Apoptosis and cell cycle regulation in the vein wall – new elements related to varicose vein occurrence
Katowice, Poland
SUMMARY
According to the majority of pathological studies in varicose vein specimens, various structural changes have been documented, including an irregular accumulation of the extracellular matrix within the vein wall and the disruption of the smooth muscle cell (SMC) bundles. Valve injury seems not to be the only factor leading to varicose vein development, and for vein dilation additional factors related to vein wall homeostasis disturbances are needed. Extracellular matrix accumulation within the vein wall related to SMC phenotype transdifferentiation and secretory activity can result in weakening of the vein wall. Among the various stimuli responsible for this process, endothelial cell hypoxia, hemodynamic disturbances, and cytokine (especially growth factors), influences are discussed. In the study, we confirm an increase in TGF-ß1 mRNA expression level and its protein product presence as well as the presence of SMC hypertrophy within the medial vein layer.
Due to the possibility of pathological cell elimination, p53 – related apoptosis is an important mechanism influencing vein wall homeostasis maintenance. In the study we documented that its activation is characteristic of the relatively early stages of varicose vein diseases. In the later course of the disease, the presence of the downregulation of SMC apoptosis within varicose vein media leads to an increase in structural changes related to local extracellular matrix accumulation.
In the proximal saphenous vein segments, despite SMC reduction and the presence of structural changes, there was no increase in programmed cell death activity. The reduction in the SMC population corresponding to cyclin dependent kinase inhibitor (p21) expression suggests a role of cell cycle disturbances that may lead to vein wall architecture destruction and further dilation.
INTRODUCTION
Although the mechanism of development of chronic venous insufficiency is becoming more and more understood, the sequence of events is still under discussion.1,2 Due to developments in angiology and molecular biology, new factors are constantly being added.3,4 The prolonged hydrostatic load in the incompetent vein, followed by activation of endothelial cells, leukocytes, macrophages, and mast cells leads to the enhanced production of cytokines, growth factors, and to adhesion molecule expression.3,4
According to the “valve failure” theory, descending vein incompetence is related to proximal valve failure.2 Despite the data documenting the possibility of congenital proximal saphenous vein valve abnormalities, in view of some clinical observations, this theory does not seem to be satisfactory.1,5,6 The presence of venous reflux or varicosities in the saphenous trunk or its collaterals without proximal valve incompetence (reported in 10% to 30% of cases), unilateral varicose veins despite bilateral saphenous junction incompetence, or the lack of varicose vein dilation in cases of insertion into the arterial highpressure system (even in cases of valve destruction by valvulotom) suggest an important role for simultaneous (or previous) vein wall injury required for its dilation.1,5-7 According to the majority of pathological studies in varicose vein specimens, various structural changes have been documented, including an irregular accumulation of the extracellular matrix within the vein wall and the disruption of the regular pattern of the smooth muscle cell (SMC) bundles.1,8-10 Moreover, significant quantitative differences in the principal vein wall compounds (SMCs, collagen, and elastin) were also reported, although contradictory results have been described. According to the literature, an increase1,9,10 or decrease11,12 in collagen in the varicose vein wall was suggested. Other authors described not only quantitative but also qualitative collagen changes. An imbalance in the collagen III:collagen I ratio, and an increase in collagen type I was documented in an ex situ study concerning vein wall SMCs as well as dermal fibroblasts, suggesting the possibility of a nonlocal but rather systemic character of the disease.1,3
Despite quantitative differences, the majority of authors report the presence of significant structural changes destroying the vein wall architecture1,8,10,11 (Figure 1). However, we still do not know whether the valve failure is the beginning or the end of the cascade of these events. Corcos et al describe the widening of the proximal saphenous vein valve annulus with structural changes within the vein wall in the majority of the investigated cases.2 According to Strucker et al, the complete destruction of the three-layer structure of the vein wall with concomitant thickening of the intima and disruption of the lamina elastica interna concerned 21% of cases.14 Varicose veins can also develop in the presence of competent valves, or can occur below a competent vein segment.5,6 Moreover, a saccular dilation of the vein can occur as a blowout on the normal vein.1,8 On the other hand, according to several other reports, in some cases, shortening, thickening, or disruption of the incompetent valve leaflets are described.5,15,16
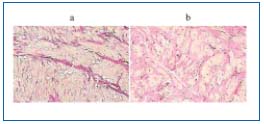
Figure 1. Verhoeff-Van Gieson staining of the normal (a) and
incompetent (b) saphenous vein – collagen deposits in the vein
media destructing regular vein wall architecture.
VARICOSE VEINS – THE ROLE OF VEIN WALL REMODELING
According to recent findings, the initial phase of the vein remodeling process can be related to endothelial cell activation.3 Shear stress disturbances, intraluminal hypertension, hypoxia of the endothelial cells, as well as ischemia of the vein medial layer (due to vasa vasorum compression) result in inflammatory reaction mediator and growth factor production.3,17 The endothelial cell activation and the release of cytokines induce the process that leads to intimal thickening and vein structure remodeling.2-4,8,19 In the mechanism leading to the vein wall dysplastic changes, its structure disorganization and intimal thickening, the possibility of SMC proliferation and migration into the vein intimal layer is taken into consideration.2-4,8,19 Another proposed mechanism can be related to the presence of SMC phenotype transdifferentiation and their secretory activity.2,3,8,20 The change in SMC phenotype from a contractile into a secretory one can result in local extracellular matrix production and its further accumulation.20-22 SMCs are also responsible for production of metaloproteinases (MMPs), and their tissue inhibitors (TIMPs), that is, for important mechanisms controlling extracellular matrix turnover in the vessel wall.23 According to the data reported by Badier- Commander, higher TIMP:MMP ratio facilitates an accumulation of fibrous tissue within the vein wall.24 The role of some other factors has also been suggested, such as neutrophil adhesion and activation or presence of monocyte and mast cell infiltrations.2,18,23
Besides the influence of cellular elements, the role of growth factors has also been investigated. Hollingsworth documented altered VEGF and VEGF receptor transcription in the initial stage of the disease with femoral junction incompetence.25 Michiels suggested the role of the release of ßFGF and PGF-_2 from activated endothelial cells in ischemic conditions.4 In a paper by Badier- Commander the presence of ßFGF and TGF-ß1 in the hypertrophic varicose vein segments was documented, whereas the lack of the increased PDGF activation (responsible for SMC migration and proliferation) was reported.20
VENOUS TONE – SMC ROLE IN THE VEIN WALL HEMOSTASIS MAINTENANCE
SMCs, being a part of the local contractile units, are responsible for active venous tone maintenance. The passive venous tone is related to vessel wall structure and to the mechanical properties of collagen and elastin fibers. Contradictory reports concerning SMC amounts within varicose vein walls can be found. According to some authors, the SMC content in the vein wall can be reduced.1,26 Others have reported unchanged or increased SMC amounts within the vein wall.12,17 These differences could probably be related to the unhomogenicity of the investigated material, often containing hypertrophic or atrophic varicose vein segments. In our study, concerning the patients in relatively early stages of the disease with femoral junction incompetence and crural varicose veins (without dilation of femoral segment of the saphenous vein), the reduction in the SMC amount within the vein media was confirmed in both proximal and distal vein segments (with simultaneous thickening of the intimal layer related to SMC and extracellular matrix accumulation).27
As was previously mentioned, SMCs are one of the most important factors controlling not only venous tone but also vein wall homeostasis.2,8,20,21,27 Their role in extracellular matrix and growth factor production has been described in many models of other vascular diseases.21,22 The possibility of phenotypic transformation of the SMC into the secretory type within the vein wall was also discussed. Jurkova documented the presence of altered collagen containing SMCs within varicose vein intima.28 Porto described the presence of structurally different subtypes of SMC in the injured vein wall, explaining these differences by SMC phenotype transdifferentiation.29 In the paper by Kockx the presence of hypertophic modified SMCs surrounded by extracellular matrix deposits was reported.30 SMC hypertrophy in varicose vein specimens was also documented by Wali and Knaapen.3,31 These observations were also confirmed in our study. Morphometric analysis of the longitudinal transsections of the vein wall specimens confirmed the local decrease in medial layer cell nucleus density in the areas qualified as SMCs, suggesting the presence of cell hypertrophy within the medial layer of incompetent veins.27,32
The mechanism of the SMC phenotype change in the arterial wall has been meticulously investigated in injured arteries or veins implanted into the arterial system.21,22 Although some factors had previously been suggested, the mechanism of the proposed phenotypic turnover remains unclear within the wall of the incompetent veins, especially in the presence of hypertrophic or atrophic segments within the same vein.2,4,20 Among various stimuli responsible for SMC dedifferentiation, hypoxia, hemodynamic disturbances, and cytokine (especially growth factors), influences were discussed.2,4 In our study, we confirm an increase in transforming growth factor-ß1 (TGF-ß1) mRNA expression level and its protein product presence within the wall of varicose veins, especially in the medial and intimal layer (Figure 2). TGF-ß1 is one of the multipotential cytokines regulating cell proliferation, differentiation, and apoptosis.33-36 The final effect of TGF-ß1 activity depends on the type of the cell, its surroundings, and presence of other cytokines.33-36
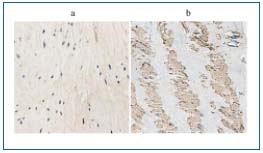
Figure 2. TGF-ß1 immunostaining – normal vein (a)
and incompetent (b) saphenous vein.
According to the literature, TGF-ß1 can be one of the factors responsible for SMCs dedifferentiation into secretory type.36,37 This cytokine also stimulates the cascade of events resulting in secretory fibroblast activity in trophic skin changes in patients with chronic venous insufficiency, and is probably one of the factors responsible for the local vein remodeling process connected with an increased extracellular matrix production within the vein wall.2,4,20,38 Beside the stimulation of collagen and fibronectin production by altered SMCs, TGF-ß1 also induces expression of TIMP and PAI-1 – two important inhibitors of extracellular matrix-degrading enzyme.38
Taking into account the role of membrane TGF-ß receptor complex activation and the method of its signal transduction, we documented the presence of increased immunoreactivity of TGF-ß receptor type I, as well as an increased transcription level of TGF-ß receptor-regulated intracellular factors – SMADs (mad related proteins) (Figure 3). According to the literature, SMAD proteins are responsible for TGF-ß1 signal transduction into the nucleus.39 According to our study, the ratio of mRNA expression level of TGF-ß1 receptor-regulated SMAD 2 to the inhibitory SMAD 7 was higher in varicose vein specimens than in the normal control veins. These data confirm the previous findings reported by Bujan documenting an increase in TGF-ß immunorectivity in the wall of varicose veins, as well as the results of Badier- Commander and coworkers, suggesting the role of TGFß1 and bFGF in the vein wall remodeling process.20,40 The coincidence of intimal hyperplasia and expression of TGF-ß1 in saphenous veins harvested for coronary artery surgery was also documented.41
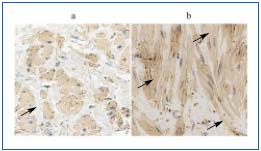
Figure 3. TGF-ß receptor type I immunostaining in the normal (a)
and incompetent (b) veins.
PROGRAMMED CELL DEATH (APOPTOSIS) IN VASCULAR REMODELING
Another important factor in embryonal, neonatal, and postnatal vessel remodeling is the programmed cell death (PCD).42,43 This method of cell elimination is one of the most important mechanisms responsible for vessel wall structure changes. Apoptosis can occur as a normal physiological process, which controls development and tissue homeostasis.42-44 PCD and its disturbances can be also involved in many pathological events such as cardiovascular diseases or malignancy.43,44 In the cardiovascular system a number of studies confirmed the role of PCD in vessels that remodel postnatally.42,43 Apoptosis can also be present in some chronic vascular conditions such as aortic aneurysm, atherosclerotic or restenotic lesions.43 PCD plays an important role in the removal of unwanted cells; however in some conditions properly functioning cells are also eliminated via apoptotic death.
Up till now, there have been very few studies performed describing the relationship between apoptotic cell death and varicose vein occurrence.19,45,46 Theoretically, an increase in PCD within the vein wall (especially SMC apoptosis), could lead to the elimination of this important component of the vein wall. Successive vein structure destruction as well as the decrease in venous tone could result in the vein wall weakening and its further dilation.
According to the very few previously performed studies, contradictory results were reported. In the paper published in 1999 by Bujan and coworkers, a relatively high apoptotic cell rate was reported, concerning 41% to 97% of cells.19 According to Ascher, in the varicose vein wall very few apoptotic cells were reported, and downregulation of the PCD within the dilated vein should be rather suspected.45,46
Verifying the previously published results by the means of TUNEL (Tdt mediated dUTP nick end labeling) method, as well as by an assessment of apoptosis-regulating genes mRNA and protein expression (FAS, p53, BAX, BCL-2), we did not confirm the hypothesis suggesting the role of the apoptosis activation as a principal mechanism leading to the reduction in smooth muscle cell number within the vein wall.27 Very low indexes (from 0.017 to 0.12) of apoptotic cells were reported within intima, media, and adventitia of both incompetent and healthy control veins. The apoptotic cells were present in the intima in 45% of the specimens only (versus 38% for the control veins). For the media and adventitia the respective values were 93.2% (versus 93.7% in control) and 82,5% (versus 73% in control) (Figure 4). Concerning patients’ age (two groups of patients were evaluated – younger and older than 50 years), in the young patient group an increase in apoptotic index within the media of the incompetent crural saphenous vein was reported. In the older patients, as well as in the prox- imal saphenous vein segments, there were no differences in the apoptotic index values. Concerning the character of the apoptotic cells, the presence of apoptotic SMCs in the intima and media, as well as apoptotic SMCs and macrophages within vein adventita was documented.
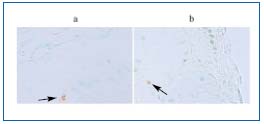
Figure 4. Apoptotic cells within the media of incompetent
saphenous vein (TUNEL–Tdt mediated dUTP nick end
labeling – in situ study).
THE ROLE OF PROGRAMMED CELL DEATH IN VARICOSE VEIN WALL REMODELING: APOPTOSIS – REGULATING PATHWAYS
Various factors influencing the activation of PCD were discovered.43,47 Among many pathways leading to apoptotic cell death activation, two were most commonly investigated: the “death receptor” pathway (related to the presence of so called “death receptors” of the TNF receptor family), and the “mitochondrial” pathway (related to the activation of BCL-2 protein family members and controlled by p53 transcriptional factor activity) (Figure 5).43,44,47,48 All of them activate the cascade of intracellular apoptosis executory enzyme – caspases, however, in the initial stage of the apoptotic changes, a balance between pro- and antiapoptotic signaling regulates cell viability.47,48
According to our study, in young patients with saphenous vein incompetence and shorter disease duration an increase in p53 activity (mRNA expression level and p53 – positive cell number in the immunostaining in the vein media) in the distal (crural) saphenous vein segment was present.27 In these specimens higher (proapoptotic) BAX to (antiapoptotic) BCL-2 mRNA expression ratio was also found, suggesting the role of “mitochondrial” pathway activation in this group. These findings correlated with a statistically significant increase in the media apoptotic cell index in this group. In the proximal vein segments, as well as in older subjects, despite previously mentioned structural changes related to extracellular matrix accumulation, the lack of enhanced apoptotic activity was reported. Simultaneously, in the older population with consequently longer disease duration, the total SMC amount within the vein media of incompetent veins was bigger than in young subjects, although in the morphometric analysis. In both groups the presence of SMC hypertrophy (in the morphometric analysis) the vein media was reported that suggest the accumulation of hypertrophic, secretory SMCs along the disease course.
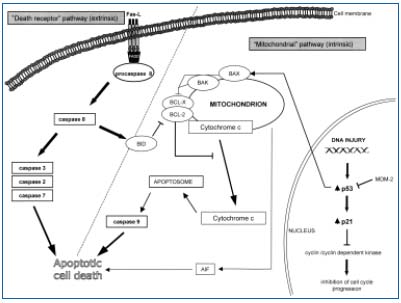
Figure 5. Apoptosis activation
pathways (“mitochondrial” and
“death receptor”pathways)
BAX, BAK, BCL-2, BCL-X, BID
– members of BCL-2 protein
familiy, AIF – Apoptosis
Inducing Factor, Fas-L – ligand
of the Fas death receptor, FADD
– Fas Associated Death Domain.
According to our findings, activation of PCD seems to be related to the relatively early stages of varicose vein disease.27 In the further course of the disease, the downregulation of p53-dependent apoptotic cell death within the media of the incompetent veins influences the further structural change increase due to hypertrophic SMC accumulation and secretory activity. Of course, apoptotic elimination could also concern normal cells surrounded by accumulated extracellular matrix. However, we did not document enhanced apoptotic activity in the specimens, where extracellular matrix totally disrupted SMC bundles separating several SMCs. There was also no correlation between apoptotic cell death and FAS death, receptor mRNA expression or its protein presence, although the presence of other apoptotic pathways should also be taken into account. The lack of apoptosis upregulation within varicose vein media as well as the role of mitochondrial pathway of the programmed cell death activation was also confirmed in the study published recently (2005) by Ducasse and coworkers.49
As we could not confirm any differences concerning apoptotic activity in the proximal incompetent saphenous vein segments, where the reduction in SMC amount within the vein media was also observed, we looked for other mechanisms that could control in SMC population within the vein wall.32
According to many authors, during varicose vein development the thickening of the vein intima occurs due to extracellular matrix accumulation that results in the course of intimal and medial SMC transdifferentiation, proliferation, and migration into the subendothelial layer.2,20,28,30 On the other hand, in some previous studies conflicting data concerning proliferating activity within the varicose vein wall were reported, especially if we take into consideration the possibility of the presence of atrophic (with complete lack of cellular elements) and hypertrophic segments within the same dilated vein. An increase in the number of the proliferating cells (assessed by the means of anti-PCNA immunostainings) was suggested in the paper published by Bujan.19 In another study (Bader-Commander and coworkers) using anti- PCNA and anti-Ki67 immunostainings, the lack of an increased rate of proliferating cells was reported.20
The role of disturbances of the molecular regulation of cell cycle within the varicose vein wall was for the first time investigated by Papas the means of retinoblastoma protein (Rb) assessment.50 Phosphorylation of Rb induces release of transcriptional factors (E2F) that activate the genes required for the cell cycle progression. The authors suggested a role of Rb not only in cell cycle control, but also in the dedifferentiation and phenotype change within the vein wall.50 Asher and coworkers assessing cyclin D1 expression (the molecule playing an important role in the cell cycle arrest mediation via p53 dependent pathway), suggest the deregulation of the cell cycle progression in varicose veins.45
In our study, an expression of another important molecule p21 was investigated.27,32 Protein product of p21 (Waf/Cip1) gene was previously identified as a cyclindependent kinase inhibitor controlling cell proliferation due to inhibition of the cell cycle progression from phase G1 to phase S.51 P21 is also a downstream regulator of the p53 tumor suppressor gene which can control not only apoptosis but also the cell cycle.32,52 The negative regulation of the cell growth by p53 activity is related to p21 induction.52 In both control and incompetent veins, very low levels of p21 immunopositivity was present.32 However, in the harvested proximal saphenous vein segments p21 mRNA expression levels as well as the number of p21-positive cells within the vein media were significantly higher than these of the control veins. This corresponded to the decrease in the SMC amount within the vein media of the proximal long saphenous vein, although previously described morphological changes, related to the local extracellular matrix accumulation and vein structure disorganisation, were present also in these vein segments.
The lack of the enhanced apoptotic activity in the region of the proximal valve can suggest the role of cell cycle regulation disturbances or SMC transdifferentiation but not apoptosis in the vein wall destructive changes leading to this valve incompetence. In particular, if the presence of unchanged, in pathological examination, proximal valve cusps could be found in some patients in whom an incompetence of proximal valve was clinically and sonographically confirmed (with simultaneously microscopically documented vein wall remodeling in the region of valve annulus).
As there is still a lack of consensus as to what is the proper sequence of the events in the initial phase of the disease (primary valve incompetence or primary vein wall injury), the local differences of the reported results (between proximal and distal vein segments) could also be related to the hemodynamic conditions along the dilating incompetent vein.1,26,27 The role of the previously discussed factors should be also taken into account (eg, TGF-ß). According to the literature there are some reports describing the possibility of TGF ß1 related and p53-depenedent or independent induction of p21, which is one of the antiapoptotic mechanisms by which this multipotential cytokine (TGF-ß1) can control SMC viability.53,54 In both distal and proximal incompetent saphenous vein segments the presence of TGF-ß1 expression was confirmed, although, as previously mentioned, the role of other cytokines produced by activated endothelial cells, smooth muscle cells, or cells of inflammatory reaction should be evaluated.
CONCLUSIONS
Despite many papers suggesting the role of valve dysfunction in vein incompetence, valve injury seems not to be the only factor leading to varicose vein development. For vein dilation additional factors related to vein wall homeostasis disturbances are needed, such as SMC transdifferentiation. One of the important mechanisms influencing vein wall homeostasis maintenance is p53-related apoptosis; however, its activation is characteristic to the relatively early stages of the diseases. Further dowregulation of SMC apoptosis within vein media leads to an increase in structural changes and vein wall weakening related to extracellular matrix accumulation.
The reduction of the SMC population corresponding to the p21 expression in the proximal saphenous vein segments suggests the role of the cell cycle disturbances that may lead to vein wall architecture destruction. However, further studies examining the role of other cell cycle-related molecules are needed. As was previously mentioned we still do not know what is the sequence of the events in the vein and valve injury process, although, according to the performed study, some new data can be introduced into varicose vein theory. The necessity of the presence of not only valve incompetence but also the molecular defect of the vein wall homeostatic mechanism can be an important argument explaining the progressive character of vein incompetence in patients with varicose veins. This observation can provide new arguments for discussion of the character of chronic venous insufficiency, which has to be evaluated not as a single vein problem but more as a systemic disease.
The project was funded by the SERVIER AWARD of the International Union of Phlebology (2001).

“Special acknowledgments for B. Skop, R. Widerkiewicz, K. Ziaja, T. Wilczok, T. Lebda – Wyborny and K. Pawlicki from Medical University of Silesia for their help and cooperation in research and manuscript preparation.”
