Benefits of micronized purified flavonoid fraction in the reduction of symptoms after operation for hemorrhoidal disease
Dmitry Timchenko
Hospital of the Ministry of Defense
of the RF, Moscow, Russia
ABSTRACT
Analysis of the treatment of patients with chronic hemorrhoids was carried out in the department of abdominal surgery at the N.N. Burdenko Main Military Clinical Hospital. The use of micronized purified flavonoid fraction (contained in Detralex) in 56 patients with stage II to IV hemorrhoidal disease in the pre- and postoperative period resulted in improved treatment outcomes.
The term “hemorrhoids” comes from the Greek words “haema” (blood) and “rhoos” (flowing). This term was first used by Hippocrates to describe bleeding from veins of the anus.1 The term “hemorrhoids” in English-language literature stands for both pathological and normal anatomical structures of the anal canal, which can be found in persons of any age. In developed countries, hemorrhoidal disease (HD) is the most prevalent disease of the rectum.2 The estimated population of people with hemorrhoids at any given time in Russia reaches 35% to 47% of all coloproctology disorders and the number of new cases of hemorrhoids diagnosed each year is 140 to 160 cases per 1000 in the adult population.3 Prevalence in Austria was estimated to be 39% of the adult population,4 and a high body mass index (BMI) was found to be an independent risk factor for developing HD. According to estimates from the United States up to 50% of the adult population will develop hemorrhoids, usually after the age of 30.5
The term “hemorrhoids,” commonly encompasses two different vascular structures: (i) internal hemorrhoidal plexus, which is submucosal; and (ii) external hemorrhoidal plexus, which is subcutaneous.
Histological analysis of the hemorrhoidal plexus shows clear predominance of a venous (cavernous) component. Veins have thick walls composed of an endothelium lined with collagen fibers. This fibrous layer comprises a framemade of elastic fibers and is not associated with the mucosa. The veins form vascular “lakes” representing fusiform, sacciform, or serpiginous extensions with a structure resembling the structure of veins in cavernous bodies. There are also thin-walled capillaries consisting of simple endothelium surrounded by sparse collagen fibers, and they are hardly distinguishable from the surrounding connective tissue membrane, which has no elastic frame. These capillaries run between the mucous membrane and the cavernous bodies. Obviously, these tissues have independent vascularization. Another feature of hemorrhoidal plexuses is arteriovenous anastomoses (shunts).6 Their presence helps to explain arterialization of the venous blood contained in the cavernous bodies. It is also supposed that anastomoses allow for a more rapid filling of cavernous bodies during arterial blood inflow, observed immediately after defecation.
Therefore, the presence of hemorrhoidal tissue with dilated venous structures (caverns) in the anal canal is a normal situation. These veins are characterized by a particular structure and have no valves.
The available evidence strongly suggests that the function of the internal sphincter itself is not sufficient to ensure complete closure of the anal canal. Internal hemorrhoids play an important role to keep intestinal contents in the rectum. At rest, vascular “lakes” (caverns) are filled with blood and are in contact with each other; as a result, the pressure in the anal canal is only 15% of the pressure in hemorrhoids.7 During defecation, longitudinal anal muscles woven with the submucousal layer contracts, which squeezes and moves the internal hemorrhoidal structures toward the internal sphincter, which then relaxes.8 The contraction of the longitudinal anal muscle leads to a shortening of the anal canal, devastation of the hemorrhoidal plexus, and inversion of the anal margin. After defecation, hemorrhoids are not under pressure anymore and gradually begin to fill with blood. This process is facilitated by the opening of precapillary sphincters, which enable blood flow through arteriovenous shunts. The volume of hemorrhoidal plexuses rise causing the anal canal to close and the pressure in the sphincter to increase.
What factors lead to the transformation of this normal physiological process into a pathological one? The generally accepted pathological mechanism of the development of clinical symptoms of HD is that hemorrhoids increase in size and become displaced with time. As a result, lesions occur in the mucous membrane that lines the hemorrhoidal plexuses, which causes bleeding from the rectum.
Research from many authors have demonstrated that hemodynamic and degenerative factors are the main causes of hemorrhoid development.3 Hemodynamic factors are represented by vessel dysfunction, which provides blood inflow and outflow in cavernous formations, and results in their overflow, thereby contributing to the abnormal increase of hemorrhoids. The development of dystrophic processes in the common longitudinal muscle of the submucosal layer of the rectum and the Parks ligament, which holds the cavernous bodies in the anal canal, results in a progressive and irreversible hemorrhoid displacement (prolapse).
Current concepts on the HD pathogenesis unalterably include venous components of hemorrhoids as one of the major substrates of the disease. Increase in the pressure in hemorrhoidal plexuses and their venous structures (corpora cavernosa) leads to lesion formation in the vascular wall, which accounts for the occurrence of consequences such as: (i) inflammation processes; (ii) swelling of the perivascular connective tissue; (iii) hemorrhoid thrombosis; and (iv) local arterial bleeding.
Doctors should direct their efforts, if possible, toward the above mentioned pathological mechanisms to more effectively relieve these manifestations. Therefore, we paid considerable attention to the drug containing micronized purified flavonoid fraction (MPFF)*. This oral venoactive drug, which consists of 90% micronized diosmin and 10% flavonoids expressed as hesperidin, diosmetin, linarin, and isorhoifolin, has been shown to effectively reduce symptoms of HD. Several randomized double-blind placebo-controlled trials have evaluated the efficacy of oral MPFF in the management of acute internal hemorrhoids.9-12 It is acknowledged that MPFF counteracts pathological mechanisms of HD at an early stage of clinical manifestation and has a positive impact on the local hemodynamic component due to normalization of venous tone in hemorrhoidal plexuses,13,14 a reduction in excessive capillary permeability and capillary walls fragility,14,15 an improvement in lymphatic drainage,16 and an inhibition of local inflammation processes.14,17,18
Patients who continue to have symptoms of acute internal hemorrhoids while receiving medical treatment may require instrumental or surgical treatment.14 Surgical treatment is required in a small fraction of patients (5% to 10%) with chronic external or internal hemorrhoids.19 The addition of MPFF to surgical procedures appears to be cost effective. MPFF may reduce the duration and/or intensity of post-hemorrhoidectomy symptoms20-23 and reduce patient recovery time. Hemorrhoid grade was not known in the previous trials.
The aim of the present trial is to know whether the association of MPFF with surgery for hemorrhoid stages II to IV may be more effective at reducing post-hemorrhoidectomy symptoms.
MATERIALS AND METHODS
For patients who came in for outpatient consultation, MPFF was prescribed at 2 tablets daily for 30 days before the surgery in the treatment group (MPFF treatment group). In the postoperative period, all these patients received 2 MPFF tablets twice daily for 4 days, and then 2 MPFF tablets twice daily for 3 days. The control group was comprised of 24 patients who were not treated with MPFF before surgery.
Patients with stage II HD underwent hemorrhoid dearterialization using the A.M.I. HAL-Doppler device. Patients with stage III to IV HD underwent Milligan- Morgan hemorrhoidectomy in the 2nd modification by the Institute of Coloproctology.
Statistical analysis. Comparison was made between the results of the 2 groups (MPFF-treated group and control group) using a Student t test. The results were considered significant at P
RESULTS
A total of 56 patients underwent surgery for stage II to stage IV chronic HD in the Department of abdominal surgery at the N.N. Burdenko Main Military Clinical Hospital during 2008 to 2011; 35 were males and 21 were females. The age of the patients ranged from 26 to 65 years. Median age was 58.5 years. Fifteen patients were diagnosed with stage II HD, 32 patients with stage III HD, and 9 patients with stage IV HD. The most frequent reason for seeking medical advice was rectal bleeding, and then, prolapse of internal hemorrhoids. All patients in either group presented with rectal bleeding, pain, and prolapse.
Reduction of postoperative symptoms at day 2 following the procedure was more pronounced in the MPFF treatment group compared with the control group (Table I). Compared with the control group, the stage II HD patients of the MPFF treatment group no longer had postsurgery symptoms at day 2. This was significant for both bleeding and prolapse (P=0.01), but not pain (P=NS). Symptoms were present in stage III HD patients at day 2 after surgery, which was significantly less in the MPFF-treatment group compared with the control group (P<0.02). Reduction of symptoms in stage IV HD patients was slightly better in the MPFF-treatment group compared with the control group, but the difference between groups was not significant except for bleeding (P = 0.008) (Table I). No allergic reactions were observed.
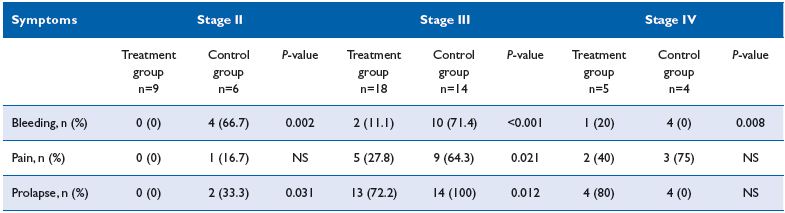
Table I. Post-surgery symptoms in each group at day 2 after intervention.
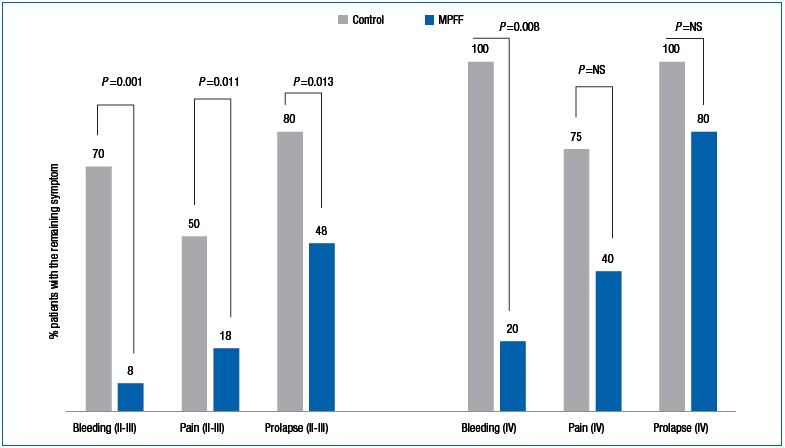
Figure 1. Comparing remaining postsurgery symptoms between groups in patients with stages II+III and stage IV hemorrhoidal disease.
We noticed that in the MPFF-treatment group, not only were symptoms reduced, but also the need for analgesics, anti-inflammatory drugs, and hemostatic agents were reduced, which may decrease the total cost of treatment substantially. Figure 1 shows the comparison of the results (presence of post-surgery symptoms) between groups in stages II + III and stage IV HD patients.
In stage III to stage IV HD control group patients, duration of wound healing lasted between 3 and 5 days, and in most cases we failed to remove all the hemorrhoids in a single operation due to the advanced stage of disease and high risk of postoperative complications.
CONCLUSION
The role of the venous component in the pathogenesis of HD should not be ignored, as well as its involvement in the occurrence of post-surgery symptoms. The early relief of symptoms when associating MPFF with HD surgery may reduce the duration of the hospital stay. In addition, the reduction in the need for analgesics, anti-inflammatory drugs, and hemostatic agents not only may decrease the total cost of treatment, but may also positively influence the psychoemotional status of the patients. Therefore, we recommend the use of MPFF in association with HD surgery.

REFERENCES
1. Leff E. Hemorrhoids. Postgrad Med. 1987;82:95-101.
2. Runzi M, Canbay A, Niebel W, et al. Hamorrhoiden und Analfissuren – das verborgene Leiden. Notfallmedizin. 2000;26:502-507.
3. Vorob’ev G.I. et al. Essentials of coloproctology, Medical news agency. 4000, Moscow. 2006
4. Riss S, Weiser FA, Schwameis K, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27(2):215-220.
5. Stock C, Haug U, Brenner H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastrointest Endosc. 2010;71(2):366-381.
6. Parnaud E, Guntz M, Bernard A, et al. Anatomie normale macroscopique et microscopique du reseau vasculaire hemorroidal [Normal macroscopic and microscopic anatomy of the hemorrhoidal vascular network]. Arch Fr Mal App Dig. 1976;65:501-514.
7. Lestar B, Penninckx F, Kerremans R. The composition of anal basal pressure. An in vivo and in vitro study in man. Int J Colorectal Dis. 1989;4:118-122.
8. Loder PB, Kamm MA, Nicholls RJ, et al. Haemorrhoids: pathology, pathophysiology and aetiology. Br J Surg. 1994;8:946-954.
9. Godeberge P. MPFF at a dose of 500 mg in the treatment of hemorrhoidal disease: a demonstrated efficacy in comparison with placebo. Angiology. 1994;45:574- 578.
10. Cospite M. Double-blind, placebocontrolled evaluation of clinical activity and safety of MPFF at a dose of 500 mg in the treatment of acute hemorrhoids. Drugs Today 1995;31:49-55.
11. Misra MC, Parshad R. Randomized clinical trial of micronized flavonoids in the early control of bleeding from acute internal haemorrhoids. Br J Surg. 2000;87:868-872.
12. Jiang ZM, Cao JD. The impact of micronized purified flavonoid fraction for the treatment of acute hemorrhoidal episodes: a prospective, randomized, double blind, placebocontrolled trial in 90 cases. Curr Med Res Opin. 2006;22:1141-1147.
13. Juteau N, Bakri F, Poimes JP, et al. The human saphenous vein in pharmacology: effect of a new micronized flavonoidic fraction (MPFF at a dose of 500 mg) on norepinephrine induced contraction. Int Angiol. 1995;14:8-13.
14. Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
15. Bouskela E, Donyo KA. Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/ reperfusion in the hamster cheek pouch. Angiology. 1997;48:391-399.
16. McHale NG, Hollywood MA. Control of lymphatic pumping: interest of MPFF at a dose of 500 mg. Phlebology. 1994;9:23-25.
17. Bergan JJ, Schmid-Schonbein GW, Coleridge-Smith PD, et al. Mechanisms of disease: chronic venous disease. N Engl J Med. 2006;355:488-498.
18. Korthuis RJ, Gute DC. Postischemic leukocyte/endothelial cell interaction and microvascular barrier dysfunction in skeletal muscle. Cellular mechanisms and effect of MPFF at a dose of 500 mg. Int J Microcirc Clin Exp 1997;17:11- 17.
19. American Society of Colon and Rectal Surgeons. Practice parameters for the treatment of hemorrhoids [online]. Available from URL: http://www.fascrs. org/ascrspp-toh.html [Accessed 2013 Nov 14].
20. Ho YH, Foo CL, Seow-Choen F, et al. Prospective randomized controlled trial of a micronized flavonidic fraction to reduce bleeding after haemorrhoidectomy. Br J Surg. 1995;82:1034-1035.
21. Ho YH, Tan M, Seow-Choen F. Micronized purified flavonidic fraction compared favorably with rubber band ligation and fiber alone in the management of bleeding hemorrhoids: randomized controlled trial. Dis Colon Rectum. 2000;43:66-69.
22. Dimitroulopoulos D, Tsamakidis K, Xinopoulos D, et al. Prospective, randomized, controlled, observerblinded trial of combined infrared photocoagulation and micronized purified flavonoid fraction versus each alone for the treatment of hemorrhoidal disease. Clinical Therapeutics. 2005;27:746-754.
23. La Torre F, Nicolai AP. Clinical use of micronized purified flavonoid fraction for treatment of symptoms after hemorrhoidectomy: results of a randomised, controlled, clinical trial. Dis of Colon and Rectum. 2004;47:704- 710.
