How to manage complications after sclerotherapy
Surgery, Hospital Central de la Cruz Roja,
Madrid, Spain
Abstract
Sclerotherapy is an effective and safe treatment when used by trained and careful hands. Good technique, satisfactory imaging, general precautions, and compliance with post treatment instructions may help avoid some of the adverse events. Even though complications can happen even to the most experienced practitioner, it is mandatory to know what they are and how to manage them. Fortunately, most of these adverse events are benign, but physicians must be aware of the potential serious events, and they should be trained to react adequately and immediately. All office settings using sclerotherapy should be equipped to administer oxygen therapy. Protocols for immediate action in case of anaphylaxis, intra-arterial injection, or neurologic deficits should be in place. A plan for transport to emergency services for further evaluation and treatment of vital emergencies, such as stroke or extended necrosis, are imperative. Access to hyperbaric oxygen therapy may be considered in this emergency planning. Minor complications require an adequate follow-up by the practitioner and adherence with post-sclerotherapy treatment by the patient. Very rare major complications could benefit from multicenter registers to provide evidence-based treatments.
Introduction
The European guidelines for sclerotherapy in chronic venous disorders recommend considering the following adverse events after sclerotherapy (Table I).1-5 Compared with liquid sclerotherapy, foam sclerosants do not result in many new or different complications, but appear to change their relative incidences.1 Most adverse effects are minor and inconsequential, such as local injection site pain, urticaria, itching, erythema, and bruising. Other common, but usually self-limiting, side effects include visual disturbances and migraines (1.4% to 14%), cutaneous hyperpigmentation (10% to 30%), and telangiectatic matting (15% to 24%) or blisters or folliculitis caused by post-sclerotherapy compression. Significant and relatively rare complications include systemic life-threatening reactions and anaphylaxis (very rare), deep venous thrombosis (1% to 3%), stroke (0.01%), tissue necrosis (variable frequency), edema of the injected extremity (0.5%), and nerve damage (0.2%).1-5
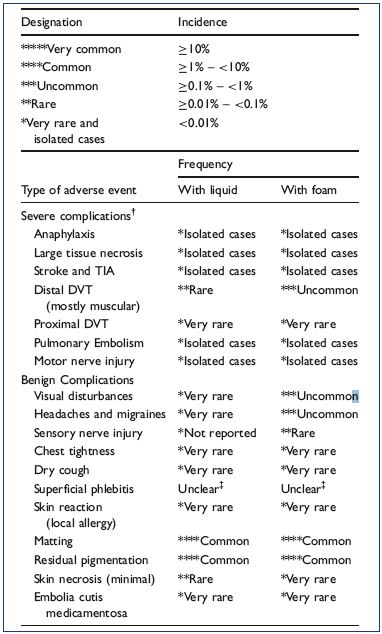
Table I. Complications observed in a prospective French Registry
of 12 173 sclerotherapy sessions.
From reference 1: Guex JJ et al. Dermatol Surg. 2005;31(2):123-
128.
Major complications
Systemic allergic reactions caused by sclerotherapy treatment occur very rarely. Local or generalized skin reactions, such as urticaria, are much more frequent (around 0.6%) than systemic involvement, and true anaphylaxis is an extremely rare complication constituting an emergency.6-10 These reactions are unpredictable. Patients who have undergone multiple previous treatments with liquid sclerosants may be at a higher risk of developing post-sclerotherapy generalized urticaria, mastocytosis, or chronic urticaria.3 Since the risk increases with repeated exposure to the antigen, it is important to always be prepared for this reaction.6 Foam sclerosants are associated with a lower incidence of hypersensitivity reactions, and histamine release is responsible for the clinical manifestations of this reaction. Although urticaria and abdominal pain are common, the three principal manifestations of anaphylaxis are airway edema, bronchospasm, and vascular collapse.
The treatment should be tailored to the clinical features of the allergic events; it is essential to have emergency protocols in place (Figure 1).11-15 The injection should be stopped immediately and the standard emergency procedure should be followed, including the administration of oxygen and epinephrine when appropriate (grade 1A).4,5 The treatment requires: (i) putting the patient in the Trendelenburg position; (ii) keeping airways secure; (iii) giving oxygen; (iv) gaining access for IV fluids; and (iv) administering drugs. Calling for emergency services should be done in parallel with initial patient support (Figure 2).11-16
The recommended treatment is a subcutaneous injection of epinephrine 0.2 to 0.5 mL (1:1000). This treatment can be repeated three or four times at 5- to 15-minute intervals to maintain a systolic blood pressure >90 to 100 mm Hg, and it should be followed by establishing an intravenous line with a 0.9% sodium chloride solution. Intravenous injections of dexclorfeniramine 5 to 10 mg every 8 hours or intravenous diphenhydramine hydrochloride 50 mg, is given next, along with cimetidine, 300 mg; both the intravenous solution and oxygen are given at 4 to 6 L/min. An endotracheal tube or tracheotomy is necessary for laryngeal obstruction. For asthma or wheezing, use salbutamol, 4 inhalations over 10 minutes, or nebulized salbutamol, 5 mg over 3 to 4 hours. For severe respiratory symptoms, use a 4 μg/kg intravenous bolus of salbutamol and, later, it is recommended to use 5 to 10 μg/minute as a continuing infusion. At this point, it is appropriate to transfer the patient to the hospital or emergency services. Methylprednisolone sodium succinate (1 mg/kg) or hydrocortisone (250 mg) is given intravenously and repeated every 6 hours for a total of 4 doses. Corticosteroids are not an emergency medication because their effects appear only after 1 to 3 hours, and they are given to prevent the recurrence of symptoms 3 to 8 hours after the initial event. The patient should be hospitalized overnight for observation.11-15
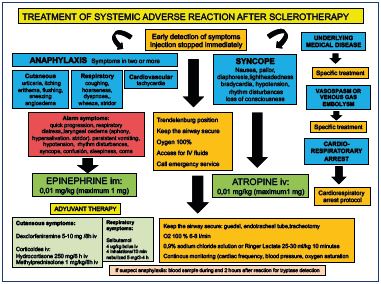
Figure 1. Local protocol for the management of systemic adverse
reactions after sclerotherapy.
Office-setting, Vascular Department, Hospital Central de la Cruz
Roja, Madrid, Spain.
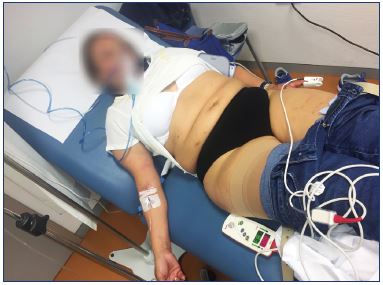
Figure 2. Transitory general effects.
The patient had painful chest tightness and an exacerbation
of an underlying stress-related allergic disease after foam
sclerotherapy of reticular veins and telangiectasia. The patient
was managed with intramuscular epinephrine, intravenous
antihistamines, bronchodilator therapy, intravenous line with
sodium chloride solution, and 100% O2, and the treatment
occurred while the patient was in the Trendelenburg position
with continuing evaluation of neurologic, cardiovascular, and
respiratory systems. Intravenous corticosteroid was administered
later. The patient was on observation until the clinical
disturbances disappeared and she was discharged.
Minor degrees of angioedema can be treated with oral antihistamines. However, if stridor is present, an intravenous injection of dexclorfeniramine 5 to 10 mg or an intramuscular injection of diphenhydramine and intravenous corticosteroids should be administered; a laryngoscope and endotracheal tube should be available.11-15 Bronchospasm has been estimated to occur after sclerotherapy in 0.001% of patients, but it usually responds to the addition of an inhaled or intravenous bronchodilator or to the already noted antihistamine-corticosteroid regimen.11-15 Minor reactions, such as urticaria, are easily treated with oral antihistamines. The addition of corticosteroids is rarely needed, but they may be needed if the reaction does not subside readily.11-15
Tissue and cutaneous necrosis
Tissue necrosis most commonly presents as an ulceration, and it can result in extensive loss of tissue. Cutaneous necrosis may occur with the injection of any sclerosing agent, even under ideal circumstances, and it does not necessarily represent a physician error. Fortunately, its occurrence is rare and usually of limited sequelae.6 Cutaneous necrosis can occur several weeks after the initial insult, and it can be associated with pain, localized inflammation, and edema.
Some classes of sclerosing agents, such as chemical irritants and osmotic agents, are more likely to cause tissue necrosis following extravasation.16 The main mechanism leading to tissue necrosis following the use of detergents is arterial occlusion, which may be caused by an inadvertent intra-arterial injection or a venoarterial reflex vasospasm.3,17-19 Passage of the sclerosants into the arterial circulation may be mediated by open cutaneous arteriovenous shunts.17-19 Venoarterial reflex vasospasm may result from a high-speed or high-pressure injection in small caliber veins, which leads to rapid dilation of the target vein and vasospasm of the associated arteries. Venoarterial reflex vasospasm clinically presents with prolonged blanching of the skin a few centimeters away from the site of injection, followed by cyanosis and reactive erythema. Prolonged arterial vasospasm may result in tissue infarction and subsequent necrosis (Figure 3).17-19
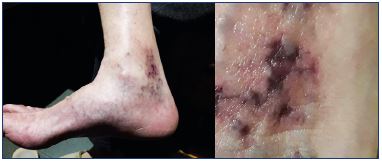
Figure 3. Cutaneous necrosis after extensive sclerotherapy with
foam in an older woman with long-term reticular veins and
telangiectasia in retromaleolar area.
The patient also suffered from severe edema and inflammation
of the ankle, which improved with nonsteroidal anti-inflammatory
drugs, medical compression stockings, and local corticosteroids.
Extravasation
A vigorous massage where extravasation has occurred may decrease tissue damage. The solution must be diluted as soon as possible. Hypertonic solutions should be diluted with copious amounts of normal saline solution (at least 10 times the volume of extravasated solution). Dilution with hyaluronidase in normal saline solution limits the extent of the necrosis and prevents the development cutaneous necrosis when using a 3% solution with sodium tetradecyl sulfate.20 Hyaluronidase should be reconstituted with a 0.9% sodium chloride solution immediately before use (75 U in a volume of 3 mL), and it is recommended to inject the diluted solution into multiple sites around the area where extravasation has occurred within 60 minutes of extravasation.21
Venoarterial reflex vasospasm
Treatments for venoarterial reflex vasospasm include topical vasodilators (2% nitroglycerine ointment), which are applied with a vigorous massage, oral antiplatelets, and oral non-steroidal anti-inflammatory drugs (NSAIDS). Systemic anticoagulant agents and systemic steroids may be used when extensive necrosis is anticipated.6
For all causes of ulceration, it must be treated as soon as it occurs. Fortunately, ulcerations are usually small, averaging 4 mm in diameter. At this size, primary healing usually leaves an acceptable scar. As ulcers may take 4 to 6 weeks to heal completely, even under ideal conditions, excision and occlusive dressing of these lesions are recommended at the earliest possible time, which gives the patient the fastest healing time, with decreased pain and an acceptable scar.6
Large tissue necrosis: inadvertent intra-arterial injection
Direct arterial/arteriolar injections are exceptionally rare. In fact, less than 70 cases have been described to date,22-26 most of which occurred after an injection in the ankle region and in the site of perforating veins above the medial ankle. Other risk areas include the cross-section of the small saphenous vein and the cross-section of the great saphenous vein. Several cases have involved arterioles of the medial thigh.22-26 Ultrasound guidance has helped minimize the occurrence of this catastrophic event, which most frequently results in limb amputation (52.5%).9,22 Intraarterial injections commonly present with severe sudden pain at the injection site, which propagates along the artery distribution. Pain can happen quickly or progress over several hours. Rarely, patients have no complaints of pain and demonstrate only a mild, sharply demarcated erythema that becomes dusky and cyanotic after a few hours.22
As endothelial damage occurs within the first minutes after injecting the sclerosant, prompt realization of the arterial complication and immediate therapy is essential to reduce the risk of subsequent amputation.22 There are no evidence-based or consensus guidelines on the optimal management of this complication.24 The European guidelines recommend that, if severe pain occurs, to stop the injection immediately, aspirate the sclerosant if possible, use local catheter-directed anticoagulation and thrombolysis if applicable, and possibly follow-up with systemic anticoagulation. Early administration of systemic steroids may help reduce the subsequent inflammation that causes tissue damage (grade 1C).3-5 Aspiration of blood with any remaining sclerosant followed by local intraarterial administration of heparin has not been identified in a single case report.24
Bergan et al16 recommended 6 days of therapeutic heparin to treat arterial injury following sclerotherapy. The in-house protocol by Parsi and Hannaford includes a subcutaneous injection of enoxaparin at 1 mg/kg over 12 hours aiming for an anti-factor Xa level in the therapeutic range of 0.5 to 1.2 IU/mL for 1 to 4 weeks depending on the extent of the injury.24 Anticoagulation may be complemented with an antiplatelet therapy of acetylsalicylic acid. Immediate intravenous application of acetylsalicylic acid, at a dosage similar to coronary events with an injection of 500 mg, might be beneficial, followed by 100 mg or 325 mg uncoated tablets of acetylsalicylic acid daily for the same period as the anticoagulation.22,24
Thrombolysis was used in four cases of inadvertent arterial injection. Complete recovery was only reported in one case, whereas amputation could not be prevented in two cases.24 In cases where cellular lysis has already taken place and microcirculatory obstruction is caused by a sludge of cellular degradation, thrombolysis might be ineffective. Therefore, it should be especially considered in the very early phase and in proximal thrombosis.24 Several authors recommend administering intravenous dextran (10%), 500 mL per dose for 3 days.6
Parsi and Hannaford published three cases that were treated with systemic steroids.24 Vessel occlusion results in an inflammatory process that will ultimately lead to skin necrosis. Their current in-house protocol includes an intravenous administration of a systemic steroid for at least 48 hours before switching to oral prednisone at 0.75 to 1 mg/kg/day (maximum dose, 50 mg daily), with a gradual reduction over the course of 12 weeks. This protocol is based on anecdotal experience and should be tested in future cases.24 In localized and less extensive cases, potent topical steroids, such as clobetasol, have been used with reported success.22
Another therapeutic goal is pain control. Given the proximity of nerves to arteries, arterial injury can result in perineural swelling and significant neuropathic pain. Pain contributes to significant morbidity, which must be managed carefully.22 Parsi and Hannaford found that shorter-acting NSAIDS, such as ibuprofen, were more effective than longer-acting NSAIDS. Gabapentin was not particularly useful in any of their patients.24 One patient found that electrostimulation therapy provided adequate pain relief.24
Hyperbaric oxygen may minimize reperfusion tissue injury by optimizing the oxygenation, inhibiting neutrophil migration, and minimizing proinflammatory cytokine production.27 This treatment has been successfully used to prevent necrosis following a single case of an intra-arterial injection of sclerosants.25 As its occurrence is extremely rare, it is recommended that an emergency flow sheet be readily accessible (Table II).
Neurological complications
The overall frequency of neurological complications of sclerotherapy is around 0% to 2%,<28,29 and they include transient events, such as visual disturbances and migraine, and ischemic events, such as transient ischemic attacks and stroke, which is an event with a lower frequency that can result either from a paradoxical clot or a gas embolism. Patent foramen ovale and other cardiopulmonar right-to-left shunts are the most consistent risk factors.9 The etiology of neurological symptoms following sclerotherapy is currently unknown.
A systematic review found that visual disturbances may occur in up to 14% of patients undergoing foam sclerotherapy,30 but a recent systematic review found the overall incidence to be 1.4%.29 The clinical presentation of these visual disturbances is similar to the aura of a migraine.30 Transient neurologic events may be observed after any kind of sclerotherapy, although they occur more commonly after foam sclerotherapy and after treatment of reticular and spider veins.1,2,4,5,30 All cases spontaneously regressed without after effects.
A patent foramen ovale or another right-to-left shunt, which is present in approximately 30% of the general population,31 may be one etiologic factor. The pulmonary filter is short-circuited, which allows foam bubbles or endothelin-1 to be released from the vessel injected with sclerosants32 and to pass into the arterial circulation.28,30,32-36
Gillet et al hypothesized that endothelin-1 reaches the cerebral cortex and induces a cortical spreading to trigger a migraine.30 Frullini et al believes that endothelin-1 provokes a vasospasm, which is the key to understanding migraines, chest tightness, retinal transient ischemia, and neurologic ischemia.32,33 There is no clear evidence for a relationship between bubbles and visual or neurological disturbances.4,5,35-37 Bubbles are known to cause vasospasm, which may trigger migraine-type symptoms and other general transient effects, such as chest tightness.28 Other factors, such as bubble load, treatment parameters, and patient factors, may be important.28
The presence of a right-to-left shunt, particularly a patent foramen ovale, is the most consistent risk factor in patients with ischemic neurologic events (transient ischemic attacks and stroke). There are only a few published reports of transient ischemic attacks following sclerotherapy.28 All reported cases were associated with a right-to-left shunt, had an immediate onset, and followed the use of air-based foam sclerosants. It has been suggested that rightto- left shunts might be a factor, allowing foam bubbles to pass into the arterial circulation.28,35-37
Stroke is a very rare, but significant, complication of sclerotherapy.38-42 Ma et al reported two cases of stroke following 4059 foam procedures in a 6-year period, yielding an incidence of 0.01%.41 Parsi reviewed 13 cases of stroke occurring after sclerotherapy that were published since 1994.28 Four cases followed liquid sclerotherapy and nine followed foam sclerotherapy; 3 patients had a partial recovery, while the others had a complete recovery. Cases with an immediate onset following foam sclerotherapy were due to a paradoxical gas embolism,28,38-41 while cases with a delayed onset of a few days were due to a paradoxical clot embolism.28,41,42 A right-to-left shunt, particularly a patent foramen ovale, was the most consistent risk factor in all reported cases.28
The mechanism of infarction in a paradoxical gas embolism may be due to direct physical occlusion of intracranial arteries by the gas bubbles or the bubbles induce vasospasm and activation of the coagulation system, resulting in secondary thrombotic occlusion.28,41 No gas or clot embolism could be demonstrated in 5 of the 13 patients with stroke reviewed.28,41 The release of cellderived sclerosant by-products may play a crucial role in the pathogenesis of neurological and other sclerotherapy complications.28,32,33 Finally, a coincidental event due to general causes of stroke should be considered.28
A venous gas embolism presents with dyspnea, continuous cough, hypotension, dizziness, and substernal chest pain. A “mill wheel” murmur may be produced by movement of bubbles in the right ventricle.28 A cerebral gas embolism can present with confusion, focal neurological symptoms, and stroke.28,38-41
The neurological events may be self-limiting, which would necessitate a nonspecific treatment. A complete ophthalmologic and neurologic examination is recommended. In addition, patients should be evaluated carefully for deep venous thrombosis, a pulmonary embolism, and a right-to-left shunt. Migraine-like symptoms are mostly self-limiting and resolve with time, but also by applying 100% oxygen with the patient in the Trendelenburg position. Headaches generally resolve with analgesia, and triptans may be considered in selected cases.43 Patients with a suspected venous gas embolism should be placed immediately in the left lateral decubitus position to reduce entry into the pulmonary arteries and a possible subsequent right ventricular outflow obstruction.28
If a neurological event occurs after foam sclerotherapy, then the possibility of a gas embolism increases28 and the following steps should be taken immediately40: (i) administer 100% oxygen immediately; (ii) place the patient in a head-down position for up to 10 minutes to clear bubbles from the cerebral circulation; however, holding this position for >10 minutes can worsen cerebral edema, meaning that the patient should be returned to a supine position28,39; (iii) a paradoxical gas embolism stroke should be confirmed by imaging of bubbles in the intracranial arterial circulation as soon as possible28; (iv) transfer the patient to a hyperbaric chamber40; (v) start anticoagulation with heparin with a partial thromboplastin time >2 times the baseline to prevent progression of the thrombus beyond the occlusion39,40,45; and (vi) thrombolytic therapy with tissue plasminogen activator may be beneficial in selected cases, according to the standard stroke guidelines.40,42,45
Increasing the inspired oxygen decreases the partial pressure of dissolved nitrogen, which allows for a more rapid diffusion of nitrogen from the cerebral arterial air embolism. Hyperbaric oxygen has been recommended in the immediate treatment of a gas embolism to enhance the diffusion of nitrogen into the blood, compression of existing bubbles, improving the oxygenation of ischemic tissues and lowering the intracranial pressure.40 Some authors consider hyperbaric oxygen as the first-line treatment of choice for an arterial gas embolism.38 Sixteen patients who underwent hyperbaric oxygen therapy for a cerebral air embolism resulting from invasive medical procedures obtained the best results when therapy was started within 6.5 hours.44 Some studies, however, have found that hyperbaric treatment does not influence the clinical outcomes; therefore, its routine use has not been universally advocated.28
For an immediate stroke after liquid-based sclerotherapy and for a delayed-onset stroke, the management should follow the standard stroke guidelines; selected patients may benefit from thrombolytic therapy.28,45 In patients with a patent foramen ovale and a paradoxical gas embolism, percutaneous closure of the patent foramen ovale is a second step in the management.38,40 Patients with a cryptogenic stroke can benefit from this treatment; however, closure procedures are not risk free.46
Venous thromboembolism
Severe deep venous thrombosis, proximal or extensive, is rare. The vast majority of reported deep venous thrombosis cases are localized to the lower legs. The overall frequency of deep venous thrombosis is <1%.29,47 The incidence is possibly higher as a significant number of procedural deep venous thromboses may be silent; most reports only include symptomatic cases. The incidence of symptomatic deep venous thrombosis is 0.02% to 0.6%1,29,47 and the incidence with duplex ultrasound follow-up is 1.07% to 3.2%.1,2,48-52 Most of the cases detected by duplex ultrasound during routine follow-up were asymptomatic.1,2,48-52 Medial gastrocnemius vein thrombosis was a complication more commonly associated with foam sclerotherapy of the small saphenous vein than with the great saphenous vein, likely due to the anatomy of the small saphenous vein.52 Pulmonary embolisms occur very rarely after sclerotherapy. In the study by Gillet et al,2 1 case of pulmonary embolism was reported in 1025 patients. In the French registry of 12 173 procedures, no cases of pulmonary embolism were reported.1 There is no data regarding the incidence of postoperative silent pulmonary embolism.
There are no evidence-based recommendations. The treatment depends on the presence of risk factors for venous thromboembolism and the extension and severity of deep venous thrombosis. A nonocclusive, postsclerotherapy, deep venous thrombosis located in the lower legs has a benign evolution and a rapid recanalization with ambulation, compression, NSAIDS, or short treatments with anticoagulation, usually with low-molecular-weight heparin.2 Coleridge Smith managed distal and not completely occlusive deep venous thrombosis without anticoagulation.53 Gillet et al showed that, in asymptomatic patients with nonocclusive distal thrombosis, a follow-up with duplex ultrasound and no anticoagulation is probably the best option, except for patients with risk factors for venous thromboembolism.52 Oral anticoagulation for 3 weeks to 3 months has also been successfully used.54 For extended deep venous thrombosis, Guex suggests looking for risk factors for venous thromboembolism.55
Superficial venous thrombosis
The definition of phlebitis after sclerotherapy in the literature is controversial. It is considered an adverse event if there is an extension beyond the treated area or an excessive inflammatory reaction.4,5 Although venous sclerosis (collagen deposition resulting in scar formation), venous thrombosis (intravascular fibrin clot formation), and venous thrombophlebitis (clot formation accompanied by an inflammatory infiltrate) are histologically separate entities, these conditions cannot always be clinically or sonographically differentiated. Hence, the incidence depends on individual understandings and the real frequency is unknown4,5; the frequencies vary between 0% and 45.8%, with a mean of 4.7%.1,3,5,29 Thrombophlebitis is a complication that should not be taken lightly. If untreated, the inflammation and clot may spread through perforating veins to the deep venous system. Patients with superficial venous thrombosis have a 5% to 40% chance of developing deep venous thrombosis.56
Deep venous thrombosis can be ruled out in patients with superficial phlebitis using an ultrasound evaluation.56 Most patients have minimal phlebitis and require no treatment or a simple drainage of an associated coagulum with a 22-gauge needle.6 In symptomatic patients, drainage of the thrombi after liquefaction, approximately 2 weeks after sclerotherapy, hastens resolution of the otherwise slow and painful resorption process.6 Adequate compression and frequent ambulation should be maintained until the pain and inflammation resolve. NSAIDS may be helpful in limiting both the inflammation and the pain.6,54 Low molecular-weight heparin is rarely required; however, it may be used in cases with extensive involvement, particularly into the proximal part of the saphenofemoral junction. In those patients with concurrent deep venous thrombosis, anticoagulation for 3 to 6 months resolved the deep and superficial venous thrombosis, while preventing a pulmonary embolism. In addition, the use of low-molecular-weight heparin in patients with superficial venous thrombosis may decrease perivascular inflammation.54
Postablation superficial thrombus extension
A postablation superficial thrombus extension (PASTE) from the great saphenous vein into the common femoral vein occurs due to endovenous ablation of the great saphenous vein with thermal or sclerotherapy treatments. The PASTE entity was discovered after follow-up examinations with duplex ultrasound in the immediate posttreatment period. The thrombi are apparent by ultrasound within 3 to 7 days of treatment, are nonocclusive, asymptomatic, and rarely identifiable after 14 days. They do not cause venous obstruction or symptomatic pulmonary embolisms. Anticoagulation treatment was used at the beginning, but experience has shown that these thrombi are usually benign, harmless, and asymptomatic; therefore, it seems that no therapy, only observation, is needed in these cases (Figure 4).57
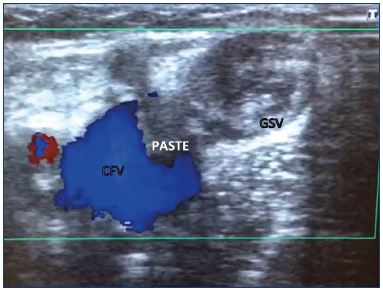
Figure 4. Postablation superficial thrombus extension into the
common femoral vein after foam sclerotherapy of the great
saphenous vein.
The thrombus was apparent on ultrasound after 3 days of
treatment. The thrombus was nonocclusive and asymptomatic.
The patient was treated with bemiparine 5000 UI for
7 days, and thereafter, bemiparine 3500 UI until the thrombus
disappeared 20 days later, with a weekly duplex follow-up.
Nerve injury
Sclerotherapy using liquid or foam sclerosants is associated with both sensory and motor nerve damage that is usually transient in nature. The incidence is very rare (0.02%) with paresthesia and dysesthesia as the main presenting complaints.58 Due to their close proximity to the veins, the saphenous and sural nerves may be inadvertently injected during sclerotherapy. Injection into a nerve is reportedly very painful and, if continued, may cause anesthesia and sometimes a permanent interruption of nerve function. Occasionally, a patient complains of an area of paresthesia that is probably caused by perivascular inflammation extending from the sclerosed vein to adjacent superficial sensory nerves.6 Nerves are readily visualized on most modern ultrasound systems and inadvertent damage can be mostly avoided. While this is usually self-limiting, it may take 3 to 6 months to resolve.6 Decreasing inflammation with NSAIDS, which speeds up the resolution in minor cases, and possible long-term therapy using neurotropic agents are the recommended treatments. Treatment may also include local infiltration of corticosteroids and local anesthetics.6
Temporary swelling: edema and lymphedema
The incidence of lower limb edema following sclerotherapy is rarely reported and probably underestimated, but the incidence is around 0.5%.59 This complication is possibly more frequent following the obliteration of the small saphenous vein due to the contiguity of this vein with the superficial lymphatic vessels. Localized lymph stasis may occur due to sclerotherapy-induced chemical phlebitis. Extensive sclerotherapy may result in transient lymph stasis in predisposed patients, such as those with latent congenital lymphatic system abnormalities.3
Edema may also be due to deep vein occlusion (thrombosis or sclerosis). Extensive sclerotherapy of the superficial incompetent veins followed by occlusion of small segments of lower limb deep veins, such as the posterior tibial or peroneal veins, may contribute. In some patients, the etiology is multifactorial and involves a combination of obesity, lack of exercise, concomitant drugs, such as calcium channel blockers, and a lack of compliance with the use of medical compression systems.3 This complication may be minimized by using careful techniques to avoid phlebitis and deep vein occlusion. Perivascular inflammation must be limited. Ankle edema occurs much less frequently if the sclerosing solution is limited to 1 mL per ankle. A topical application of a strong-potency corticosteroid cream, lotion, or gel has been found useful.6
Systemic causes should be identified and excluded. Lymphedema may be investigated by lymphoscintigraphy and treated with a combined decongestive treatment. These patients should be encouraged to resume regular exercise and lose weight. Adequate compression is important in reducing edema and phlebitis in general.3,6,60 Irrespective of the underlying cause, postsclerotherapy edema is mostly transient in nature.3
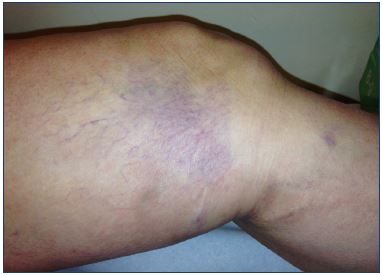
Figure 5. Matting after sclerotherapy of reticular veins in the
medial lateral thigh that resolved after sclerotherapy of
an unrecognized underlying reflux in a collateral of great
saphenous vein.
Minor complications
Telangiectatic matting is the proliferation of new small vessels (<0.2 mm) in the area of a sclerosed vein that typically appears 4 to 6 weeks after sclerotherapy.61 The most common locations are on the inner and outer thighs and near the knees and calves. Unfortunately, even in the most expert hands, telangiectatic matting occurs in a significant percentage of patients. Telangiectatic matting may affect one-third of patients undergoing sclerotherapy, and usually resolves spontaneously in 3 to 12 months.62 In many cases, inadequate or no treatment of the underlying reflux is the cause of telangiectatic matting (Figure 5).63,64 The precise cause of telangiectatic matting remains unknown, but its development is attributed to a reactive inflammatory or angiogenic mechanism, and it is more prevalent with high concentrations or volumes of sclerosant or high-infusion pressures that can result in inflammation o excessive vein obstruction (Figure 6).63,64 Patient risk factors include excessive body weight, female sex, hormone treatments with estrogens, a longer duration of spider veins, and a family history of telangiectasia.61,63,64
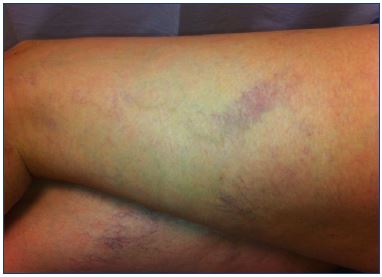
Figure 6. Matting after extensive foam sclerotherapy of
telangiectasia in the lateral thigh with underlying reflux in the
reticular veins.
The underlying reflux resolved with treatment of the reticular
veins at a lower volume and concentration of sclerosant and the
use of medical compression stockings, and finally resolved with
sclerotherapy of telangiectasia using glycerin.
reatment of telangiectatic matting should concentrate on the underlying reflux and residual patent veins using noninflammatory concentrations of sclerosants or phlebectomy.6 If there is no identifiable feeding vessel, instead of succumbing to the immediate urge for multiple treatments with stronger liquid sclerosants, often reassurance and passage of time is all that is required to resolve telangiectatic matting.63 The patient can be given a mild anti-inflammatory cream; photographs are taken at 6- to 8-week intervals until resolution occurs. If it does not resolve within a reasonable time, inject any remaining telangiectatic matting with glycerin or low concentration of detergent sclerosant; 31- to 33-gauge needles can facilitate cannulation of these extremely small vessels. Treatment with a 595-nm or 1064-nm laser can also be useful if the vessels are too small to cannulate.61
Residual pigmentation
Postsclerotherapy hyperpigmentation refers to the appearance and persistence of pigmentation along the course of a treated vein (Figure 7). Hyperpigmentation occurs in 10% to 30% of patients in the short term, and it is usually noticed within 3 to 4 weeks after sclerotherapy. Although spontaneous resolution occurs in 70% of cases at 6 months, pigmentation may persist longer than 1 year in up to 10% of patients.2,6,63 Hyperpigmentation is usually due to a combination of both melanin and hemosiderin pigment deposits secondary to either direct hemosiderin deposition, post-inflammatory processes, or a combination of the two. The red blood cells extravasate after rupture of treated vessels or perivenulitis. The red blood cell dies and the hemoglobin is released into the dermis and degrades into hemosiderin.5,6
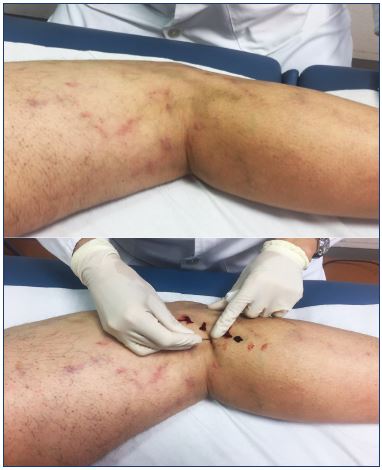
Figure 7. Hyperpigmentation after foam sclerotherapy of
varicose veins in the lateral thigh that resolved with intravascular
coagula drainage and medical compression stockings.
Time is the first-line intervention. Most patients will have spontaneous resolution of hyperpigmentation within 1 year.62,63 Untreated refluxing veins that connect to the affected area should be sought and treated.5 Extracting the intravascular coagulum expedites the resolution of hyperpigmentation (Figure 7).64,65 Medical compression systems have anti-inflammatory effects, decrease chronic venous hypertension, and help resolve the intravascular coagula.5 The evidence from two randomized clinical trials comparing the effects of compression vs no compression after sclerotherapy on the side effects (hyperpigmentation, bruising, migraine, and edema) is poor; there were no differences in the treatment of telangiectasia and reticular veins66 or saphenous veins.67 Nonrandomized studies have shown that compression decreases side effects from the sclerotherapy of telangiectasia and reticular veins.60,68,69
As this pigmentation is caused primarily by hemosiderin deposition and not melanin, bleaching agents that affect melanocytic function are usually ineffective. Treatments that may have some value include exfoliation with mild peeling agents and Q-switched laser therapy.62,63 The exfoliants trichloroacetic acid and mercaptoacetic acid are of particular interest since hemosiderin is soluble in acids.70 Izzo et al showed that the combination of 20% trichloroacetic acid, 0.05% retinoic acid, and 2% hydroxyquinoline successfully achieved a totally faded pigmentation in 76% of patients whose pigmentation persisted for 6 months to 5 years.70 A treatment using 10% to 20% mercaptoacetic acid is the most effective and safe because of its affinity to ionize iron and bind it to the hemosiderin, ensuring good efficacy even at low concentrations.70 Goldman has treated patients who have had pigmentation for >3 months with topical retinoic acid with good results and without any adverse sequelae.6 Chelation of the subcutaneous iron deposition with intradermal injections of deferoxamine mesylate appears to be somewhat effective, but these are painful and expensive.6 Weekly administration of 500 mg of deferoxamine mesylate reduced the time to depigmentation by 82%, although further studies are needed to determine the optimum dose.71 The topical iron chelator 2-furildioxime may also be useful to treat cutaneous hemosiderin pigmentation.72
Hyperpigmentation is similar to tattooing with hemosiderin; thus, lasers may offer a reasonably effective therapy. It is believed that laser treatment causes physical fragmentation of pigment granules that are later removed by phagocytosis. In the past, a copper vapor laser73 and a 510 nm flashlamp– excited pulsed-dye laser74 proved the most effective with 69% and 45% efficacy in patients with pigmentation lasting 12 or 6 months, respectively. The Q-switched ruby laser (694 nm) is also effective in removing recalcitrant pigmentation with a high rate of resolution (≈90%).75,76 The Q-switched 532/1064-nm Nd: YAG laser, with its longer wavelengths, can safely treat darker skin and penetrate into the deeper dermis with a 75% resolution in persistent hyperpigmentation lasting 18 months with 2.8 treatments.77 Most recently, Q-switched lasers that generate picosecond domain pulses have been introduced with an even greater ability to target and destroy cutaneous pigment.78 A Polish group has achieved a complete regression of hyperpigmentation in 90% of cases using an intense pulsed light that is equipped with radio waves.79
Intravascular coagulum
Sclerotherapy frequently results in the formation of a coagulum within the treated vessel. Intravascular coagulum/coagula/hematoma or microthrombi appear 1 to 6 weeks after sclerotherapy. Such coagulum, which is trapped between the two ends of a treated vein, tends to remain liquefied. In a systematic review of four randomized controlled trials on foam sclerotherapy, the frequency of retained coagulum ranged from 7.8% to 55.1%.29 The larger the vessel size, the more frequently intravascular coagulum occurs.
The retention of coagulum is usually associated with tenderness and may predispose patients to post-treatment hyperpigmentation. Evacuation of the intravascular coagula reduces tenderness and inflammation and it may help prevent discoloration.3,65,70 Microthrombi in veins ≤1 mm can be evacuated by puncture with a No.65 beaver blade.65 Larger veins can be punctured with a 16- or 18-gauge needle, and the intravascular coagulum manually expressed or aspirated. Intervention is recommended within 2 to 4 weeks after sclerotherapy, while the thrombus is gelatinous and not yet organized (Figure. 7).65 Continued use of compression is recommended, and evaluation for an underlying source of venous insufficiency is indicated for persistent intravascular coagulum.65
Transitory general effects
Transitory general effects are short-lasting disturbances and recovery occurs within minutes. Chest tightness and dry cough are reported the most (Figure 1); nausea and a metallic taste can also occur. The physiopathology is not clear. In chest tightness, it is suggested that a coronary vasospasm is provoked by air bubbles35 or endothelin-1 release33; however, it does not seem to be related to a myocardial infarction and no increase in troponin levels has been observed.80 The management is similar to that of transient neurologic events (Figures 1 and 2). Apply 100% oxygen, put the patient in the Trendelenburg position, and evaluate their neurological and cardiovascular state. If a venous gas embolism is suspected, apply the aforementioned maneuvers.
Stress-related symptoms
Vasovagal reflex is nonspecific and benign, but does increase the risk of falling. It is the most common cause of a simple loss of consciousness.11 The vasovagal reflex is a common adverse sequelae of any surgical or invasive procedure. It has been estimated to occur in 1% of patients during sclerotherapy6 and must be managed according to the protocol for the management of syncope (Figure 1).11 A characteristic of a vasovagal response is dysfunction of the autonomic nervous system, with parasympathetic activation, which results in an initial bradycardia and loss of sympathetic stimulation that results in initial hypotension. An environmental trigger, such as a needle stick, is a common cause.11
The patient should be placed in the Trendelenburg position and observed. If the reaction persists or intensifies, consider a subcutaneous injection of 1 mL atropine 0.4 mg/mL (Figure 1).6,11 This safe and effective treatment rapidly reverses the vasovagal reaction and prevents its progression.
Sclerotherapy can exacerbate certain underlying medical diseases. Patients with a history of asthma may start wheezing (Figure 2), or angina may develop in patients with cardiovascular disease. Polidocanol is a negative inotropic agent and slows cardiac contractility in a dose dependent manner.
Urticaria and periorbital edema may be related to histamine release from irritated perivascular mast cells. Rarely, an urticarial reaction has been noted when using graduated compression stockings. Urticaria is easily treated with an oral antihistamine, but may be a sign of a systemic allergy.
Transitory local side effects
Transitory local side effects are common to all sclerosants; they tend to be mild, transient, and somewhat expected. Such complications are usually self-limiting and transient in nature, and they can be treated with topical agents.3 The possible side effects include: (ii) injection site reactions (injection pain, pruritus, minor bruising, wheals, local swelling, indurations, and erythema) that are self-limited; (ii) skin irritation (itching and an irritant contact dermatitis may follow the use of compression stockings) and excessive skin xerosis that can be treated effectively with emollient creams or oils; (iii) tape compression blister that can be prevented by using a tubular support bandage, and the resolution occurs within 1 to 2 weeks without any adverse sequelae. To aid healing, prevent infection, and alleviate any pain, the use of an occlusive hydroactive dressing is helpful; (iv) tape compression folliculitis that can be treated by removing the occlusive dressing and applying a topical treatment with an antibacterial soap or a topical antibiotic gel, such as a 2% erythromycin or 1% clindamycin phosphate topical solution. The folliculitis usually resolves within a few days, and systemic antibiotics are rarely necessary6; (v) localized urticaria, often in the form of wheal associated with itching, is usually relieved within 30 minutes, and it can be diminished by applying topical steroids and by limiting the injection quantity per injection site.6
Conclusion
Bad results are usually the consequences of an inappropriate use or indication. The best treatment is prevention. If performed properly, sclerotherapy is an efficient treatment method with a low incidence of complications, but some can be vital emergencies. Our improved knowledge of complications allows us to implement the treatment carefully. This article addressed the treatments that should be used for sclerotherapy complications. Early interventions may minimize possible sequelae. Physicians who perform sclerotherapy should have an emergency plan in the event of neurological deficits, intra-arterial injections, severe systemic adverse reactions, or anaphylaxis, including transport to emergencies services for further evaluation and treatment of vital emergencies. Access to hyperbaric oxygen therapy may also be considered in emergency planning. All office settings using sclerotherapy should be equipped with the ability to administer oxygen therapy.
Serious adverse events are very rare, meaning that there are no evidence-based recommendations to manage them, and most management options are based on anecdotal experience or data extrapolated from others pathologies. As other very rare entities, they would benefit from a multicenter register coordinated by an international phlebological association, to obtain enough numbers to provide management recommendations based on evidence or consensus. Minor complications, such as telangiectatic matting and hyperpigmentation, require time, a side-by-side follow-up by the practitioner, and a careful examination and treatment of residual inadvertent vein reflux that may cause these minor, but worrisome side effects. Compared with liquid sclerotherapy, foamed sclerosing agents do not cause many new or different complications, but it does appear to change their relative incidences (Table I),1 for example, neurological complications are more prevalent with foam vs liquid sclerotherapy.
REFERENCES
1. Guex JJ, Allaert FA, Gillet JL, Chleir F. Immediate and midterm complications of sclerotherapy: report of a prospective multicentre registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31(2):123-128.
2. Gillet JL, Guedes JM, Guex JJ, et al. Side effects and complications of foam sclerotherapy of the great and small saphenous veins: a controlled multicentre prospective study including 1,025 patients. Phlebology. 2009;24(3):131- 138.
3. Cavezzi A, Parsi K. Complications of foam sclerotherapy. Phlebology. 2012;27(suppl 1):46-51.
4. Gillet GL. Complications and side effects: European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(suppl 1):34-38.
5. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338-354.
6. Goldman MP. Complications and adverse sequelae of sclerotherapy. In: Goldman MP, Weiss R, eds. Treatment of Varicose and Telangiectatic Leg Veins. 6th ed. Elsevier Inc; 2017;200-261.
7. Bunke-Paquette N. Complications of liquid sclerotherapy. Bergan JJ, Bunke- Paquette N, eds. The Vein Book. Second Ed. New York, USA: Oxford University Press; 2014:115-126.
8. Brzoza Z, Kasperska-Zajac A, Rogala E, Rogala B. Anaphylactoid reaction after the use of sodium tetradecyl sulphate: a case report. Angiology. 2007;58(5):644- 646.
9. Parsi K. Emergencies in phlebology: anaphylaxis, intra-arterial injection, neurological and cardiac repercussions. Phlebolymphology. 2014;21(1):65-66.
10. Scurr JRH, Fisher RK, Wallace SB, Gilling-Smith GL. Anaphylaxis following foam sclerotherapy: a life threatening complication of a non invasive treatment for varicose veins. EJVES Extra. 2007;13(6):87-89.
11. Mowatt-Larssen E. Syncope for phlebologists. Phlebology. 2014;29(8):517-521.
12. Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis. J Allergy Clin Immunol. 2006;117(2):391-397.
13. Lieberman PL. Recognition and first-line treatment of anaphylaxis. Am J Med. 2014;127(suppl 1):S6-S11.
14. Simons FE, Ardusso LR, Bilò MB, et al. World Allergy Organization anaphylaxis guidelines: summary. J Allergy Clin Immunol. 2011;127(3):587-593.
15. Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477-480.
16. Bergan JJ, Weiss RA, Goldman MP. Extensive tissue necrosis following highconcentration sclerotherapy for varicose veins. Dermatol Surg. 2000;26(6):535- 542.
17. Schuller-Petrović S, Pavlović MD, Neuhold N, Brunner F, Wölkart G. Subcutaneous injection of liquid and foamed polidocanol: extravasation is not responsible for skin necrosis during reticular and spider vein sclerotherapy. J Eur Acad Dermatol Venereol. 2011;25(8):983-986.
18. Miyake RK, King JT, Kikuchi R, Duarte FH, Davidson JR, Oba C. Role of injection pressure, flow and sclerosant viscosity in causing cutaneous ulceration during sclerotherapy. Phlebology. 2012;27(8):383-389.
19. Tran D, Parsi K. Veno-arteriolar reflex vasospasm of small saphenous artery complicating sclerotherapy of the small saphenous vein. Aust N Z J Phlebology. 2007;10(1):29-32.
20. Zimmet SE. The prevention of cutaneous necrosis following extravasation of hypertonic saline and sodium tetradecyl sulphate. J Dermatol Surg Oncol. 1993;19(7):641-646.
21. Brown AS, Hoelzer DJ, Piercy SA. Skin necrosis from extravasation of intravenous fluids in children. Plast Reconstr Surg. 1979;64(2):145-150.
22. Hafner F, Froehlich H, Gary T, Brodmann M. Intra-arterial injection, a rare but serious complication of sclerotherapy. Phlebology. 2013;28(2):64-73.
23. Fegan WG, Pegum JM. Accidental intraarterial injection during sclerotherapy of varicose veins. Br J Surg. 1974;61(2):124- 126.
24. Parsi K, Hannaford P. Intra-arterial injection of sclerosants: report of three cases treated with systemic steroids. Phlebology. 2016;31(4):241-250.
25. Nitecki SS, Bass A. Inadvertent arterial injury secondary to treatment of venous insufficiency. Vascular. 2007;15(1):49-52.
26. Biegeleisen K, Neilsen RD, O’Shaughnessy A. Inadvertent intraarterial injection complicating ordinary and ultrasound-guided sclerotherapy. J Dermatol Surg Oncol. 1993;19(10):953- 958.
27. Thom SR. Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg. 2011;127(suppl 1):131S-141S.
28. Parsi K. Paradoxical embolism, stroke and sclerotherapy. Phlebolology. 2011;27(4):147-167.
29. Jia X, Mowatt G, Burr JM, Cassar K, Cook J, Fraser C. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94(8):925-936.
30. Gillet JL, Donnet A, Lausecker M, Guedes JM, Guex JJ, Lehmann P. Pathophysiology of visual disturbances occurring after foam sclerotherapy. Phlebology. 2010;25(5):261-266.
31. Lynch JJ, Schuchard GH, Gross CM, Wann LS. Prevalence of right-to-left atrial shunting in a healthy population: detection by Valsalva maneuver contrast echocardiography. Am J Cardiol. 1984;53(10):1478-1480.
32. Frullini A, Barsotti MC, Santoni T, Duranti E, Burchielli S, Di Stefano R. Significant endothelin release in patients treated with foam sclerotherapy. Dermatol Surg. 2012;38(5):741-747.
33. Frullini A, Felice F, Burchielli S, Di Stefano R. High production of endothelin after foam sclerotherapy: a new pathogenetic hypothesis for neurological and visual disturbances after sclerotherapy. Phlebology. 2011;26(5):203-208.
34. Gillet JL. Neurological complications of foam sclerotherapy: fears and reality. Phlebology. 2011;26(7):277-279.
35. Parsi K. Venous gas embolism during foam sclerotherapy of saphenous veins despite recommended treatment modifications. Phlebology. 2011;26(4):140-147.
36. Raymond-Martimbeau P. Transient adverse events positively associated with patent foramen ovale after ultrasoundguided foam sclerotherapy. Phlebology. 2009;24(3):114-119.
37. Morrison N, Neuhardt DL. Foam sclerotherapy: cardiac and cerebral monitoring. Phlebology. 2009;24(6):252- 259.
38. Asbjornsen CB, Rogers CD, Russell BL. Middle cerebral air embolism after foam sclerotherapy. Phlebology. 2012;27(8):430-433.
39. Leslie-Mazwi TM, Avery LL, Sims JR. Intraarterial air thromnogenesis after cerebral air embolism complicating lower extremity sclerotherapy. Neurocrit Care. 2009;11(2):247-250.
40. Bush RG, Derrick M, Manjoney D. Major neurological events following foam Sclerotherapy. Phlebology. 2008;23(4):189-192.
41. Ma RW, Pilotelle A, Paraskevas P, Parsi K. Three cases of stroke following peripheral venous interventions. Phlebology. 2011;26(7):280-284.
42. Hanisch F, Müller T, Krivokuca M, Winterholler M. Stroke following variceal sclerotherapy. Eur J Med Res. 2004;9(5):282-284.
43. International Headache Society. ICHD guidelines. http://www.ihs-headache. org/ichd-guidelines/national-society guidelines. Accessed July 21, 2017.
44. Murphy BP, Harford FJ, Cramer FS. Cerebral air embolism resulting from invasive medical procedures. Treatment with hyperbaric oxygen. Ann Surg. 1985;201(2):242-245.
45. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2013;44(3):870-947.
46. Carroll JD, Saver JL, Thaler DE, et al; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368(12):1092-1100.
47. Dermody M, Schul MW, O’Donnell TF. Thromboembolic complications of endovenous thermal ablation and foam sclerotherapy in the treatment of great saphenous vein insufficiency. Phlebology. 2015;30(5)357-364.
48. Myers KA, Jolley D, Clough A, Kirwan J. Outcome of ultrasound-guided sclerotherapy for varicose veins: mediumterm results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33(1):116-121.
49. Kulkarni SR, Messenger DE, Slim FJ, et al. The incidence and characterization of deep vein thrombosis following ultrasound-guided foam sclerotherapy in 1000 legs with superficial venous reflux. J Vasc Surg Venous Lymphat Disord. 2013;1(3):231-238.
50. Guex JJ, Schliephake DE, Otto J, Mako S, Allaert FA. The French polidocanol study on long-term side effects: a survey covering 3,357 patient years. Dermatol Surg. 2010;36(suppl 2):993-1003.
51. Gillet JL. Foam sclerotherapy of saphenous veins–results and side effects. Rev Vasc Med. 2013;1:24-29.
52. Gillet JL, Lausecker M, Sica M, Guedes JM, Allaert FA. Is the treatment of the small saphenous veins with foam sclerotherapy at risk of deep vein thrombosis? Phlebology. 2014;29(9):600- 607.
53. Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32(5):577-583.
54. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
55. Guex JJ. Complications and side-effects of foam sclerotherapy. Phlebology. 2009;24(6):270-274.
56. Galanaud JP, Gentry C, Sevestre MA, et al; OPTIMEV SFMV Investigators. Predictive factors for concurrent deepvein thrombosis and symptomatic venous thrombotic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost. 2011;105(1):31-39.
57. Wright D, Morrison N, Recek C, Passariello F. Post ablation superficial thrombus extension (PASTE) into the common femoral vein as a consequence of endovenous ablation of the great saphenous vein. Acta Phlebologica. 2010;11(3):59-64.
58. Bergan J, Pascarella L, Mekenas L. Venous disorders: treatment with sclerosant foam. J Cardiovasc Surg (Torino). 2006;47(1):9-18.
59. Cavezzi A, Frullini A, Ricci S, Tessari L. Treatment of varicose veins by foam sclerotherapy: two clinical series. Phlebology. 2002;17(1):13-18.
60. Weiss RA, Sadick NS, Goldman MP, Weiss MA. Post-sclerotherapy compression: controlled comparative study of duration of compression and its effects on clinical outcome. Dermatol Surg. 1999;25(2):105-108.
61. Goldman MP, Sadick NS, Weiss RA. Cutaneous necrosis, telangiectatic matting, and hyperpigmentation following sclerotherapy. Etiology, prevention, and treatment. Dermatol Surg. 1995;21(1):19-29.
62. Munavalli GS, Weiss RA. Complications of sclerotherapy. Semin Cutan Med Surg. 2007;26(1):22-28.
63. Palm MD, Guiha IC, Goldman MP. Foam sclerotherapy for reticular veins and nontruncal varicose veins of the legs: a retrospective review of outcomes and adverse effects. Dermatol Surg. 2010;36(suppl 2):1026-1033.
64. Kern P, Ramelet AA, Wutschert R, Bounameaux H, Hayoz D. Singleblind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30(3):367-372.
65. Scultetus AH, Villavicencio JL, Kao TC, et al. Microthrombectomy reduces postsclerotherapy pigmentation: multicentre randomized trial. J Vasc Surg. 2003;38(5):896-903.
66. Kern P, Ramelet AA, Wütschert R, Hayoz D. Compression after sclerotherapy for telangiectasia and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45(6):1212-1216.
67. Hamel-Desnos CM, Guias BJ, Desnos PR, Mesgard A. Foam sclerotherapy of the saphenous veins: randomised controlled trial with or without compression. Eur J Vasc Endovasc Surg. 2010;39(4):500- 507.
68. Goldman MP, Beaudoing D, Marley W, Lopez L, Butie A. Compression in the treatment of leg telangiectasia: a preliminary report. J Dermatol Surg Oncol. 1990;16(4):322-325.
69. Nootheti PK, Cadag KM, Magpantay A, Goldman MP. Efficacy of graduated compression stockings for an additional 3 weeks after sclerotherapy treatment of reticular and telangiectatic leg veins. Dermatology Surgery. 2009;35(1):53-57.
70. Izzo M, Mariani F, Binaghi F, Amitrano M. Postsclerotherapy hyperpigmentation: incidence, clinical features, and therapy. In: Negus D, Jantet G, Coleridge-Smith PD, eds. Phlebology ’95. Springer-Verlag; 1995:550-551.
71. Lopez L, Dilley RB, Henriquez JA. Cutaneous hyperpigmentation following venous sclerotherapy treated with deferoxamine mesylate. Dermatologic Surgery. 2001;27(9):795-798.
72. Bissett DL, Oelrich M, Hannon DP. Evaluation of a topical iron chelator in animals and in human beings: short-term photoprotection by 2-furildioxime. J Am Acad Dermatol. 1994;31(4):572-578.
73. Thibault P, Wlodarczyk J. Postsclerotherapy hyperpigmentation. The role of serum iron levels and the effectiveness of treatment with the copper vapor laser. J Dermatol Surg Oncol. 1992;18(1):47-52.
74. Goldman MP. Postsclerotherapy hyperpigmentation: treatment with a flashlamp-excited pulsed dye laser. J Dermatol Surg Oncol. 1992;18(5):417- 422.
75. Tafazzoli A, Rostan EF, Goldman MP. Q-switched ruby laser treatment for postsclerotherapy hyperpigmentation. Dermatol Surg. 2000;26(7):653-656.
76. Weiss RA, Weiss MA. Treatment of severe post-sclerotherapy hyperpigmentation with Q-switched ruby laser. Lasers Med Surg. 2004;34(suppl 16):55.
77. Beasley KL, Weiss RA, Weiss MA, Munavalli G. Treatment of postsclerotherapy hyperpigmentation with a novel Q-switched combination 532/1,064 nm Nd:YAG laser. Lasers Med Surg. 2004;34(suppl 16):15.
78. Freedman JR, Kaufman J, Metelitsa AI, Green JB. Picosecond lasers: the next generation of short-pulsed lasers. Semin Cutan Med Surg. 2014;33(4):164-168.
79. Mlosek RK, Woźniak W, Malinowska S, Migda B, Serafin-Król M, Miłek T. The removal of post-sclerotherapy pigmentation following sclerotherapy alone or in combination with crossectomy. Eur J Vasc Endovasc Surg. 2012;43(1):100-105.
80. Hamel-Desnos CM, Desnos PR, Ferre B, Le Querrec A. In vivo biological effects of foam sclerotherapy. Eur J Vasc Endovasc Surg. 2011;42(2):238-245.


