.
8. Maintz D, Landwehr P, Gawenda M, Lackner K. Failure of Wallstents in the subclavian vein due to stent damage. Clin Imaging. 2001;25:133-137.
9. AbuRahma AF, Robinson PA. Effort subclavian vein thrombosis: Evolution of management. J Endovasc Ther. 2000;7:302- 308.
10. Raju S, Neglén P, Carr-White PA, et al. Ambulatory venous hypertension: Component analysis in 373 limbs. Vasc Surg. 1999;33:257-267.
11. Neglén P, Raju S. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583-589.
12. Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132:46-51.
13. Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106-113.
14. Negus D, Cockett FB. Femoral vein pressures in post-phlebitic iliac vein obstruction. Br J Surg. 1967;54:522-525.
15. Rigas A, Vomvoyannis A, Giannoulis K, et al. Measurement of the femoral vein pressure in edema of the lower extremity. J Cardiovasc Surg. 1971;12:411-416.
16. Albrechtsson U, Einarsson E, Eklöf B. Femoral vein pressure measurements for evaluation of venous function in patients with postthrombotic iliac veins. Cardiovasc Intervent Radiol. 1981;4:43-50.
17. Juhan C, Hartung O, Alimi Y, Barthélemy P, Valerio N, Portier F. Treatment of nonmalignant obstructive lesions by stent placement: mid-term results. Ann Vasc Surg. 2001;15:227-232.
18. Neglén P, Raju S. Proximal lower extremity chronic venous outflow obstruction: Recognition and treatment. Semin Vasc Surg. 2002;15:57-64.
19. Neglén P, Raju S. Balloon dilation and stenting of chronic iliac vein obstruction: technical aspects and early clinical outcome. J Endovasc Ther. 2000;7:79-91.
20. Neglén P, Raju, S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694- 700.
21. Forauer AR, Gemmete JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) Syndrome. J Vasc Interv Radiol. 2002;13:523-527.
22. Neglén P, Berry MA. Raju S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Eur J Vasc Endovasc Surg. 2000;20:560-571.
23. Raju S, McAllister S, Neglén P. Recanalization of totally occluded iliac and adjacent venous segments. J Vasc Surg. 2002;36:903-911.
24. Ouriel K, Kandarpa K, Schuerr DM, Hultquist M, Hodkinson G, Wallin B. Prourokinase vs. urokinase for recanalization of peripheral occlusions, safety and efficacy: the PURPOSE Trial. J Vasc Intervent Radiol. 1999; 10:1083-1091.
25. Raju S, Owen S Jr, Neglén P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002; 35:8-15.
26 Thorpe PE. Endovascular therapy for chronic venous obstruction. In: Ballard JL, Bergan JJ, eds. Chronic Venous Insufficiency. New York: Springer;1999:179-219.
27. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39-49.
28. Nazarian GK, Austin WR, Wegryn SA, et al. Venous recanalization by metallic stents after failure of balloon angioplasty or surgery: four-year experience. Cardiovasc Intervent Radiol. 1996;19:227-233.
29. O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. JVIR. 2000;11:823-836.
30. Binkert CA, Schoch E, Stuckmann G, et al. Treatment of pelvic venous spur (May-Thurner syndrome) with selfexpanding metallic endoprostheses. Cardiovasc Intervent Radiol. 1998;21:22-26.
31. Nazarian GK, Bjarnason H, Dietz CA Jr, et al. Iliofemoral venous stenosis: Effectiveness of treatment with metallic endovascular stents. Radiology. 1996;200:193-199.
32. Lamont JP, Pearl GJ, Patetsios P, Warner MT, et al. Prospective evaluation of endoluminal venous stents in the treatment of the May-Thurner syndrome. Ann Vasc Surg. 2002;16:61-64.
33. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539-554.
34. Scott J, Huskisson EC. Graphic presentation of pain. Pain. 1976;2:175-184.
