Benefit of MPFF at a dose of 500 mg in the reduction of chronic venous disease–related symptoms.
Zrinka STANIC ZGOMBIC
Teo MANESTAR BLAZIC
Ines BRAJAC
Darinka PERISA
SUMMARY
Lower limb symptoms have frequently been associated with increased severity of venous disease. In clinical practice they are reported by patients at all stages of chronic venous disease. This study assessed the contribution of MPFF at a dose of 500 mg in improving venous symptoms and promoting venous ulcer healing in patients with primary chronic venous disease.
Patients aged ≥18 years, with at least 3 symptoms (including pain, sensation of heaviness in the legs, sensation of swelling, and night cramps), assigned to clinical classes C0 to C6 of the Clinical (severity)–Etiology– Anatomy–Pathophysiology (CEAP) classification following leg examination (recording the highest CEAP class for each patient), and presenting either with or without venous reflux, but not with obstruction, were included in the trial and received MPFF at a dose of 500 mg 2 tablets per day for 6 months.
Between month 3 and month 6, there was a significant (P<0.05) improvement in patients’ symptoms (sensations of heaviness in the legs, swelling, pain, and cramps) following treatment with MPFF at a dose of 500 mg. After 6 months, 13% of recalcitrant ulcers had healed, which was deemed satisfactory by the health care team.
Selected abbreviations and acronyms
CEAP Clinical (severity)–Etiology–Anatomy–Pathophysiology
CVD chronic venous disease
MPFF micronized purified flavonoid fraction
PCVD primary chronic venous disease
VAD venoactive drug
INTRODUCTION
The term “chronic venous disease” (CVD) refers to any long-standing morphological and functional abnormality of the venous system that manifests with symptoms and/or signs indicating the need for investigation and/or care.1
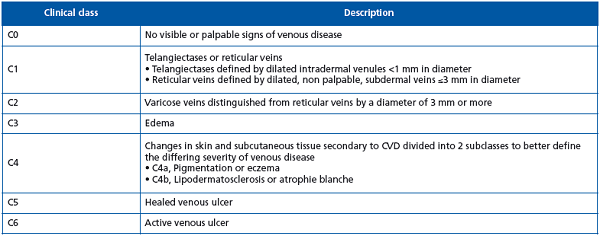
Primary chronic venous disease (PCVD) includes patients presenting with reflux but not with obstruction. Signs of PCVD may be present on clinical examination. The venous signs that are visible manifestations of CVD are described in the Clinical (severity)–Etiology– Anatomy–Pathophysiology (CEAP) classification and include dilated veins (telangiectasia, reticular veins, varicose veins), leg edema, skin changes, and ulcers.2 According to this classification, clinical signs are categorized into seven classes ranging from C0 to C6.2 Affected limbs may either be symptomatic (S) or asymptomatic (A), regardless of their clinical class. A review of the literature shows that the most common symptoms of PCVD are tingling, aching, burning, pain, muscle cramps, swelling, sensations of throbbing or heaviness, itchy skin, restless legs, and leg tiredness/fatigue. Although not pathognomonic, these symptoms may be suggestive of CVD, particularly if they are exacerbated by heat, vary during the course of the day, and are relieved with leg rest and/or elevation.1 The presence of venous signs and/or (noninvasive) laboratory evidence is required to attribute these symptoms to PCVD.
PCVD encompasses the full spectrum of signs and symptoms associated with classes C0 to C6. (Table I)
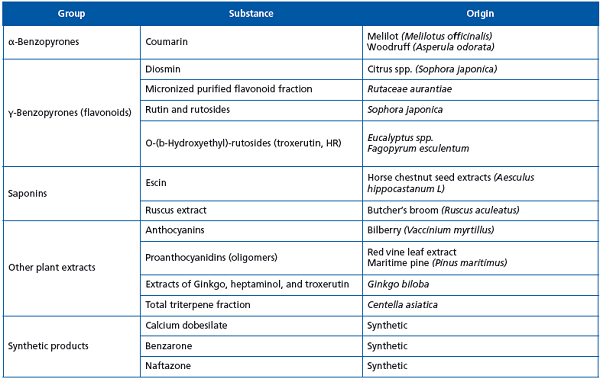
Venoactive drugs (VADs) belong to several different chemical classes. The majority of them are plant-derived compounds, like benzopyrones, saponins, anthocyanins, proanthocyanidins, and Ginkgo biloba,3 while some are produced by chemical synthesis, like calcium dobesilate, benzarone, and naftazone. (Table II)
Micronized purified flavonoid fraction (MPFF at a dose of 500 mg), an oral VAD consisting of 90% micronized diosmin and 10% flavonoids expressed as hesperidin, diosmetin, linarin, and isorhoifolin,4 improves venous tone and lymphatic drainage, and reduces capillary hyperpermeability by protecting the microcirculation from inflammatory processes.5 Recent research has highlighted the central role of inflammation in the progression of PCVD and elucidated some of the processes involved.6 MPFF at a dose of 500 mg is the only currently available drug to have shown an anti-inflammatory effect in acute venous hypertension, in a model induced by the creation of a venous fistula in rats.7 In this model, treatment with MPFF at a dose of 500 mg reduced reflux flow in a dose-dependent manner.7 In addition, MPFF at a dose of 500 mg reduced the release of inflammatory mediators such as oxygen free radicals, prostaglandins, and thromboxane in animal models of ischemia reperfusion.8 In MPFF, the absorption of diosmin is improved by its micronization to particles with a diameter <2μm.9
Given the comprehensive evidence about it, we chose to use MPFF at a dose of 500 mg in our trial assessing the contribution of a pharmacological treatment in symptom improvement, venous edema reduction, and venous ulcer healing in patients with PCVD.
MATERIAL AND METHODS
From January 2007 to December 2009, we enrolled outpatients consulting for venous leg problems in the outpatient clinic of the dermatovenerology ward of the Clinical Hospital Center Rijeka, Rijeka, Croatia.
Patients, aged ≥18 years, with at least 3 symptoms (including sensation of heaviness in the legs, sensation of swelling, and night cramps), assigned to clinical classes C0 to C6 of the CEAP classification following leg examination (recording the highest CEAP class for each patient), and presenting either with or without venous reflux, but not with obstruction, were included in the study. They received MPFF at a dose of 500 mg 2 tablets per day for 6 months. For all CEAP clinical classes, the end point was the disappearance of clinical symptoms.
In addition to MPFF at a dose of 500 mg 2 tablets per day, patients with active venous ulcers (class C6) underwent standard therapy, which consisted of elastic bandages and silverreleasing foam dressings, as adjunctive treatment. Complete wound reepithelisation was considered successful healing. Symptoms were assessed after 3 and 6 months of therapy. The percentage of healed ulcers was determined after 6 months of treatment.
RESULTS
A total of 1212 patients showing no obstruction on pocket Doppler ultrasound were enrolled in the trial. Most patients (90.1%) were women and the average patient age was 53.5 years (ranging from 28 years to 79 years). The majority of patients were assigned to the C0, C1 or C2 stages of the CEAP classification (42.90%), 34.5% to either the C3 or C4 stages, and 22.6% to either the C5 or C6 stages. The majority of patients had an occupation that required them to stand or sit for sustained periods of time. All patients reported sensations of heaviness, pain, and nocturnal cramps that were unresponsive to elastic bandage.
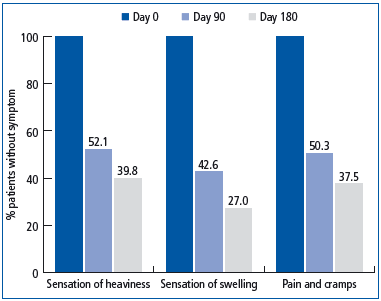
Based on our analysis, symptoms like sensations of heaviness, pain, nocturnal cramps, and edema disappeared in 60.2%, 62.5%, and 73% of patients, respectively, after 6 months of therapy with MPFF at a dose of 500 mg. (Figure 1).
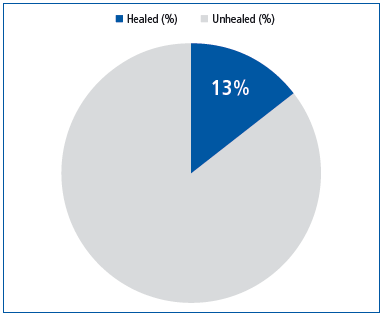
Out of the 274 patients in the C5 or C6 classes, 115 presented an active venous ulcer which had either failed to heal over the past 6 months or had recurred. These recalcitrant venous ulcers healed completely in 13.1% of patients following a 6-month adjunctive treatment with MPFF at a dose of 500 mg. (Figure 2).
DISCUSSION
Despite the open design of the trial, we can assume that treatment with MPFF at a dose of 500 mg improves patients’ quality of life, since venous symptoms have a very negative impact on the daily life of those suffering from PCVD.10 Moreover, venous leg ulcers, the most severe manifestation of CVD, are usually painful and affect quality of life.11 The impairment associated with CEAP classes C5 and C6 has been likened to the impairment associated with heart failure.12
The results of our trial adds weight to previous substantial evidence from randomized trials,13 metaanalyses, and a large observational study,14 in favor of the efficacy of MPFF at a dose of 500 mg in relieving venous symptoms such as pain, sensations of heaviness and swelling, and cramps, and in reducing PCVD-related lower limb edema. However, there is little evidence indicating which CEAP clinical class benefits the most from treatment with MPFF at a dose of 500 mg, since the majority of studies were carried out before the creation of the CEAP classification. Based on our results, it is reasonable to assume that patients at all stages of the disease, including the early CEAP stages, may benefit. Acceleration of venous leg ulcer healing (CEAP stage C6) was demonstrated in a double-blind study using MPFF at a dose of 500 mg in combination with compression.15 This was confirmed in 2005 by a meta-analysis of 5 trials with adjunctive MPFF in 723 patients with venous ulcers.16 Since our trial focused on difficult-to-heal venous ulcers, it was deemed satisfactory to have achieved a 13% rate of healing.
The results of our trial are encouraging and larger randomized controlled trials should be performed to better specify which CEAP clinical class would benefit the most from such a treatment. This would give further strength to the current recommendation of MPFF at a dose of 500 mg as an adjuvant to standard treatment in PCVD to relieve clinical symptoms and edema (grade 1B recommendation)17 and heal ulcers.18

REFERENCES
1. Eklöf B, Perrin M, Delis KT, et al. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49:498-501.
2. Eklöf B, Rutherford RB, Bergan JJ, et al; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders. Consensus statement. J Vasc Surg. 2004;40:1248-1252.
3. Ramelet AA, Kern P, Perrin M. Varicose veins and telangiectasias. Paris, France: Elsevier; 2004.
4. Paysant J. Sansilvestri-Morel P, Bouskela E, et al. Different flavonoids present in the micronized purified flavonoid fraction (MPFF at a dose of 500 mg) contribute to its anti-hyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81- 85.
5. Lyseng-Williamson KA, Perry CM. Micronised Purified Flavonoid Fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
6. Bergan JJ, Schmid-Schonbein GW, Coleridge Smith PD, et al. Chronic venous disease. N Engl J Med. 2006;355:488-498.
7. Bergan JJ, Pascarella L, Schmid- Schönbein GW. Pathogenesis of primary chronic venous disease: insights from animal models of venous hypertension. J Vasc Surg. 2008;47:183-192.
8. Nicolaides A, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
9. Garner RC, Garner JV, Gregory S, et al. Comparison of the absorption of micronized (MPFF at a dose of 500 mg) and nonmicronized 14C-diosmin tablets after oral administration to healthy volunteers by accelerator mass spectrometry and liquid scintillation. J Pharm Sci. 2002;91:32-40.
10. Kaplan RM, Criqui MH, Denenberg JO, et al. Quality of life in patients with chronic venous disease: San Diego Population Study. J Vasc Surg. 2003;37:1047-1053.
11. Franks PJ, Moffatt CJ. Health related quality of life in patients with venous ulceration: use of the Nottingham health profile. Qual Life Res. 2001;10:693-700.
12. Andreozzi GM, Cordova RM, Scomparin A, et al. Quality of life in chronic venous insufficiency: an Italian pilot study of the Triveneto Region. Int Angiol. 2005;24:272-277.
13. Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7:303-308.
14. Jantet G. Chronic venous insufficiency: worldwide results from the RELIEF study. Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids. Angiology. 2002;53:245-256.
15. Guilhou JJ, Dereure O, Marzin L, et al. Efficacy of MPFF at a dose of 500 mg in venous leg ulcer healing: a double-blind, randomised, controlled versus placebo trial in 107 patients. Angiology. 1997;48:77–85.
16. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198-208.
17. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41:117- 125.
18. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006;129:174-181.
