Benefit of MPFF at a dose of 500 mg in combination with sclerotherapy of telangiectasias of the lower limbs: results from the SYNERGY and SATISFY surveys
Françoise PITSCH
Suresnes, France
INTRODUCTION
Telangiectasias are defined as a confluence of dilated intradermal venules less than 1 mm in caliber. Synonyms include spider veins, hyphen webs, and thread veins.1 Telangiectasias are common in adult populations. In the Basle study,2 telangiectasias were combined with varicose veins in 36% of men and 44% of women. In a study of 696 women working in a department store in Slovakia,3 46% of women were found to have telangiectasias. In Poland, Jawien found telangiectasias without varicose veins in 16.5% of consecutive patients seeking medical help at primary outpatient clinics,4 while the prevalence rises to 59% in the adult general population of Bonn.5
PATHOPHYSIOLOGY OF TELANGIECTASIAS
One common area for the development of telangiectasias is the medial thigh. It was thought this would be a result of pressure exerted by crossing the legs, but no formal study has confirmed this hypothesis. Many causes are associated with telangiectasias, including genetic and congenital factors, acquired (disease of vascular collagen) or primary cutaneous disease (varicose veins) , hormonal factors (pregnancy and estrogen therapy), and physical factors trauma, infection, etc.).6
TREATMENT OF TELANGIECTASIAS
Sclerotherapy or microsclerosis is the first choice treatment of telangiectasias. Sclerotherapy is carried out from the lumen of the venule and results in its occlusion. Use is made of chemical sclerosing agents (liquid or foam) or thermal energy, such as laser energy.
The mechanism of action for sclerosing agents consists in generating endothelial damage (endosclerosis) which causes endofibrosis. The extent of damage to the blood vessel wall determines the effectiveness of the solution. Total endothelial destruction results in the exposure of subendothelial collagen fibers, causing platelet aggregation, adherence, and release of platelet-related factors. This series of events initiates the intrinsic pathway of blood coagulation by activating factor XII. Ideally, sclerosing agents should not trigger thromboplastic activity because this would initiate the extrinsic pathway of blood coagulation. Excessive thrombosis is detrimental to the production of endofibrosis because it may lead to recanalization of the vessel as well as excessive intravascular and perivascular inflammation.7
Laser treatment of telangiectasias uses thermal energy from a laser fiber and generator. Electromagnetic energy from the laser acts on the vessel wall, which shows loss of intima, thickening, and inflammatory changes. Usually, many fibroblasts and inflammatory cells are present, and collagen is the predominant histological finding.8 There is a body of evidence that inflammation is involved whatever the process, chemical or thermal.
Some studies have compared sclerotherapy using sodium tetradecyl sulfate (STS) or polidocanol (Pol) with laser therapy. In a study of 20 patients with telangiectasias of 0.1 to 1.5 mm, 0.25% STS was more rapidly effective than Nd-YAG laser therapy.9 Another study of 14 patients comparing 1) 0.5% Pol and then Nd-YAG laser therapy, 2) Nd-YAG laser therapy and then 0.5% Pol, 3) 0.5% Pol only, and 4) Nd-YAG laser therapy only, showed that 0.5% Pol as first choice was the most costeffective.10
Side effects are more frequent with STS than with Pol or placebo,11 the most important being pigmentation (92% with 0.25% STS) and ulceration (7% with 0.25% STS, and none with Pol).
The superiority of foam sclerotherapy over liquid sclerotherapy still remains controversial. Pigmentation, matting, and thrombi may be more frequent with foam than with liquid. The second Consensus Meeting on Foam Sclerotherapy, Tergersee, Germany, 2006, recommends the use of foam second line for the treatment of telangiectasias.12
RATIONALE FOR THE USE OF MPFF at a dose of 500 mg IN COMBINATION WITH SCLEROTHERAPY
Microsclerosis is based on the chemical destruction of the venule endothelium. This may cause post-intervention complications such as pain, induration of the treated venule, swelling, and pigmentation.
Two trials in patients with varicose veins who underwent phlebectomy have evaluated the benefits of the micronized purified flavonoid fraction (MPFF, MPFF at a dose of 500 mg), which consists of 90% diosmin and 10% other flavonoids expressed as hesperidin, diosmetin, linarin, and isorhoifolin,13 as part of the pharmacological post-operative recovery.14-17 In both studies, MPFF at a dose of 500 mg helped attenuate pain, decrease postoperative hematomas and accelerate their resorption, and increase exercise tolerance in the early post-operative period.
On the other hand, successful microsclerosis depends on elimination of sources of venous hypertension to prevent reflux before starting such treatment. MPFF at a dose of 500 mg experimentally reduced reflux through pressurized veins in an animal model of acute venous hypertension.18 MPFF at a dose of 500 mg is the only available venoactive drug known to modify the chain of events leading to chronic venous hypertension. Therefore MPFF at a dose of 500 mg currently possesses the most appropriate profile for use with microsclerosis to reinforce the latter’s effect on telangiectasias, with beneficial effects on clinical severity, symptoms, and quality of life.19
OBJECTIVES
To assess the impact of microsclerosis plus MPFF at a dose of 500 mg on telangiectasias:
• symptoms: pain, sensation of swelling and heaviness, by means of the visual analogue scale20
• change in the quality of life using the CIVIQ-1421
The secondary objectives will be to assess:
• Investigators’ overall evaluation of the benefit of the combination
• Frequency of use of available microsclerosis techniques (foam, liquid, or laser)
• Breakdown of sclerotherapy indications
• Patients’ global satisfaction by means of the visual analogue scale20 and pictures (additional survey called “SATISFY” in 240 practitioners)
METHODS
Open trial of patients undergoing microsclerosis with foam, liquid sclerosing agents, or laser, combined with MPFF at a dose of 500 mg, 2 tablets daily, from the first session to the last.
The study did not alter the normal patient pathway in the management of chronic venous disease. More particularly, the reported data were those usually collected during examination of patients for chronic venous disease.
• adult of any race
• aged between 30 and 60 years
• having given written informed consent
• with no known allergy to sclerosing agents
• having taken no phlebotropic during the 4 weeks before selection
• presenting with symptomatic telangiectasias, the diagnosis of venous disease according to the CEAP classification: C1s with or without complications (±C2, ±C3), the absence of deep venous reflux confirmed by duplex ultrasonography (measured in upright position at 3 cm below the saphenofemoral junction and after the Valsalva maneuver; the patient will stand on the contralateral limb)
• available for at least 2 of 3 sclerotherapy sessions at an interval of 3 weeks
• phlebotropic treatment in the 4 weeks before selection
• history of alcohol or drug abuse, known history of allergy or intolerance to diosmin or any other phlebotropic agent and to sclerosing agents
• asymptomatic telangiectasias
• participation of the patient in another clinical trial during the previous 3 months
RESULTS
The SYNERGY survey was performed in several centers in France, between January and November 2009. A total of 392 phlebologists participated in the study and 3202 patients were included.
• The mean number of sclerotherapy sessions performed per phlebologist and per week was 57.4 ± 32.9. Extrapolating for the total number of phlebologists practicing in France, this means that there are more than 3 million sclerotherapy sessions every year in France.
• Liquid sclerotherapy remains the most often practiced in France: in 100 patients, 73 underwent liquid sclerotherapy, 23 foam sclerotherapy, and 4 a combination of the two.
• Sclerotherapy is most often performed on varicose veins in France: 66% of patients underwent sclerotherapy for varicose veins, 57% for telangiectasias, and 2% for other reasons (total might be >100%). In fact, at the inclusion visit, 84% of patients were already assigned C2s or C3s of the CEAP classification, while 16% were C1s patients.
• 90% of patients seeking sclerotherapy were women. Sedentary patients requested treatment the most (28% were employees, 20% retired, 13% executives or white-collar workers, 8% blue-collar workers or farmers)
• Mean duration of chronic venous disease-related symptoms was 14.7 years ±11.6, and mean duration of edema was 9.7 years ±10.1 at the inclusion visit
• Prevalence of risk factors:
– Family history of chronic venous disease, 82.4%
– Number of pregnancies >1, 76.8%
– Prolonged standing position, >6 hours per day, 42.2%
– Sedentary lifestyle and prolonged standing position, >6 hours per day, 40% and 28%, respectively
– BMI>30, 36%
• Most patients were already managed for chronic venous disease with:
– Venoactive drugs, 73.2%
– Sclerotherapy, 71.5%
– Compression therapy, 58.7%
Before sclerotherapy, patients were expecting treatment not only to improve the cosmetic appearance of their legs, but also to get rid of all symptoms related to chronic venous disease. Leg pain and heaviness were the symptoms patients complained of most (Figure 1).
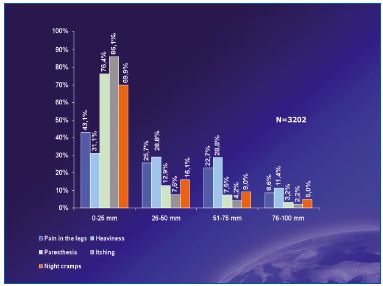
Figure 1. Pre-treatment intensity and frequency of symptoms
Treatment = combination of sclerotherapy + MPFF at a dose of 500 mg
After sclerotherapy in combination with MPFF at a dose of 500 mg, side effects were present in 2.4% of cases only. These were essentially hematomas (0.4%), post-procedure pain (0.3%), and inflammation (0.3%).
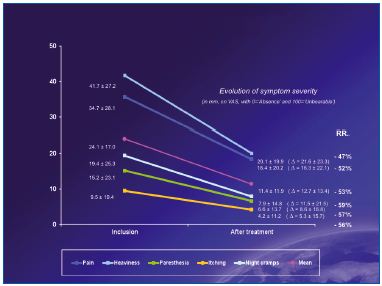
Figure 2. Intensity of symptoms before and after treatment
Treatment = combination of sclerotherapy + MPFF at a dose of 500 mg
All symptoms associated with chronic venous disease were significantly decreased after treatment (Figure 2), and quality of life as assessed with the CIVIQ-14 was significantly improved (Figure 3).
Most patients (81%) were satisfied or very satisfied with the combination of sclerotherapy + MPFF at a dose of 500 mg (Figure 4).
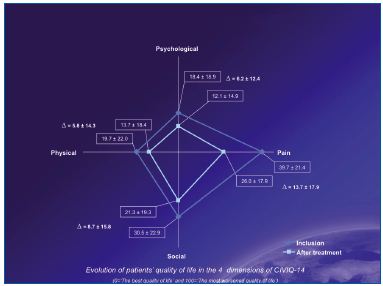
Figure 3. Quality of life scores before and after treatment
Treatment = combination of sclerotherapy + MPFF at a dose of 500 mg
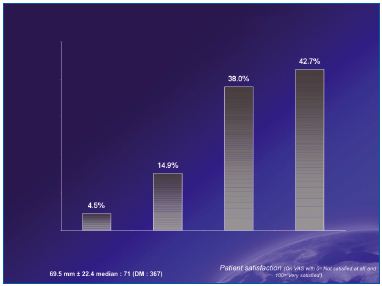
Figure 4. Patient satisfaction after treatment
Treatment = combination of sclerotherapy + MPFF at a dose of 500 mg
DISCUSSION
In the present survey, most patients consulting for treatment presented with telangiectasias combined with varicose veins and edema. This is in line with former epidemiological trials in which patients often showed a combination of telangiectasias and varicose veins.2-4 Phlebologists therefore treated a higher proportion varicose veins than telangiectasias, mostly with liquid sclerosing agents rather than foam. Despite the good results obtained with foam sclerotherapy,22 it is clear that at the time of the survey French phlebologists were not yet used to delivering such treatment, or laser therapy, which is not even mentioned.
Patients in European countries and the USA seek therapy mainly because of the unsightly appearance of telangiectasias and varicose veins. A survey in the USA has shown that American women are more concerned by telangiectasias of the lower limbs than by any other cosmetic problem.23 It should be pointed out that patients in this survey were mainly sedentary and at high risk of venous disease (with family history of disease, obesity, or prolonged standing or sitting positions). In addition, and this is shown also in the present survey, many patients suffered from venous symptoms and expected treatment to get rid of them.24
MPFF at a dose of 500 mg, 2 tablets daily for 2 months, combined with a microsclerosing treatment, significantly relieved patients’ symptoms and improved their quality of life. In addition, the frequency of side effects due to the procedure was very low (2.4%). Further study including a control group with no addition of MPFF at a dose of 500 mg is needed to confirm the results of the present survey, but there is evidence that MPFF at a dose of 500 mg may help normalize the underlying pathologic physiology, which is the primary aim of any treatment.
Telangiectasias like varicose veins are believed to be the manifestations of higher than normal venous pressure. Venous hypertension, when chronic, causes a sequence of hemodynamic and clinical disturbances, including cutaneous manifestations like telangiectasias, leg ulcer, and edema. By reducing the likelihood of leukocyte adhesion to the venous wall, MPFF at a dose of 500 mg presumably acts by hampering the cascade of events that leads to venous hypertension.
Addition of MPFF at a dose of 500 mg may increase the success of sclerotherapy and greatly relieve patients’ symptoms.
REFERENCES
1. Eklöf B, Rutherford R, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J Vasc Surg. 2004;40:1248- 1252.
2. Widmer LK. In: Peripheral Venous Disorders-prevalence and socio-medical importance. Hans Hubert Ed., Bern, 1978.
3. Stvrtinova V, Kolesar J, Wimmer G. Prevalence of varicose veins of the lower limbs of women working in a department store. Int Angiol. 1991;10:2- 5.
4. Jawien A, Grzela T, Ochwat A. Prevalence of chronic venous insufficiency in men and women in Poland: multicentre cross-sectional study in 40095 patients. Phlebology. 2003; 18:110-122.
5. Rabe E, Pannier F. What have we learned from the Bonn Vein Study? Phlebolymphology. 2006;13:188-193.
6. Goldman MP, Bergan JJ, Guex JJ eds. Pathophysiology of telangiectasias. In: Sclerotherapy. Treatment of varicose and telangiectatic leg veins. 4th edition: Mosby Elsevier, Philadelphia, PA,USA; 2007, pp 73-90.
7. Goldman MP, Bergan JJ, Guex JJ eds. Mechanism of action of sclerotherapy. In: Sclerotherapy. Treatment of varicose and telangiectatic leg veins. 4th edition: Mosby Elsevier, Philadelphia, PA,USA; 2007, pp 163-188.
8. Bush RG, Shamma HN, Hammond K. Histological changes occurring after endoluminal ablation with two diode lasers (940 and 1319 nm) from acute changes to 4 months. Lasers Surg Med. 2008;40:676-679.
9. Lupton JR, Alster TS, Romero P. Clinical comparison of sclerotherapy versus long-pulsed Nd-YAG laser treatment for lower extremity telangiectases. Dermatol Surg. 2002;28:694-697.
10. Levy JL, Elbahr C, Jouve E et al. Comparison and sequential study of long pulsed Nd-YAG 1064 nm laser and sclerotherapy in leg telangiectasias treatment. Lasers Surg Med. 2004;34:273-276.
11. Rabe E, Schliephake D, Otto J et al. Sclerotherapy of telangiectases and reticular veins: double-blind prospective comparative trial of polidocanol, sodium tetradecyl sulfate and isotonic saline (EASI study). Phlebolymphology. 2009;25:124-131.
12. Breu FX, Guggenbichler S, Wollmann JC. Second European Consensus Meeting on Foam Sclerotherapy 2006, Tegernsee, Germany; Vasa. 2008;37(Suppl 71):1-29.
13. Paysant J. Sansilvestri-Morel P, Bouskela E, Verbeuren TJ. Different flavonoids present in the micronized purified flavonoid fraction (MPFF at a dose of 500 mg) contribute to its antihyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81-85.
14. Pokrovsky AV, Saveljev VS, Kirienko AI et al. Surgical correction of varicose vein disease under micronized diosmin protection (results of the Russian multicenter controlled trial DEFANS). Angiol Sosud Khir. 2007;13(2):47-55.
15. Pokrovsky AV, Saveljev VS, Kirienko AI et al. Stripping of the great saphenous vein under micronized purified flavonoid fraction (MPFF) protection (results of the Russian multicenter controlled trial DEFANCE). Phlebolymphology. 2008; 15: 45-51.
16. Veverkova L, Kalac J, Jedlicka V et al. Analysis of surgical procedures on the vena saphena magna in the Czech Republic and an effect of Detralex during its stripping. Rozhl Chir. 2005; 84:410-412.
17. Veverkova L, Kalac J, Jedlicka V et al. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of MPFF at a dose of 500 mg to postoperative symptoms. Phlebolymphology. 2006;13:195-201.
18. Bergan JJ, Pascarella L, Schmid- Schönbein G. Pathogenesis of primary chronic venous disease: insights from animal models of venous hypertension. J Vasc Surg. 2008;47:183- 192.
19. Lyseng-Williamson A, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
20. Huskisson EC. Measurement of pain. Lancet. 1974;2:1127-1131.
21. Launois R, Mansilha A, Jantet G. International Psychometric Validation of the Chronic Venous Disease Quality of Life Questionnaire. Eur J Vasc Endovasc Surg. 2010;40:783-789.
22. Coleridge Smith P. Sclerotherapy and foam sclerotherapy for varicose veins. Phlebology. 2009;24:260-269.
23. Wilson NM, Browse NE. Venous disease. In: Clement DL, Shepherd JT, eds. Vascular diseases in the lower limbs, St Louis, LA: Mosby Yearbook; 1993.
24. Darvall KAL, Bate GR, Sam RC, et al. Patients’ expectations before and satisfaction after ultrasound guided foam sclerotherapy for varicose veins. Eur J Vasc Endovasc Surg. 2009;38:642- 647.
