Advances in the surgical treatment of postthrombotic syndrome
and Fedor LURIE**
Vascular Surgery, year 2007
** Department of Surgery, John A. Burns
School of Medicine, University of Hawaii
ABSTRACT
Postthrombotic syndrome (PTS) is the late complication of lower extremitiy deep venous thrombosis (DVT). Its incidence is approximately 3/1000 per year in the adult population. A combination of reflux and obstruction is often seen in limbs with more advanced clinical disease than obstruction alone. A thorough workup of the patient with disabling PTS is necessary to identify patients amenable to open surgical or endovascular intervention. Duplex scanning is the gold standard for diagnosis of chronic venous disease. The superficial system should be addressed first, followed by or in conjunction with the perforator and deep systems. Chronically obstructed veins are amenable to endovascular interventions, sometimes in combination with disobliteration of the veins (“endophlebectomy”), bypasses or valvular repair. A novel autologous valve reconstruction (the Italian neo-valve) that involves the construction of a valve in postthrombotic veins by using an intimal flap has been performed with satisfactory results. A percutaneously-implantable, nonimmunogenic venous valve that remains patent and competent is an attractive alternative to deep venous reconstructions. Results from animal studies with a bioprosthetic valve (the Portland valve) are encouraging.
INTRODUCTION
Postthrombotic syndrome (PTS) is the late complication of lower extremitiy deep venous thrombosis (DVT). Its manifestations are lower extremity edema, pain, eczema, hyperpigmentation, lipodermatosclerosis, and stasis ulcers. The estimated incidence of DVT is approximately 3/1000 per year in the adult population, and the cumulative incidence of PTS after DVT is 22.8% after 2 years, 28% after 5 years, 29% after 8 years,1 and 40% (10% with, 30% without ulcer) after 13 years.2 There are no validated measures to predict which patients will develop PTS. Therefore, a patient with acute DVT can expect an approximate global risk of 30% to 40% of developing significant postthrombotic sequelae, most likely within the first 2 years.
Venous thrombi originate from the valve sinus. As the thrombus resolves, damage to the venous valves and walls occurs, leading to outflow obstruction and valvular insufficiency. Re-canalization of old organized thrombus results in formation of synechiae and septae. In addition to increased resistance, these structures restrict the movements of venous wall, significantly limiting the ability of the affected vein to adjust to outflow changes. These two processes are cumulatively known as “venous obstruction.” Valves eventually become incompetent in both the deep and perforating veins, aggravating ambulatory venous hypertension. The combination of reflux and obstruction is seen more frequently in limbs with more advanced clinical disease than obstruction alone.3
The CEAP (Clinical, Etiologic, Anatomic, and Pathophysiologic) classification discriminates patients with secondary chronic venous disease (or postthrombotic syndrome) who may have obstruction, reflux, or a combination of reflux and obstruction in deep veins (Es, Ad, Pr; Es, Ad, Eo; Es, Ad, E r+o), from patients with primary chronic venous disease (Ep) who always have superficial reflux, and do not have deep vein obstruction. The third group includes patients with a combination of primary and secondary diseases, who have postthrombotic changes in deep veins, and reflux in superficial veins. Duplex scanning is the gold standard for diagnosis of chronic venous disease and constitutes level 2 investigation of the CEAP. It is sufficient for identification and anatomical classification of reflux. Venous obstruction, however, is not as clearly identifiable and quantifiable as reflux, and to-date there is no set of duplex criteria for venous obstruction. Therefore, thickened walls and valves as well as luminal narrowing with poor flow and reduced augmentation after manual compression are often considered as signs of venous obstruction on duplex scans. Plethysmography is another noninvasive tool commonly used for the diagnosis of chronic venous insufficiency, as it may provide an overall assessment of the physiological function of the lower extremity venous system, but is not able to determine the anatomical level of the disease precisely. Ascending and descending venography are invasive methods, performed when vascular or endovascular options are being considered, in order to evaluate the site and extent of both reflux and obstruction. These invasive techniques should be combined with and considered as complementary to duplex studies. Magnetic resonance and computed tomography are evolving techniques that provide a 3D view of the lower extremity venous systems.
In general, conservative management aimed at decreasing ambulatory venous pressure is attempted first. Graduated compression stockings, Unna Boots, leg elevation and local wound care are effective, if ulcers are present. When these noninvasive therapies are unsuccessful (failed ulcer healing or recurrence), due either to poor patient compliance or significant reflux and/or venous obstruction, then surgical therapy should be considered.
SURGERY FOR PTS
Planning for surgical treatment of patients with postthrombotic syndrome requires a thorough and thoughtful diagnostic workup. All sites of reflux and obstruction should be identified and evaluated. Ideally, a “hemodynamic map” should be created for each patient outlining major and minor outflow tracks and reflux sites. This may require a sequence of noninvasive and invasive techniques including dynamic ascending and descending venography. In general, the more accessible superficial system should be treated first, followed by, or at the same time as the perforator system. However, in the presence of significant proximal deep vein stenosis or occlusion associated with severe obstructive symptoms such as venous claudication, deep venous recanalization by means of angioplasty and stenting may be considered early in the management algorithm (Figure 1).
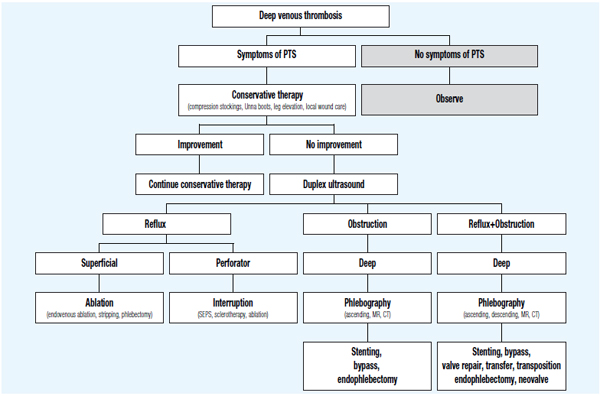
Figure 1. Management algorithm of postthrombotic syndrome (PTS).
If the deep system is not significantly obstructed, removal of a refluxing great saphenous vein (GSV) is indicated in symptomatic patients. The procedure is generally well tolerated and is associated with improvement in reflux parameters without significant worsening of objective measures of obstruction.4,5 Thromboprophylaxis with subcutaneous low-molecular-weight heparin should be instituted in this group of patients even during superficial vein surgery. More recent endovenous techniques like endovenous laser therapy (EVLT) and radiofrequency ablation (RFA) have been successfully employed by some authors, even in postthrombotic limbs,5,6 while others have considered this as a contraindication.7,8 Therefore, given the paucity of data and the serious concern of recurrent DVT, use of endovenous techniques should be carefully considered in this group of patients. Leg ulcers are often due to localized venous hypertension originating in an incompetent perforator, which can be treated by minimally invasive surgery (subfascial endoscopic perforator vein surgery – SEPS) or percutaneous procedures (sclerotherapy, avulsion, RFA). Interruption of incompetent perforators with SEPS in limbs with PTS is followed by good early outcomes (72-86% ulcer healing rate),9,10 but long-term follow-up data have shown that over 50% of healed ulcers recur within 5 years in this group of patients.9,11 We have used duplex-guided sclerotherapy to treat incompetent perforating veins with good short-term results, also in patients with PTS.12 RFA13 has also been recently described as a means of ablating incompetent perforating veins, but its role in PTS has yet to be defined. Moreover, while the advantages of SEPS over conservative treatment have been demonstrated by at least one prospective randomized trial that included 60 limbs with PTS,10 such comparisons are not available for percutaneous techniques of perforator ablation.
The mainstay of treatment for iliocaval obstruction nowadays is percutaneous angioplasty and stenting. If endovascular recanalization of unilateral iliac vein obstruction has failed, use can be made of a crossover saphenous vein transposition (Palma-Dale procedure), which utilizes the GSV of the contralateral limb to be anastomosed distal to the iliac obstruction. When this type of reconstruction is not feasible or not indicated (ie, GSV not available, bilateral iliac vein or caval obstruction not amenable to stenting), an iliocaval or femorocaval expanded polytetrafluoroethylene bypass graft with the adjunct of a distal arteriovenous fistula is a viable option. Primarily incompetent valves without significant structural changes are sometimes found in limbs affected by PTS. Competence can be restored by internal or external valvuloplasty, both of which were introduced by R. Kistner14,15 (Figure 2). However, it should be pointed out that the durability of such repairs and symptom-free interval are shorter in limbs with PTS than in those with primary valvular incompetence.16 In the absence of a reconstructable valve, a very select group of patients can be treated by transposition of an incompetent femoral vein to a competent saphenous or profunda vein,17 or by autotransplantation of upper extremity valved vein segments (axillary, brachial or basilic) to postthrombotic veins of the lower extremity.18
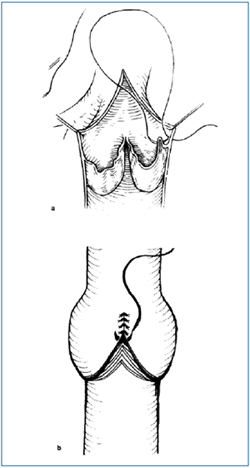
Figure 2. Kistner valvuloplasties.
a) Internal valvuloplasty (transvalvular approach) and
b) External valvuloplasty.
“Reprinted from Cardiovascular Surgery, Vol. 3, No. 2, RL.
Kistner, B. Eklof and EM. Masuda, Deep venous valve
reconstruction, pp 129-140, Copyright (1995), with permission
from Paula Mucci, BC Decker Inc.”
Reflux in postthrombotic veins is a challenging problem. Because the valves are frequently destroyed, deep venous reconstruction requires valve substitution rather than repair. Surgical options include transplantation of a venous segment containing an intact valve,19 or transposition of an adjacent segment containing a competent valve.20 The long-term success rate of these procedures has been consistently reported as close to 50%.21
RECENT ADVANCES
Endophlebectomy
In 2003 we described how surgical disobliteration, or “endophlebectomy”, of chronically obstructed venous segments can be performed to increase the flow through previously obstructed veins during various kinds of procedures, including deep venous reconstructions and iliocaval stenting.22,24 The main indications were: to allow valve repair, increase inflow for iliac vein stenting, increase outflow for vein valve transposition or transfer, and increase calf outflow. With this technique, postthrombotic veins and their major branches are surgically exposed and longitudinal venotomy is carried out at a variable length. The synechiae and masses attached to the intimal layer are carefully excised (Figure 3).
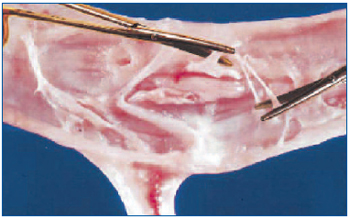
Figure 3. Endophlebectomy. The postthrombotic vein is open longitudinally and the synechiae attached to the intimal layer are carefully removed with scissors.
“Reprinted from J Vasc Surg, Vol. 39, No. 5, Puggioni A,
Kistner RL, Eklof B, Lurie F, Surgical disobliteration of
postthrombotic deep veins – endophlebectomy –is feasible,
pp 1048-1052, Copyright (2004), with permission from
Elsevier Science Ltd.”
After removal of the synechiae, an increase in vein diameter is observed as a result of the release of constricting bands, and this contributes to improved vessel compliance. The venotomy is then repaired primarily with a longitudinal running suture. In our series of 13 patients, surgical disobstruction of 23 deep venous segments was performed in association with 14 deep venous reconstructions. In 10 patients (77%) the treated segments remained primarily patent at mean follow-up of 11 months, while overall secondary patency rate was 93%. Synechiae were removed at the base of their tenuous attachments. In order to minimize the risk of thrombosis, we tried to preserve as much endothelium as possible. Recently, a case-series of 8 patients who underwent endophlebectomy in conjunction with iliocaval vein stenting has been reported from the Mayo Clinic.23
The authors used venoplasty with a bovine pericardial patch to increase vein diameter at the venotomy site. At a mean follow-up of 10 months, 3 occlusions and 2 restenoses were identified, all successfully treated with endovascular interventions.
The Italian neovalve
A novel autologous valve reconstruction that involves the construction of a neovalve in postthrombotic veins by using vein wall dissection and an intimal flap has been performed on 16 limbs and described by an Italian group of vascular surgeons.25 The technique consists of surgical exposure of the vein, followed by longitudinal or T-shaped venotomy. The thickened intima is carefully dissected and a bi- or monocuspid valve obtained by creating an intimal flap with an ophthalmic blade or microscissors (Figure 4). The flaps have to be accurately sized in order to prevent valve prolapse, and therefore incompetence. At the end of reconstruction, valve function is assessed by using the strip test.
At a median follow-up of 22 months, clinical improvement was observed in 89% of cases, overall patency was 83.3%. All patent valves remained competent at follow-up.
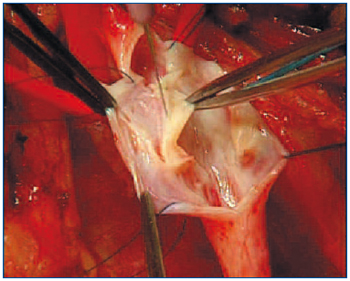
Figure 4. The Italian neovalve. The thickened intima is carefully dissected and a bi- or monocuspid valve obtained by creating an intimal flap with an ophthalmic blade or microscissors.
“Reprinted with verbal consent from the authors
(Maleti and Lugli) – Unpublished.”
Cryopreserved venous valves
Short-term results of cryopreserved vein valves in humans have not been favorable. Neglen and Raju26 described a high morbidity of 48% after the procedure was performed in 25 patients due to seroma formation, with wound infection and poor patency/competency rates at 2 years (41% and 27%, respectively). Clinical results paralleled poor technical results as pain and swelling did not ameliorate significantly. At this point in time, it seems that improved cryopreservation techniques, immunologic modifications, or better matching are required before cryovalves can be reconsidered as an alternative to deep venous reconstruction.
Experimental artificial venous valves
Percutaneously implanted artificial venous valves were frist attempted in animal models, with various degrees of success. The small-intestinal submucosa square-stent bicuspid venous valve—the Portland valve—has given very promising results.27 Long-term results of the valve implanted in the sheep’s internal jugular resulted in 88% success as the valves maintained good function after 6 months.28 Malfunction of the remaining valves (12%) was due to valve tilting; 4% had thrombi in the tilted valve. A second-generation valve consisting of a square stent submucosa attached to a second square stent (DS BVV) or Z-stent was then developed (Figure 5). No tilting was seen at 6 weeks and angiographic competency was over 90%.
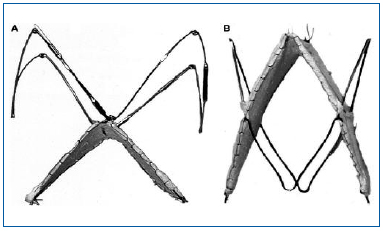
Figure 5. Second-generation bioprosthetic venous valves. A stainless steel Z-stent with barbs is attached to the apex of the square stent valve. B, A nitinol double-stent bioprosthetic venous valve with 4 barbs.
“Reprinted from J Vasc Surg, Vol. 40, No. 6, Pavcnik D,
Kaufman J, Uchida BT, et al, Second-generation percutaneous
bioprosthetic valve: a short-term study in sheep,
pp 1223-1227, Copyright (2004), with permission from
The Society for Vascular Surgery.”
CONCLUSION
A thorough workup of patients with disabling PTS is necessary to identify which patients are amenable to open surgical or endovascular intervention. The superficial system should be addressed first, followed by or in conjunction with the perforator and deep systems.
In those who have mainly deep valvular incompetence, valvuloplasty can be expected to yield good long-term results in a high percentage of patients. Chronically obstructed veins are amenable to endovascular interventions, sometimes in combination with open disobliteration of the veins, bypasses or valvular repair.A percutaneously implantable, nonimmunogenic venous valve that remains patent and competent is an attractive alternative to deep venous reconstructions. The results of current studies are encouraging and warrant an experimental trial in humans.

REFERENCES
2. Eichlisberger R, Widmer MT, Frauchiger B, et al. The incidence of post-thrombotic syndrome. Wien Med Wochenschr. 1994;144:192-195.
3. Neglén P, Thrasher TL, Raju S. Venous outflow obstruction: an underestimated contributor to chronic venous disease. J Vasc Surg. 2003;38:879-885.
4. Raju S, Easterwood L, Fountain T, et al. Saphenectomy in the presence of chronic venous obstruction. Surgery. 1998;123:637-644.
5. Neglen P, Hollis KC, Raju S. Combined saphenous ablation and iliac stent placement for complex severe chronic venous disease. J Vasc Surg. 2006;44:828-833.
6. Puggioni A, Kalra M, Carmo M, et al. Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. J Vasc Surg. 2005;42:488-493.
7. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003;38:207-214.
8. Huang Y, Jiang M, Li W, et al. Endovenous laser treatment combined with a surgical strategy for treatment of venous insufficiency in lower extremity: a report of 208 cases. J Vasc Surg. 2005;42:494-501.
9. Kalra M, Gloviczki P. Surgical treatment of venous ulcers: role of subfascial endoscopic perforator vein ligation. Surg Clin North Am. 2003;83:671-705.
10. Van Gent WB, Hop WC, Van Praag MC, et al. Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg. 2006;44:563-571.
11. Puggioni A, Kalra M, Noel A, et al. Ulcer healing and recurrence after subfascial endoscopic perforator surgery (SEPS). Paper presented at the 17th American Venous Forum Meeting; March 9-13, 2005; San Diego-CA.
12. Masuda EM, Kessler DM, Lurie F, Puggioni A, et al. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43:551- 556.
13. Whiteley, MS, Holstock JM, Price BA, et al. Radiofrequency ablation of refluxing great saphenous systems, Giacomini veins and incompetent perforating veins using VNUS Closure and TRLOP Technique. J Endovasc Ther. 2003;10:1-46.
14. Kistner RL. Valve reconstruction for primary valve insufficiency. In: Bergan JJ, Kistner RL, eds. Atlas of Venous Surgery Philadelphia: WB Saunders, 1992:125-130.
15. Kistner RL. Surgical technique of external venous valve repair. The Straub Foundation Proceedings. 1990;55:15-16.
16. Raju S, Fredericks RK, Neglen PN, Bass JD. Durability of venous valve reconstruction techniques for “primary” and postthrombotic reflux. J Vasc Surg. 1996;23:357-366.
17. Kistner RL, Sparkuhl. Surgery in acute and chronic venous disease. Surgery. 1979;85:31-43.
18. Raju S, Neglen P, Doolittle J, et al. Axillary vein transfer in trabeculated postthrombotic veins. J Vasc Surg. 1999;29:1050- 1062.
19. Taheri SA, Pendergast DR, Lazar E, et al. Vein valve transplantation. Am J Surg. 1985;150:210-212.
20. Ferris EB, Kistner RL. Femoral vein reconstruction in the management of chronic venous insufficiency. Arch Surg. 1982;117:1571-1579.
21. Lurie F, Kistner RL, Eklof B. Surgical management of deep venous reflux. Seminars in Vascular Surgery. 2002;15:50- 56.
22. Kistner RL, Lurie F, Puggioni A, Eklof B. Seven cases illustrating the feasibility of surgical disobliteration of deep veins (“Endophlebectomy”) in postthrombotic disease. Paper presented at the 15th American Venous Forum Meeting; February 20-3, 2003; Cancun-Mexico.
23. Oderich GS, Noel AA, Bjarnasson H, et al. Open endophlebectomy and endovenous stenting for chronic thrombosis of the inferior vena cava and the iliofemoral veins. Presented at the 18th annual American Venous Forum meeting, Feb 22-26, 2006 Miami, FL.
24. Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of postthrombotic deep veins –endophlebectomy – is feasible. J Vasc Surg. 2004;39:1048-1052. 25. Maleti O, Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794-799.
26. Neglen P, Raju S. Venous reflux repair with cryopreserved vein valves. J Vasc Surg. 2003;37:552-557.
27. Pavcnik D, Uchida BT, Timmermans HA, et al. Percutaneous bioprosthetic venous valve: a long-term study in sheep. J Vasc Surg. 2002;35:598-602.
28. Pavcnik D, Kaufman J, Uchida BT, et al. Second-generation percutaneous bioprosthetic valve: a short-term study in sheep. J Vasc Surg. 2004;40:1223-1227.
