Iliac vein compression: undervalued or overestimated?
MD, PhD
Vascular Surgeon & Head of Surgical
Department, Sint-Andriesziekenhuis,
Tielt, Belgium;
Visiting professor, Department
of Cardiovascular Science,
University of Leuven, Belgium
Abstract
Iliac vein compression (IVC) is a common anatomic disorder affecting more than 20% of the adult population, especially young females. Most of those patients are asymptomatic. Some of them will develop symptoms in their left leg, such as swelling, pain, and heaviness. But progression to venous claudication, skin changes, and even venous ulceration is possible. Intolerance to exercise is an undervalued symptom. The most feared complication is the development of a deep venous thrombosis (DVT) and pulmonary embolism (PE). In addition to the symptomatology, the diagnosis can be confirmed using duplex ultrasound, computed tomographic (CT) scan, or magnetic resonance (MR) venography. However, for the exact measurement of the degree of stenosis and indication for stenting, intravascular ultrasound (IVUS) is the preferred tool for assessing iliac vein compression. Those patients are, especially in combination with other risk factors, at higher risk for developing DVT and PE. However, it is difficult to identify the patients who will benefit from a treatment (stenting) in terms of symptomatology and quality of life (QOL) or even in effective DVT prevention. Venous stenting is the treatment of choice and seems to be safe and effective. Post stenting antiplatelet medication is most appropriate for patients with nonthrombotic IVC, whereas postthrombotic patients should preferably be treated with oral anticoagulants. Meticulous selection of patients for treatment is necessary to avoid over-treatment.
Introduction
Iliac vein compression (IVC) was until recently underestimated as a cause of venous hypertension. However, the awareness of the importance of iliac vein obstruction as a cause of lower extremity symptoms is increasing. Many patients nowadays are screened for IVC with an increasing frequency of iliac stenting as a result. The purpose of this paper is to review the pathophysiology, diagnosis, and treatment results of patients with diagnosed IVC.
History
As early as 1851, Rudolf Virchow (Figure 1) first proposed that the increased incidence of deep venous thrombosis (DVT) within the left lower extremity was a result of the right common iliac artery compressing the left common iliac vein (CIV), noting a fivefold left-sided predominance for DVT.1 In 1957, the researchers May and Thurner described the anatomical variation in the left CIV,2 now commonly named the “May-40 Thurner syndrome” (MTS) (Figure 2). They reported that 22% of 430 cadavers exhibited this anatomical variant with localized intraluminal fibrous bands, referred to as spurs, of the CIV. They postulated that the spurs were acquired from the chronic compression on the left CIV via the overriding right common iliac artery. The pulsatile compression from the right common iliac artery was thought to cause increased irritation of the endothelium, which subsequently caused cell proliferation and the development of spurs within the left CIV.2
It was Cockett and Thomas who first studied living patients presenting with an iliofemoral DVT from IVC syndrome. They used venography to describe the nature of the compression. These patients exhibited pigmentation, induration, and ulceration, as well as swelling and widespread pain of the entire left leg.3
Clinical presentation
IVC includes MTS and other conditions in which iliac veins are compressed, resulting in an impaired venous return from the affected leg to the heart. Most patients with this anatomy have no symptoms, but some can develop blood clots in the vein related to this compression. Compression can also be provoked by other anatomic structures such as lymphadenopathy, oncological disorders, arterial aneurysms, retroperitoneal fibrosis, and distended bladder and kidney.4,5 The obstruction of the iliac veins or vena cava may thus occur due to a variety of mechanisms, including postphlebetic venous thickening. In a similar manner, pregnancy may be associated with transient compression of the iliocaval venous segment.6
This compression is not exclusive to the left CIV; significant stenosis of the CIV can also occur on the right side, although clearly less frequently.
Significant compression of the left CIV is thought to be present in up to one-third of the general population. MTS is primarily seen in people aged 18-50 years old. Women are five times more likely to have MTS.7
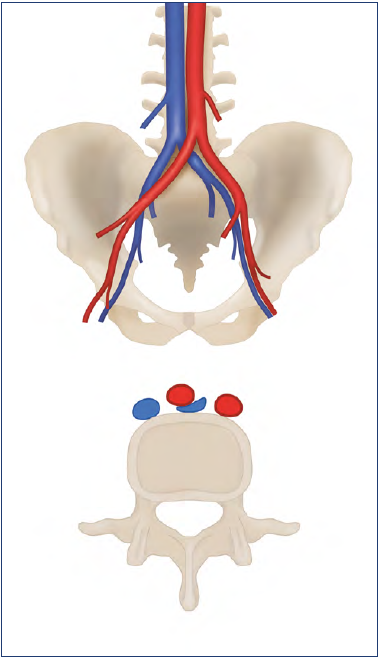
Figure 2. May-Thurner syndrome: compression of the left common iliac vein between the overriding right common iliac artery and the vertebral body.
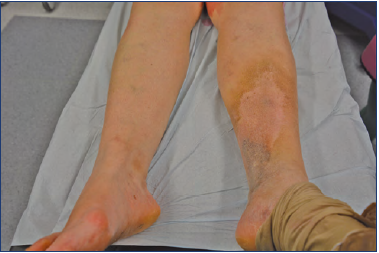
Nonthrombotic IVC is often referred to as “nonthrombotic iliac vein lesions” (NIVL). Clinical phases of IVC include a prolonged asymptomatic period followed by the gradual development of an intraluminal fibrous band (ie, spur), which can subsequently progress to an acute unilateral iliofemoral DVT that can be accompanied by a pulmonary embolism (PE).8,9 Furthermore, those patients can develop a postthrombotic syndrome (PTS) (Figure 3), which will aggravate their symptomatology and decrease their quality of life (QOL).
Venous compression usually becomes clinically significant when increased venous pressure leads to the formation of venous collaterals, signs, and symptoms of chronic venous disease (CVD).10 However, the often-silent nature of the lesion precludes accurate assessment of its true prevalence.
Symptomatic IVC most commonly occurs in women in their 20s and 30s, when they develop left leg swelling, heaviness, and pain, also known as venous congestion. Symptoms worsen through the course of the day or after prolonged standing or sitting, causing significant leg swelling or tenderness. Some patients may also experience venous claudication. The occurrence of IVC in adolescents has also been documented. While IVC does occur among men, it occurs more frequently in women, although the reason for this has not yet been completely explained.11 Research suggests that a female’s pelvis exhibits more of an accentuation of the lumbar lordosis that pushes the lower lumbar vertebrae anteriorly, thereby compressing the left CIV against the right common iliac artery.9,12 In regard to treatment, due to the mechanical pulsatile nature of the obstruction from the right common iliac artery, patients respond poorly to conservative anticoagulation therapy alone.13,14
Clinical manifestation may be acute, with venous spur development and lower limb DVT, or chronic, including unilateral limb swelling, venous claudication, varicose veins, and ulceration.15 Patients with limb ulceration and chronic venous insufficiency have a high incidence of iliac vein obstruction. In patients with advanced venous disease (Clinical, Etiological, Anatomical, Pathophysiological classification [CEAP] 5-6), reports of a former deep venous thrombosis have been noted in at least 50% of cases, and 23% had obstruction of >80%.16
Patients with IVC or even iliac vein occlusion (IVO) have exercise intolerance. Due to the impaired venous return, cardiac preload may decrease. During sustained exercise, the majority of blood flow is directed to the lower limbs, and the splanchnic blood flow is reduced to 5% to 10% of total cardiac output.17 The increased preload during exercise drives an increase in stroke volume, but obstruction of venous return may significantly limit the cardiovascular response to exercise.18 The increase in venous return during upright exercise comes predominantly from the lower limbs. Obstruction to the iliocaval veins, including compression and intraluminal pathologies can restrict outflow from lower limbs and impair venous capacitance, limiting the rate of flow back to the heart, in addition to causing venous hypertension and damage to valves in the affected limb. Those patients may present with exertional dyspnea. Kaufman reported an improvement in the cardiovascular fitness test parameters after stent recanalization of chronic iliocaval occlusion after thrombosis (PTS).19
IVC is more common in women and is at least twice as frequent in women as in men.11 Men tend to have more pain and swelling in the legs, whereas women tend to be younger and are more likely to have a pulmonary embolus on presentation.
Diagnosis
Clinical examination is insufficient to diagnose IVC. Given that most symptoms are rather atypical and could just as easily be due to CVD, the underlying cause may well be overlooked. Patients with signs and symptoms of CVD not responding to conventional management, may well suffer from IVC. Clinical presentations suggestive of IVC also include left lower extremity DVT in the absence of differential causes of iliofemoral thrombosis.
The presence of suprapubical collaterals is a very typical sign indicative of IVO. Especially in combination with other signs such as edema, skin changes, and even venous ulcerations.
IVC/IVO is a frequent and underappreciated contributor to venous hypertension in patients with venous leg ulcers. Patients with a history of DVT or duplex scan–diagnosed deep venous reflux have a higher incidence of outflow obstruction and should also be routinely examined to allow correction in this high-risk group of patients.
Duplex ultrasound has been identified as a sensitive method to identify venous stenosis; a peak velocity ratio of >2.5 across the narrowing was confirmed as the best criterium.15 However, ultrasound is highly examinator dependent. In many cases, it is technically difficult to capture an image of venous compression and stenosis via ultrasound due to the deep location of the iliac veins. Obesity and the presence of abdominal gas may complicate this examination.
Additionally, it may be overlooked in the investigation of the left iliofemoral DVT and thus underdiagnosed as an etiological factor.
Duplex ultrasound examination of the femoral vein may provide evidence of outflow obstruction with loss of respiratory variation in the femoral tracing or poor augmentation of the signal with distal limb compression.16 In patients with nonthrombotic IVC, ultrasound should be performed in supine and standing positions to identify whether the obstruction is positional or not.10
However, a negative duplex scan for outflow obstruction can be unreliable, as shown by Marston et al. In their study, 23% of cases of high-grade IVC on CT or magnetic resonance (MR) had a normal venous duplex.16
Contrast venography using transvenous pressure measurements has in the past been the gold-standard test used to diagnose IVC; the formation of collateral veins and a pressure gradient that is ≥2 mm across the iliofemoral stenosis at rest are landmarks of IVC (Figure 4). Specific findings on angiography are suggested to be associated with significant IVC. These include contrast translucency and lumen deformity, as well as axial, trans pelvic, or ascending lumbar collaterals.20
Standard venography can underestimate the magnitude of stenosis by 15% to 30% compared with intravascular ultrasound (IVUS).21-23
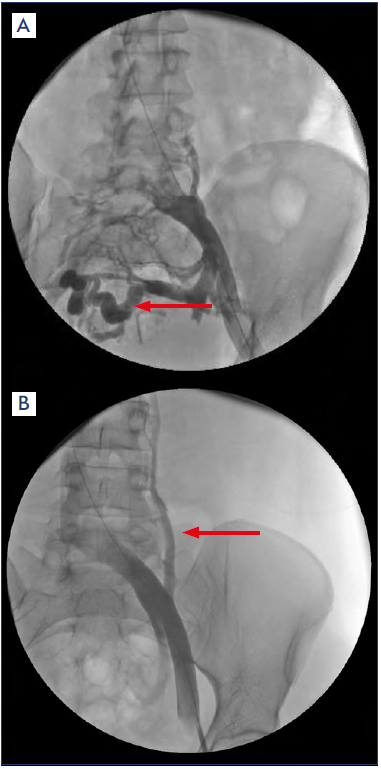
Figure 4. Venography of the left iliac vein, showing contrast translucency, obstruction, and pelvic collaterals (a, arrow) and ascending lumbar collaterals (b, arrow).
The existence of pelvic venous collaterals is a further clue for the presence of pathological IVC, suggestive of left CIV compression reaching hemodynamic significance.15
Several limitations exist in detecting IVC on CT-angiography. First, the ipsilateral reference CIV may have significant prestenotic dilatation, and this may exaggerate the compression severity. Also, IVC may involve the entire CIV in certain cases, and the compression severity can therefore be underestimated. Finally, angulation of the CIV can lead to inaccurate measurements.22
Using the contralateral CIV as a reference vessel assumes that the CIVs bilaterally are equal in size, which may be erroneous.
Because of its known limitations and lack of prospective validation studies, CT is not recommended initially to identify or exclude obstructive IVC.
Neither CT nor MR venography provide useful hemodynamic information to indicate the relevance of anatomic findings for venous function in the lower limb. In addition, CT and MR venography may not identify intraluminal webs or other chronic abnormalities that may contribute to poor venous function (Figure 5,6).16
IVUS is currently considered the gold standard in diagnosing IVC, whereas, the venography consistently underestimates the severity of the compression.
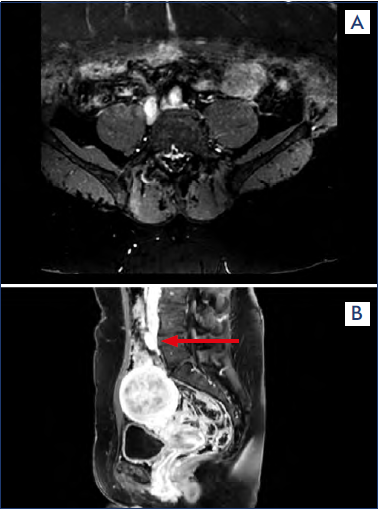
Figure 5. (a) Magnetic resonance (MR) angiography showing a bilateral iliac vein compression. (b) MR angiography showing compression of the left common iliac vein (arrow).
Figure 6. Magnetic resonance (MR) venography reconstruction showing compression of the left common iliac vein.
A minimal lumen area (MLA) of <100 mm2 seems to predict the presence of a significant compression in a symptomatic population as seen on IVUS (>50%).22
It is important to remember that all those anatomical investigations are usually performed in the supine position and may not reflect hemodynamic characteristics exacerbated by the standing position.15 Although venography and IVUS have been reported to be the gold standard for diagnosis, these are invasive, expensive studies that cannot be used to screen large numbers of patients who are potentially at risk for IVC.16,24 However, IVUS is of great value in measuring the degree of stenosis, determining the diameter of the stent required, as well as stenosis location and thus the length of the stent required (Figure 7). Therefore, it should always be used, if available, as preoperative support.
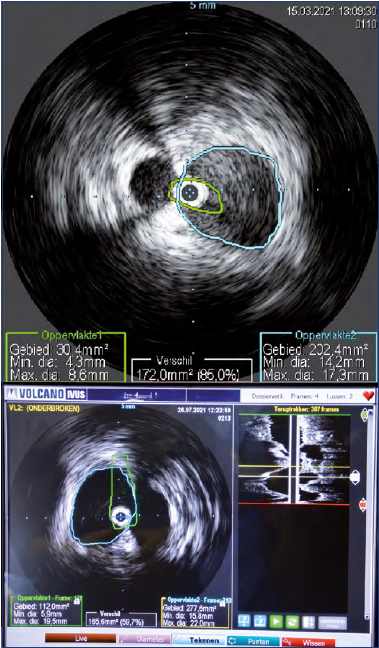
Figure 7. Intravascular ultrasound (IVUS) with measurement of the minimal lumen area and calculation of the degree of stenosis.
In young, healthy adults, a remarkably high percentage of anomalous angiographic examinations of the deep pelvic veins can be found.25 These results therefore expose a potential imbalance between clinical signs and imaging findings in suspected IVC patients, leading to a risk of overtreatment.
With the uncertainty in diagnostic examinations, clinical evaluation of suspected IVC patients becomes increasingly important.
Complications
DVT is the most serious complication of IVC. Clinically, IVC accounts for only 2% to 5% of all DVTs,8,26 but it has been speculated that the percentage of DVTs due to IVC may be much higher than clinically recognized. This suspicion is supported by the disproportionately greater incidence of leftsided DVT.27 Of all patients with isolated DVT of the leg, 55.9% (95% CI, 54.0-57.8) had a left-sided DVT. This predominance of left-sided versus right-sided DVT is not modulated by obesity, age, sex, surgery, injury, or oral contraceptive use.27
Clinical studies report that significant IVC occurs in the majority of patients with left DVT12,26 and a presence of fibrous spurs in 22% to 33% of cadavers.2,3
Oguzkurt et al reported an increased average percentage of iliac vein obstruction (mean 74%, range 45%-100%) for patients presenting with left leg DVT compared with asymptomatic controls (mean 28%, range 0%-68%).28 Notably, about 65% of their study population (DVT patients) had risk factors for DVT other than compression. However, Carr et al29 concluded from their studies, in which diameters were measured in patients with DVT and in patients without DVT as a control, that IVC was a strong independent risk factor for development of DVT. Furthermore, using multivariate regression and adjusting for risk factors, the odds of left-sided DVT increased by a factor of 2.08 for each 1-mm decrease in left CIV diameter.
Owing to the potential associated severe consequences, such as PE and death, it may be postulated that preventive treatment in severe cases of asymptomatic IVC is defendable.25
What about IVC without blood clot?
Patients with IVC are usually asymptomatic. The vein narrowing in these cases is often discovered on CT or MRI performed for other reasons. However, patients can develop symptoms.
Due to the high prevalence of IVC, even in asymptomatic patients, the IVC can be considered as a normal anatomic pattern. Kibbe et al30 found that nearly one-fourth of asymptomatic patients had a greater than 50% compression, and two-thirds of patients had a greater than 25% compression. They proposed that some degree of compression of the iliac vein may be a normal anatomic variant that in itself does not place the patient at increased risk for development of DVT.30-32 IVC can be found in many patients and many are asymptomatic. Van Vuuren et al have shown that only 63% of patients treated for IVC showed a clinical response to treatment, and 14% showed a minor symptom deterioration.25
The diagnosis lacks a precise definition for the degree of compression that may designate a patient at high risk for developing a DVT.
Carr et al29 reported an average stenosis of 68% among patients with a DVT due to IVC, whereas the age-matched controls had an average stenosis of 52%. The odds of DVT were increased by a factor of 2.18 for each 10% increase in left iliac vein stenosis. This strongly suggests that greater iliofemoral venous stenosis is associated with increased DVT risk. But the wide range of compression between patient groups suggests that the degree of stenosis alone is only one factor determining the development of DVT.9 Patients with IVC are more susceptible to developing a DVT, but this is often in combination with other risk factors such as prolonged immobilization, thrombophilia, trauma, etc.
Marston et al showed that nearly one-fourth of limbs that are affected with ulceration have IVC of 80% or more (CT venography).16 Patients with a history of DVT were positive for high-grade IVC in nearly 40% of the cases.
Studies with IVUS measurements show that IVC with 50% diameter reduction are likely to be hemodynamically significant in nonthrombotic patients. Furthermore, the VIDIO study (Venogram vs IVUS for Diagnosing Iliac vein Obstruction) showed that >54% area reduction was the optimal threshold, whereas a higher threshold of stenosis of >61% diameter stenosis was optimal to indicate significant clinical improvement at 6 months after iliac stenting in nonthrombotic patients.23,33
Treatment
In patients with NIVL, the goal of treatment is to improve venous flow through the compressed iliac vein in order to relieve these symptoms and theoretically prevent future blood clot formation.
In patients with iliac vein thrombosis, treatments are aimed at dissolving blood clots and relieving the compression that caused them to form.7 The primary goal of treatment is to restore the normal blood flow in the compressed CIV. This is very often done by thrombolysis or mechanochemical thrombus removal. However, the discussion of these treatment options is beyond the scope of this paper.
Treatment for IVC is generally reserved for patients with symptoms, often those with a new DVT. Conventional surgical procedures for the treatment of iliofemoral venous obstruction have largely been supplanted by an endovascular approach (Figure 8) relying on the deployment of dedicated venous stents (Figure 9).
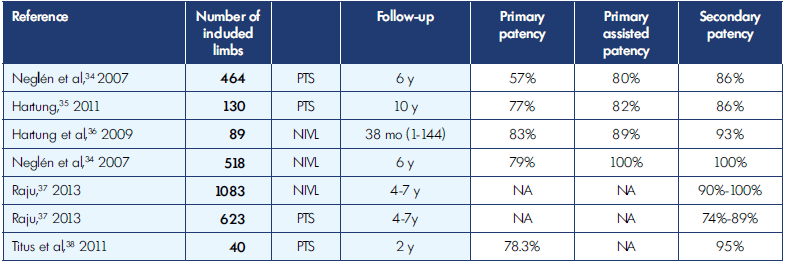
NIVL, nonthrombotic iliac vein lesions; PTS, postthrombotic syndrome.
Table I. Patency rates for venous stenting, nonthrombotic iliac vein lesions (NIVL), and postthrombotic syndrome (PTS).
Nowadays, many patients diagnosed with NIVL, are treated with endovascular techniques. Despite ongoing controversy about the exact definition of its pathological process,15 endovascular treatment has been shown to be safe and effective for treatment of acute venous thrombosis or chronic compression (Table I).34-38 Furthermore, Raju has shown that iliac vein stenting is a safe and effective treatment option.37
The primary stent patency rates in the 6-month follow-up were 98.3% in NIVL versus 90.9% in PTS, with a statistical difference showing reduced stent patency in PTS. However, there is no statistical difference when the treatment is conducted in nonthrombotic patients as compared with patients with acute lower-limb DVT. These findings favor the treatment of acute DVT instead of PTS.39
Following treatment, anticoagulation, compression stockings, and stent patency evaluation are recommended.
Anticoagulation
There is currently no consensus on the optimal medical therapy for patients after iliocaval stenting. Anticoagulant and antiplatelet agents have been used in varying dosages and durations.
In many centers, anticoagulants, such as warfarin or direct oral anticoagulants (DOACs) are prescribed in the posttreatment period, and this is done for a period of 6 months. The rationale is that the reendothelialization of the stented vein segments takes about 6 months. A thrombotic reocclusion of a stent is a nightmare for many interventional vascular surgeons. Furthermore, it has been suggested that antiplatelet therapy may play a lesser role in the slow-flow, low-shear venous system than in the arterial system.40
In a systematic review of venous stent placement trials after DVT, 86% of patients received anticoagulation and 33% received antiplatelet therapy.41 Stent patency of 98% was observed in patients with NIVL and the occlusion/restenosis rates were similar comparing the patient cohorts with PTS and those with acute DVT (14.2% versus 14.8%). Most reocclusions occurred within the first 3 months, when most patients were still on anticoagulation.40 In the studies of Raju and Neglén, only patients with thrombophilia received warfarin.42
Eijgenraam et al41 conducted a systematic review of 14 venous stent studies after DVT (including PTS). They couldn’t find any difference in outcome (reocclusion) comparing antiplatelet therapy (aspirin and/or clopidogrel) and oral anticoagulation. However, most studies were single-arm cohort studies with mostly a small sample size.
The American College of Chest Physicians guidelines recommended at least 3 months of anticoagulation in the setting of iliac vein stenting and thrombolysis for DVT.43
Antiplatelet agents are likely most appropriate for patients with primary NIVL, whereas anticoagulants probably have a greater role in (post)thrombotic disease.44
Results
IVC is commonly treated with iliac vein stenting. High patency rates have been reported.
Raju found, in a review of approximately 1500 iliac and caval stent series, a cumulative patency between 90% to 100% for nonthrombotic and from 74% to 89% in postthrombotic disease at 3 to 5 years of follow-up.37
In a systematic review and meta-analysis including 1050 patients and 1169 affected lower limbs, da Silva Rodriguez reported a primary stent patency rate in 6-month follow-up of 98.3% in nonthrombotic IVC versus 90.9% in PTS. Titus et al stress the significance of underlying disease for long-term results, as they reported a significantly better 2-year outcome in patients with MTS than in those with thrombophelia as underlying cause of disease when comparing stenting with external compression.38
Attaran et al40 found that stents that occluded had a tendency toward longer length, extension into the common femoral vein, and more hypercoagulable syndromes. Inversely, larger diameter iliocaval vein stents are associated with improved patency rates.
The degree of iliac vein stenosis does not appear to affect stent patency.45 However, patients with a ≥90% initial IVC stenosis more frequently experience recurrence of symptoms on long-term follow-up compared with mild and moderate stenosis cohorts. A hypothesis is that this symptom recurrence may be due to reduction in caliber of the stent over time, reflecting greater extrinsic compression from a greater degree of stenosis. The severity of the IVC stenosis grade is not a predictor of initial clinical symptoms.45
Venous stenting has been shown to be safe and effective in the treatment of IVC. It has a positive effect on pain, reducing swelling, healing ulcers, and improving QOL.
Conclusion
IVC is a common anatomical finding. In many cases, patients are asymptomatic. However, some of them develop symptoms, such as leg swelling, heaviness, and pain. These can evolve to skin changes and even venous ulcerations. Such patients are, especially when their condition is in combination with other risk factors, at risk of developing a DVT and PE. However, it is difficult to identify those patients who will benefit from a treatment (stenting) in terms of symptomatology and QOL or even in effective DVT prevention. Venous stenting is the treatment of choice and seems to be safe and effective.

REFERENCES
1. Virchov R. Uber die Erweiterung kleinere Befasse. Arch Path Anat. 1851;3:1.
2. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
3. Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg. 1965;52:816-821.
4. Hung JB, Hsu CW, Tsai SH. Prostatism and May-Thurner syndrome. Am J Emerg Med. 2013;31:445-el2.
5. Elsharawy MA, Moghazy KM, Alsaif HS, Al Asiri MM. Unusual case of left iliac vein compression secondary to May-Thurner syndrome and crossed fused renal ectopic. Saudi Med J. 2008;29:603-605.
6. Humphries A, Mirjalili S, Tarr G, Thompson JMD, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med. 2018;3923-3930. doi:10. 1080/14767058.2018.1478958
7. Liddell RP, Evans NS. May-Thurner syndrome. Vasc Med. 2018;23:493-496.
8. Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27:984-995.
9. Harbin M, Lutsey P. May-Thurner syndrome: history of understanding and need for defining population prevalence. J Thromb Haemost. 2020;18:534-542.
10. Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60:118-125.
11. Kaltenmeier C, Erben Y, Indes J, et al. Systematic review of May-Thurner syndrome with emphasis on gender differences. J Vasc Surg Venous Lymphat Disord. 2018;6:399-407.
12. Fraser DG, Moody AR, Martel PS. Reevaluation of iliac compression syndrome using magnetic resonance imaging in patients with acute deep venous thromboses. J Vasc Surg. 2004;40:604- 611.
13. Ibrahim W, Al Safran, Hasan H, Zeid WA. Endovascular management of May-Thurner syndrome. Ann Vasc Dis. 2012;5:217-221.
14. Meissner MH, Gloviczki P, Comerota, et al. Early thrombus removal strategies for acute deep venous thrombosis. Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55:1449-1462.
15. Hameed M, Onida S, Davies A. What is pathological May-Thurner syndrome? Phlebology. 2017;32:440-442.
16. Marston W, Fish D, Unger J, Keagy B. Incidence of and risk factors for iliocaval venous obstruction in patients with active or healed venous leg ulcers. J Vasc Surg. 2011;53:1303-1308.
17. Harper D, Chandler B. Splanchic circulation. Br J Anesthesia Educ. 2016;16:66-71.
18. Morris R, Sobotka P, Balmforth P, et al. Iliocaval venous obstruction, cardiac preload reserve and exercise limitation. J Cardiovasc Translat Res. 2020;13:531- 530.
19. Kaufman JA, Dimov IP, Kuehl K, Kanable A, Balin J. Exercise intolerance in patients with chronic iliocaval venous occlusion: initial experience with noninvasive exercise testing before and after intervention. J Vasc Interv Radiol. 2021;32:305-308.
20. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136- 143.
21. Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694-700.
22. Shammas N, Shammas B, Miller S, et al. Predicting iliac vein compression with computed tomography and venography: correlation with intravascular ultrasound. J Invasive Cardiol. 2018;30:452-455.
23. Gagne PJ, Tahara RW, Fastabend CP. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5:678-668.
24. Forauer AR, Gemeten JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13:523-527.
25. Van Vuuren TMAJ, Kurstjens RLM, Wittens CHA, van Laanen JHH, de Graaf R. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg. 2018;56:874-879.
26. Brazeau NF, Harvey HB, Pinto EG, Deipolyi A, Hesketh RL. May-Thurner syndrome: diagnosis and management. Vasa. 2013;42:96-105.
27. Thijs W, Rabe KF, Roosdaal FR, Middeldorp S. Predominance of left-sided deep vein thrombosis and body weight. J Thromb Haemost. 2010;8:2083-2084.
28. Oguzkurt L, Ozkan U, Ulusan S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patient’s with left iliofemoral deel vein thrombosis. J Vasc Interv Radiol. 2008;19:366-370.
29. Carr S, Chan K, Rosenberg J. Correlation of the diameter of the left common iliac vein with risk of lower-extremity deep venous thrombosis. J Vasc Interv Radiol. 2012;23:1467-1472.
30. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937- 943.
31. Chen F, Den J, Yuan QW, et al. Compression of the left common iliac vein is independently associated with left-sided deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1:364-369.
32. Wolpert LM, Rahmani O, Stein B, Gallagher JJ, Drezner AD. Magnetic resonance venography in the diagnosis and management of May-Thurner syndrome. Vasc Endovascular Surg. 2002;36:51-57.
33. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48-56.
34. Neglén P, Hollis KC, Olivier J, Raju S, Stenting of the venous outflow in chronic venous disease: long-term related outcome, clinical and hemodynamic result. J Vasc Surg. 2007;46:979-990.
35. Hartung O. Results of stenting for postthrombotic venous obstructive lesions. Perspect Vasc Surg. 2011;23:255-260.
36. Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling iliocaval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38:118- 124.
37. Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57:1163-1169.
38. Titus JM, Moise MA, Bena J, Lyden SP, Clair DG. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706-712.
39. Da Silva Rodriguez L, Bertanha M, El Dib R, Moura R. Association between deep vein thrombosis and stent patency in symptomatic iliac vein compression syndrome: systematic review and metaanalysis. J Vasc Surg Venous Lymphat Disord. 2021;9:275-284.
40. Attaran R, Ozdemir D, Lin I, Mena-Hurtado C, Lansky A. Evaluation of anticoagulant and anti platelet therapy after iliocaval stenting: factors associated with stent occlusion. J Vasc Surg. 2019;7:527-534.
41. Eijgenraam P, Ten Cate H, Ten Cate-Hoek AJ. Venous stenting after deep venous thrombosis and anti thrombotic therapy: a systematic review. Rev Vasc Med. 2014;2:88-97.
42. Raju S, Neglen P. Percutaneous recanalisation of total occlusions of the iliac vein. J Vasc Surg. 2009;50:360-368.
43. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: anti thrombotic therapy and prevention of thrombosis,, 9th ed: American College of Chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S-496S.
44. Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology. 2013;28(suppl 1):91-98.
45. Jayaraj A, Buck W, Knight A, et al. Impact of degree of stenosis in May-Thurner syndrome on iliac vein stenting. J Vasc Surg Venous Lymphatic Dis. 2019;7:195- 202.