Benefits of MPFF in combination with sclerotherapy
Medical University (RNRMU), Moscow
Abstract
This article presents the results of using micronized purified flavonoid fraction for preventing adverse events after sclerotherapy of reticular veins and telangiectases. Based on experimental and clinical studies, the author concludes that administration of micronized purified flavonoid fraction in a daily dose of 1000 mg for the period of the sclerosing treatment significantly reduces the severity of local vein-specific inflammation and the rates of associated typical adverse events after sclerotherapy.
Introduction
Sclerotherapy, despite its long history, remains one of the most commonly used procedures for eliminating dilated intradermal and varicose veins. Such a great popularity of sclerotherapy is explained by high rates of complaints related to the dilated reticular veins and telangiectases on the one hand and the relative simplicity of this procedure and optimal price-quality ratio on the other hand.1-2
Mechanism of sclerotherapy
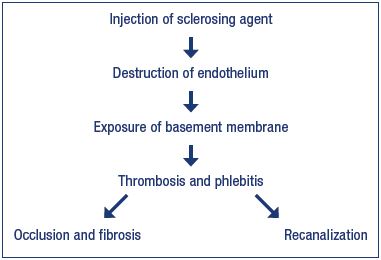
In sclerotherapy, agents with different modes of action (detergents, hyperosmotic, and corrosive agents) are used for the destruction, in one way or another, of the endothelium and creating conditions for the fast parietal thrombosis resulting in ablation and fibrosis of the target vein. In particular, a chain of sequential events taking place during sclerotherapy of a vein of any caliber includes chemical injury or burn to the endothelium, exposure of the collagen-rich basement membrane, thrombus formation, obliteration followed by fibrosis or recanalization of the target vein (Figure 1). Moreover, these processes occur regardless of the type of sclerosing agent, route of its administration, presence or absence of compression of the target vein, and other factors.
Obviously, the endothelial lesion caused by a sclerosing agent, with the formation and evolution of a thrombus, is accompanied by an inflammatory reaction, the severity of which is determined by a number of factors. The most important of them are caliber, location and length of the target vein, type of sclerosing agent and its aggregate form (liquid or microfoam), and the presence and level of compression.
Adverse events after sclerotherapy
Sclerotherapy demonstrates high efficacy in eliminating reticular veins and telangiectases. However, fast and reliable ablation of target veins is accompanied by various adverse reactions, the most frequent of which are ecchymosis, hyperpigmentation, and neovascularization (matting), with the total incidence of 10% to 30% or more.3 The cause of the development of ecchymosis is a mechanical and chemical lesion to the vessel wall in combination with the anticoagulant effect of sclerosing detergent solutions.
Routine prevention of ecchymosis includes the use of thin needles, slow injection of the sclerosing agent, which prevents hydraulic rupture of the target vein, and immediate external compression. In addition to these measures, the precooling of the sclerosing agent and syringe, as well as the use of external cooling of the sclerotherapy area using a thermal gel or a jet of cold air from special generators also prevents the formation of ecchymosis. To speed up the resorption of ecchymosis, various local treatments based on heparin and venoactive drugs are commonly used.
Hyperpigmentation is caused by penetration of hemoglobin into paravasal tissues, where it is converted into the dark pigment hemosiderin, which gives the skin a brown or reddish brown color of varying intensity. With the natural desquamation of the epithelium, hyperpigmentation gradually disappears; however, this process can last for several months to a year or more. It is obvious that hyperpigmentation after sclerotherapy, performed according to cosmetic indications, significantly reduces the patient’s quality of life, and sometimes the newly acquired cosmetic defect exceeds the problems associated with dilated veins. General recommendations for the prevention of hyperpigmentation include careful performance of sclerotherapy, use of adequate concentrations of the sclerosing agent, prolonged compression, and timely removal of coagula. For the treatment of persistent hyperpigmentation, various methods of medical and laser peeling or masking cosmetics are used.
Neovasculogenesis with the formation of small red intracutaneous vessels (matting) in the sclerotherapy area is associated with the development of local hypoxia, leading to the activation of vascular endothelial growth factor (VEGF) and other vasoactive substances. For the prevention of matting, it is proposed to use low concentrations of detergents or hyperosmotic sclerosing agents. Usually, matting disappears spontaneously within a few months after sclerotherapy. In case of persistent matting, repeated sclerotherapy or percutaneous laser coagulation is used.
Hyperpigmentation and matting are often preceded by phlebitis of a sclerosed vein. Treatment of hyperpigmentation and matting can be time consuming and require significant additional costs, which is why the search for new methods of preventing adverse events after sclerotherapy is highly relevant.
The role of MPFF in the prevention of adverse events after sclerotherapy
A number of experimental and clinical studies has shown that micronized purified flavonoid fraction (MPFF) has a pluripotent mode of action, the main components of which are an increase in tolerance of the venous wall to mechanical damage, suppression of vein-specific inflammation with a reduction in leukocyte activity and a proinflammatory endothelium phenotype. These features make it possible to actively use MPFF to reduce the incidence of adverse reactions during stripping of varicose veins. Earlier studies have shown that administration of MPFF in the perioperative period significantly reduces the severity of pain, intensity, and duration of ecchymosis, and it prevents posttraumatic edema.
The effects of adjuvant therapy with MPFF during sclerotherapy have been evaluated in several studies. In an experimental study, 22 rabbits were allocated into two groups of 11 animals (or 22 ears) each. The study group received MPFF at a dose of 300 mg/kg/day (2 mL/kg of body weight of the working solution) starting 7 days before the procedure. The control group received a similar volume of 10% lactose as a placebo. After preliminary local application of anesthesia with 5% prilocaine, 5% ethanolamine oleate was injected into the dorsal vein of the rabbit ear. The diameters of venules and arterioles, functional capillary density, microvascular permeability, as well as severity of rolling and leukocyte adhesion to the endothelium were evaluated at 24 hours and 8 days after sclerotherapy.4
The increase in diameter of venules and arterioles was found to be significantly lower in the MPFF group compared with the control group. The decrease in the number of functional capillaries occurred in both groups, but was greater in the control group. During the first 2 and 24 hours, the abnormal microvascular permeability was significantly lower in the study group. However, 8 hours after the injection of the sclerosing agent, there were no significant differences in the microvascular permeability between the study and control groups. The leukocyte-endothelial reaction at 2 hours after sclerotherapy in the study group was significantly less prominent. After 24 hours, it was impossible to assess the severity of rolling and leukocyte adhesion in the control group due to severe edema. After 8 days, the number of rolling and adherent leukocytes in the study and control groups was comparable. The photography of the rabbit ears performed on day 14 showed an occlusion and partial disappearance of the dorsal vein in the MPFF group and a persistent paravasal inflammatory process in the control group.
In a clinical study, 60 female patients with reticular veins and telangiectases (CEAP clinical class C1) located on the lateral side of the thighs were divided into 2 groups of 30 people each. In the main group, MPFF at a daily dose of 1000 mg was prescribed 2 weeks before the scheduled sclerotherapy and continued for 2 months after the procedure. Prior to the injection of sclerosing agent into the target intracutaneous vessels, blood was taken with a vacutainer from the “central” vein for measuring the levels of high-sensitivity C-reactive protein (hsCRP), histamine, interleukin 1 (IL-1), tumor necrosis factor α(TNF-α), and VEGF. To monitor the systemic inflammatory response, blood from a forearm vein was taken in 15 patients of the control group.5 Sclerotherapy was performed using the standard method and with the same sclerosing agent (0.2% Fibrovein or 0.5% aethoxysklerol). The repeated blood sampling from the “central” vein was carried out at day 10 and followed by sclerotherapy of this vein using the agent in a higher concentration.
In blood samples obtained from the “central” vein of the target vascular cluster before microsclerotherapy, there were no statistically significant differences between the main and control groups in the basal levels of inflammatory and endothelial dysfunction markers. In the blood samples obtained from the “central” vein on day 10 postsclerotherapy, a statistically significant increase in the levels of key markers of endothelial damage was recorded. At the same time, in blood samples obtained from a forearm vein, the levels of proinflammatory cytokines before and after sclerotherapy were not different. Therefore, standard doses of low-concentration sclerosing agents caused only a local proinflammatory response, and the markers of this response deserve special discussion.
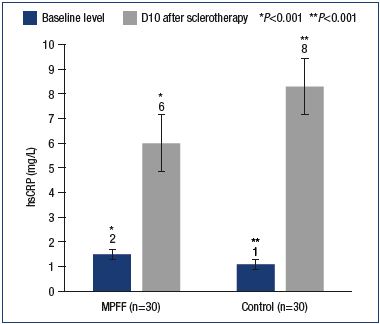
hsCRP stimulates a number of proinflammatory cytokines, such as IL-1, TNF-α, and especially interleukin 6 (IL-6). CRP is involved in the activation of complement (a group of proteins that are part of the immune system), monocytes and stimulation of the expression of the adhesion molecules ICAM-1, VCAM-1, and E-selectin on the surface of the endothelium. According to recent studies, persistent inflammation in the vein wall leads to the development of varicose fibrosis. The normal CRP value is 1.0 mg/L. In our study, with similar baseline levels in the main and control groups, a significant local increase in CRP was observed on day 10 postsclerotherapy, which was greater in the control group (6.0±0.9 mg/L vs 8.3±1.0 mg/L). The differences are significant with a P value <0.001 (Figure 2).
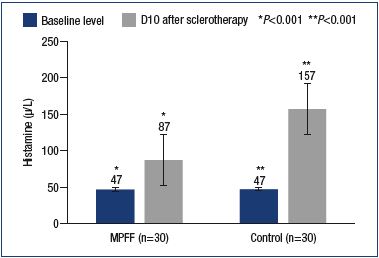
Another proinflammatory agent produced due to the endothelial-leukocyte interaction is histamine. Histamine causes a variety of systemic and local reactions. In relation to sclerotherapy, a local increase in histamine levels leads to an increase in the permeability of the vascular wall and the development of edema. On day 10 postsclerotherapy, a significant (P<0.001) increase in local histamine levels was observed in the main and control groups compared with the baseline values (87.0±9.8 μg/L vs 156.9±33.9 μg/L). At the same time, with comparable baseline values of histamine, in patients in the main group, the histamine level was almost 2 times lower in comparison with the control group (Figure 3).
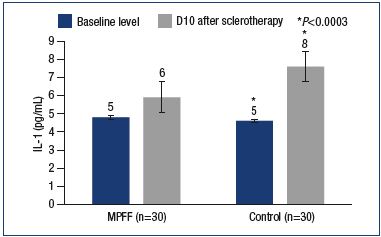
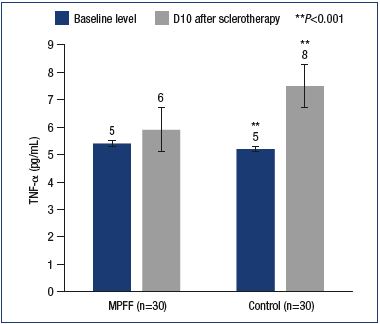
MPFF provided a reduction in the level of IL-1. IL-1 is one of the important factors for the activation of leukocytes and macrophages, as well as the stimulation of the development of venous thrombosis and thrombophlebitis. In this study, the IL-1 levels significantly increased in the main and control groups, reaching 5.9±0.4 pg/mL and 7.6±0.6 pg/mL, respectively (Figure 4). Meanwhile, in the main group, the IL-1 level was significantly lower than in the control group (P<0.0003). Inhibition of the expression of certain interleukins is a feature of MPFF that has been shown in vitro and in animal experiments. TNF-α is produced by activated macrophages and largely duplicates the actions of IL-1. In particular, TNF-α activates leukocytes, dramatically increasing the formation of hydrogen peroxide and other free radicals by macrophages and neutrophils. High levels of TNF-α have been associated with adverse effects of sclerotherapy, such as phlebitis, thrombophlebitis, and skin necrosis. After sclerotherapy, an increase in TNF-α levels was reported in both the main and control groups (5.9±0.9 pg/mL vs 7.5±0.4 pg/mL). However, compared with the control group with a significant increase in TNF-α level versus baseline (P<0.001), no significant differences versus both baseline and induced levels were observed in the main group (P=0.49) (Figure 5).
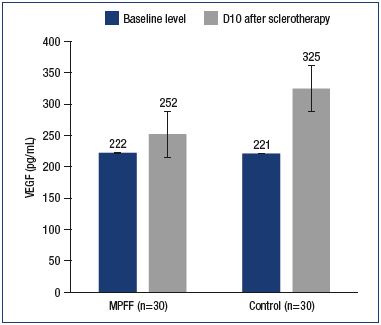
Various impairments in neovasculogenesis have been associated with an increase in the VEGF levels. In the early stage after sclerotherapy, an increased expression of VEGF stimulates the occurrence of matting, while, in the late stage, it, presumably, causes the recurrence of telangiectases. An increase in VEGF levels in response to sclerotherapy was reported in both the main and control groups (to 252.3±26.0 pg/mL and 325.1±47.7 pg/mL, respectively). At the same time, in contrast to the control group, the increase in VEGF level in the main group was not significant (P=0.5) (Figure 6).
The assessment of MPFF efficacy was carried out based on the rates of adverse events (Table I). There where significantly less ecchymoses, phlebitis, hyperpigmentation and neoangiogenesis in patients treated with MPFF when compared with the control group.
The rationale for the MPFF use in routine clinical practice was demonstrated in the observational program Vein Act Pro-C1, with participation of 70 doctors from various regions of the Russian Federation who included 1150 patients (79 men and 1071 women) with chronic venous disease of CEAP class C1s who were undergoing sclerotherapy.6 In a proportion of patients, the doctors prescribed MPFF at a daily dose of 1000 mg at their own discretion. The patients started to take MPFF 2 weeks before the scheduled sclerotherapy and continued it for the next 6 weeks after the procedure. The primary efficacy end point for the MPFF treatment was the rate of adverse reactions in patients in the study and control groups at 4 weeks after the completion of sclerotherapy (Table II). There was significantly less pigmentation in patients treated with MPFF at 60 days when compared with the control group.
Discussion
The main mechanism of sclerotherapy, regardless of the type of sclerosing drug, is the irreversible lesion of the endothelium of the target vein. At the same time, an acute local vein-specific inflammatory reaction develops, which is accompanied by the synthesis of various proinflammatory cytokines and leukocyte activation. It is obvious that the cause of adverse events after sclerotherapy is extravasation of inflammation with damage to the paravasal tissues.
Based on the presented experimental and clinical data, the beneficial effects of MPFF during sclerotherapy may include prolongation of the noradrenergic activity of smooth muscle fibers, preventing dilatation and hydraulic rupture of the target vein, which increases the efficacy of the sclerosing agent and reduces the likelihood of leukocyte and erythrocyte extravasation. MPFF reduces the severity of rolling and leukocyte adhesion, which prevents the occlusion of microvasculature vessels and reduces local hypoxia. Due to the nonspecific antihistamine activity of MPFF, vascular wall permeability decreases, preventing local edema and extravasation of erythrocytes, along with the development of ecchymosis and hyperpigmentation.
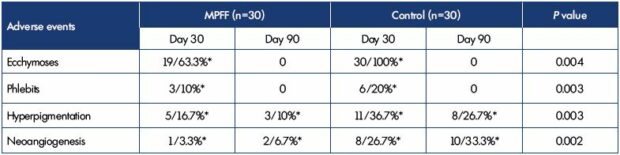
Table I. Rates of adverse events occurred in patients treated and not treated with MPFF during the sclerotherapy.
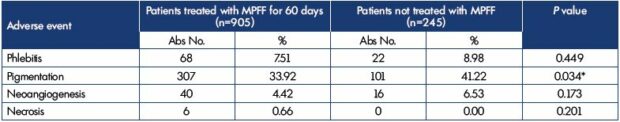
Table II. Comparative evaluation of adverse reactions for phlebosclerosing treatment with or without MPFF.
MPFF is able to maintain the functional capillary density, the reduction of which is accompanied by a violation of soft tissue metabolism and increased inflammation with possible skin necrosis. MPFF reduces the synthesis of VEGF and provides better oxygenation of the interstitium around the sclerosed vein, which prevents the development of matting. Suppression of VEGF and TNF-α synthesis in patients receiving MPFF can prevent the recurrence of reticular veins and telangiectases in the future.
Analysis of the clinical outcomes of using MPFF during sclerotherapy on reticular veins and telangiectases has shown a reduction in the rates of most typical adverse effects, such as ecchymosis, matting, phlebitis, and hyperpigmentation. At the same time, both of these clinical studies have reported a significant decrease in the rate of hyperpigmentation after sclerotherapy. As for the discrepancy in the significant reduction in the rate of other adverse events noted in the above-mentioned clinical studies, this fact can be explained by a greater heterogeneity of the clinical observational study Vein Act Pro-C1. At the same time, a clear trend toward a reduction in the rate of all adverse events after sclerotherapy, noted in the Vein Act Pro-C1 study, suggests the efficacy of MPFF in sclerotherapy of the dilated reticular veins and telangiectases.
Conclusion
Due to the complex and unique mode of action, MPFF is able to reduce the severity of vein-specific inflammation associated with the effects of sclerosing agents and prevent extravasation of the agent. This effect of MPFF provides a reduction in the rate of typical adverse events, such as the formation of ecchymosis, hyperpigmentation, and matting, with no negative influence on the time and quality of ablation of the target vein. Thus, the appointment of an intraoperative positioning system during sclerotherapy of reticular veins and telangiectases can be recommended for routine clinical practice. Therefore, the MPFF could have a role in preventing adverse events after sclerotherapy in routine clinical practice settings.

REFERENCES
1. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP Coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31(2):105-115.
2. Rabe E, Breu F, Cavezzi A, et al; Guideline Group. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338- 354.
3. Reina L. How to manage complications after sclerotherapy. Phlebolymphology. 2017;24(3):130-143.
4. de Souza MD, Cyrino FZ, Mayall MR, et al. Beneficial effects of the micronized purified flavonoid fraction (MPFF, MPFF at a dose of 500 mg) on microvascular damage elicited by sclerotherapy. Phlebology. 2016;31(1):505-506.
5. Bogachev V, Boldin B, Lobanov V. Benefits of micronized purified flavonoid fraction as adjuvant therapy on the inflammatory response after sclerotherapy. Int Angiol. 2018;37(1):71- 78.
6. Bogachev V, Boldin B, Turkin P. Administration of micronized purified flavonoid fraction during sclerotherapy of reticular veins and telangiectasias: results of the national, multicenter, observational program VEIN ACT PROLONGED-C1. Adv Ther. 2018;35(7):1001-1008.