Benefits of MPFF on primary chronic venous disease-related symptoms and quality of life: the DELTA study
2 Belarusian State University of Medicine
3 Vitebsk State University of Medicine
4 Brest Regional Hospital
Abstract
Aim: To assess the benefits of MPFF treatment on symptomatic chronic venous disease (CVD) patients, in terms of symptom improvement, amelioration of daily activity, quality of life (QOL), patient satisfaction, and tolerability in the framework of venous oriented consultations.
Methods: Male or female patients consulting a venous specialist for symptomatic chronic venous disease (CVD), aged over 18 years, not having ongoing treatment for CVD, not consulting for an emergency or for the acute episode of an ongoing event, free of concomitant diseases that might interfere with venous treatment, informed of their involvement in the program and agreeing to take part were enrolled in the trial and started MPFF treatment, 2 500 mg tablets per day for 2 months. Patient’s clinical presentation, presence of CVD signs and/or symptoms, and QOL score using CIVIQ were reported at the selection visit and at the 2- and 6-month follow-up visits. Side effects, if any, were also reported.
Results: The most significant regression of the disease-related symptoms was reported at the end of the 2nd month of MPFF treatment, with a 76% decrease in patients with night cramps, -75% in those with itching, -66% in patients with pain along the vein, -81% with feeling of burning, -66% with swelling, -59% with leg pain, and -38% with feeling of leg heaviness. All of these changes were significant (P<0.01). In the meantime, the percentage of patients with edema was reduced from 42% to 36%. The global index score (GIS) equaled 32.9±21.0 at baseline and decreased to 14.6±14.7; P<0.0001, after 2 months, reflecting the patients’ QOL improvement after treatment. 94% of patients and 96% of physicians assessed MPFF efficacy as high and very high. The most common adverse events with MPFF were gastrointestinal.
Conclusion: A 2-month treatment with MPFF, 2 tablets per day, significantly reduces the frequency of many CVD symptoms and improves patients’ QOL.
Introduction
Chronic venous disease (CVD) of the lower limbs is an umbrella term that encompasses a wide spectrum of pathology. 1,2 CVD is defined as long-lasting morphological and functional abnormalities of the venous system manifested by symptoms (tingling, aching, burning, pain, muscle cramps, swelling, sensations of throbbing or heaviness, itching skin, restless legs, leg tiredness and/or fatigue) with or without signs indicating the need for investigation and/or care.3
Chronic venous disease (CVD) is a major cause of morbidity in the Western world, comprising medical, social, and economic aspects.4-7 In the European adult population, the prevalence of varicose veins has been estimated to be 25% to 50% for all types and degrees of varicosities, 10% to 15% for marked varicose veins, and 5% to 15% for chronic venous insufficiency (CVI).4-6 Recent surveys have sought the prevalence of venous symptoms either in the general population (ie, 3072 people of Bonn)8 or in consecutive subjects consulting their general practitioner for any medical reason (ie, 40 095 Polish adults9 and 91 545 subjects in 20 countries of the Vein Consult Program [VCP]).10 The results showed that 49% of the male and 62% of the female population of Bonn had leg complaints related to symptoms of venous diseases (eg, heaviness, sensation of swelling).8 Leg complaints were reported in up to 81% of the varicose veins group and up to 35% of the varicose-free patients in Poland,9 and almost 80% of the VCP subjects reported venous symptoms.10 Such studies show that CVD is a global phenomenon not solely limited to the western countries, as previously thought.
Daily activities and quality of life (QOL) are worse in patients suffering from CVD.11,12 The QOL impairment associated with venous edema has been shown to be equal to the QOL impairment in cancer and diabetes, and the impairment in venous ulcer patients is equal to that of heart failure.12 Initially, the progression of CVI is related to venous hypertension. The earliest complaints or symptoms, as well as vessel wall deterioration, valve restructuring, and, eventually, varicose veins, result not only from elevation of pressure, but also from a cascade of biochemical and inflammatory events related to both the macro- and microcirculation. Thickening and remodeling of the venous wall are influenced by venous inflammation. The subsequent capillary leakages and lymphatic overload resulting from higher than normal venous pressures are part of the disease.1,13
Micronized purified flavonoid fraction (MPFF*), which consists of 90% micronized diosmin and 10% flavonoids (hesperidin, diosmetin, linarin, and isorhoifolin),14 has been shown to effectively reduce symptoms related to CVD and improve the QOL of CVD patients.13,15,16 This would result from the ability of MPFF to increase venous tone, to reinforce capillary resistance, and to improve lymphatic drainage in humans and animals. The anti-inflammatory effect of MPFF has been demonstrated in a number of studies.16 The comprehensive mechanism of action of MPFF, in addition to its micronized form, provides an explanation for its clinical efficacy at all stages of venous disease.17 Therefore, MPFF may improve major symptoms of CVD and was chosen for this trial.
Aim of the program
In a first step of this program, the detection of ambulatorycare patients in specialized consultations presenting with CVD–related symptoms and signs was enhanced. This program helped widen the scope of management of symptomatic CVD patients through the assessment of leg symptoms and characterization of their venous origin, using a validated, simple symptom screening questionnaire relevant to the clinical practice. For reporting venous signs, the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification was used. This also contributed to better appraisals of the actual prevalence of CVD symptoms and signs in daily venous practice. Results of the first step have already been published.18
The aim of the present analysis was to assess the effects of MPFF treatment on symptomatic CVD patients, in terms of symptom improvement, amelioration of daily activity and QOL, patient satisfaction, and tolerability of the MPFF treatment in the framework of venous oriented consultations.
Material and methods
The survey was organized within the framework of specialized consultations in 37 healthcare facilities in the Belarusian State, and was surveyed and coordinated by venous specialists. Patients were selected from among those who complained of symptoms in the lower limbs and who consulted a venous specialist because of clinical presentations related to CVD. The suitability of the patients for involvement in the program was assessed using set criteria: (i) women or men over 18 years old (not having ongoing treatment for CVD); (ii) informed of their involvement in the program and agreeing to take part; (iii) informed that they have the right to refuse to participate fully or partly; (iv) not consulting for an emergency or for an acute episode of an ongoing event; and (v) free of concomitant diseases that might interfere with venous treatment.
If these criteria were met, patients were asked about venous signs and symptoms, then underwent a leg examination, and a case report form was completed with the following information: patient’s clinical presentation, presence of CVD signs and/or symptoms, QOL score at the selection visit by using the patients’ self-reported specific ChronIc Venous dIsease quality of life Questionnaire (CIVIQ),19 and mention of the MPFF treatment prescribed (ie, at the dose of 1000 mg per day, meaning 2 tablets of 500 mg daily, for 2 months). CIVIQ is made of 4 dimensions: physical, psychological, social, and pain. In our analysis, the index 0 represented the best QOL score and 100 the worst score.
Patients were advised to come for two follow-up visits, the first one scheduled at month 1 and the second one at month 2 of MPFF treatment. In the follow-up consultations, the effect of treatment was assessed on symptoms, signs, and QOL scores together with patient satisfaction. Side effects, if any, were reported.
Statistical analysis
All patients included in the survey and complying with the selection criteria were included in the analyzed population. Excel software has been used for the statistical analyses. These were performed by pooling all the data from all of the centers. The prevalence of CVD by item (CEAP class and symptoms) was assessed. Descriptive statistics are presented as mean, standard deviation (SD), minimum, maximum for continuous data, and number of cases and percentages for categorical data. A student t test was used for the confidence estimation and nonparametric statistics (Wilcoxon test) was used for bidimensional analysis.
Results
The trial was conducted between April and August 2009 by 83 venous specialists. 557 patients were enrolled, but only 522 of the patients were in compliance with the protocol requirements and therefore were analyzed. There were 423 women and 99 men. The mean age of the studied population was 51.1±13.7 years (SD, 20 to 94 years).
CVD prevalence by CEAP stage
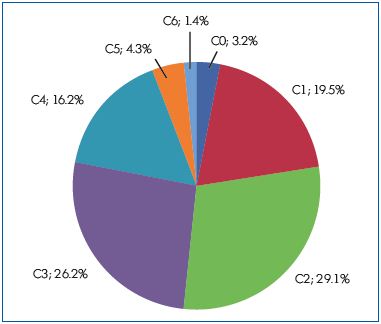
The distribution of patients according to the CEAP classification was as follows: C0, 3.2%; C1, 19.5%; C2, 27.5%; C3, 26.2%; C4, 16.2; C5, 4.3; C6, 1.4%. (Figure 1).
Symptoms prevalence
The most often encountered symptoms were in ranking order: heaviness (93.7%), leg pain (84.5%), sensation of swelling (75.5%), night cramps (65%), pain along the course of the vein (56.3%), itching (51.2%), and sensation of burning (49.2%).
Assessment of MPFF treatment on venous–related symptoms
Assessment of patients still complaining of venous symptoms (heaviness, leg pain, pain along the veins, sensation of swelling or burning, and night cramps) at 2 and 6 months after MPFF treatment (Figure 2). The most significant regression of the disease-related symptoms was registered at the end of the 2nd month of the MPFF treatment, with a significant decrease in the percent of patients with night cramps (-76.2%), itching (-74.8%), pain along the vein (-66.4%), feeling of burning (-81.3%) and swelling (-66.2%), leg pain (-59.1%). The less dynamic change was the feeling of leg heaviness which decreased by 38.4%. All changes were significant (P<0.01) at month 2. (Figure 2). In the meantime, the percentage of patients with edema was reduced after a 2 month treatment from 42% to 36%.
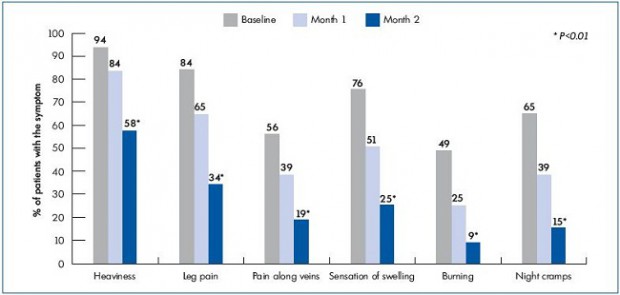
Figure 2. Percentage of patients complaining of venous symptoms at baseline, and at 2 and 6 month MPFF 500 mg treatment, 2 tablets daily.
Evaluation of the patients’ quality of life after treatment
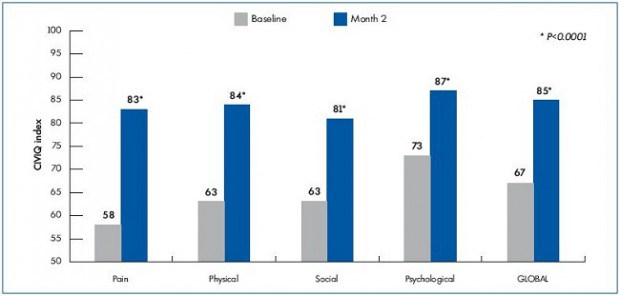
QOL was also improved as assessed by the global index score and by dimension. The global index score (GIS) equaled 67±21 at baseline and increased to 85±15; P<0.0001, after 2 months, reflecting the patients’ QOL improvement after treatment. This was also observed for the physical parameter of CIVIQ with an increase in score from 63±23 to 84±18, P<0.0001; the psychological score, from 73±22 to 87±15, P<0.0001; and the social and pain scores, from 63±27 to 81±21 and 58±20 to 83±15, respectively; P<0.0001). Results are illustrated in Figure 3.
Patients’ and physicians’ satisfaction
Among the patients who completed the 2-month course of treatment, a total of 493 patients (94.4%) assessed the treatment efficacy as “good” or “very good.” Twenty-seven patients (5.1%) assessed the treatment efficacy as satisfactory and only 2 patients noticed no improvement. 96.0% of the physicians assessed MPFF efficacy as high or very high.
Tolerability of treatment
MPFF was associated with a small incidence of adverse events (in 4.2% of cases). It was not possible to verify whether the incidence would have been similar to that seen with a placebo. The most common adverse events with MPFF were gastrointestinal, appearing after 2 to 3 days and disappearing at the end of treatment; one single case of urticarial was reported.
Discussion
Special emphasis is to be placed on the high percentage (77.3%) of patients with advanced stages of the disease (C2-C6) at baseline, which reflects the tendency that patients have of consulting their physician very late in the disease process. This corresponds with the results of the VCP that found that general practitioners (GPs) do not refer patients to a venous specialist before the C2 stage (varicose veins), which demonstrates the critical role GPs may have in the management of CVD.10 As concluded in the VCP, public awareness campaigns are needed to alert patients to contact their physicians for care. Educational programs are also needed so that primary care physicians recognize early cases of CVD.
The VCP symptom distribution by decreasing frequency was as follows: heavy legs, pain, sensation of swelling, night cramps, sensation of pins and needles, and burning and itching in the legs. The VCP symptom prevalence was similar to our study. Venous symptoms seem to be universally felt and cannot be seen as a cultural phenomenon reserved to restricted populations.
This trial confirms that CVD causes physical and psychological suffering for patients, not to mention the social handicap and the painful and unpleasant sensations they feel, which is reflected by a worse QOL.
Results of the present trial confirm the previous findings on MPFF regarding venous symptom alleviation and QOL amelioration. 11,15,16 Duration of MPFF treatment in most of the previous trials was 2 months. Our study shows that symptom relief occurs from the first month of MPFF treatment. In addition, the number of symptoms considered in our trial was extensive and concerned the 6 most often encountered symptoms, as identified in the Worldwide Vein Consult Program,<sup>10</sup> that is to say, heaviness, leg pain, pain along the veins, sensation of swelling and burning, and night cramps. The efficacy of MPFF treatment is particularly comprehensive considering that these symptoms are the most frequent reason for visiting a physician.
The improvement of pain and physical components of the CIVIQ leads to the restoration of patients’ usual everyday activities and to the improvement of their psychoemotional status, allowing them to feel more confident and actively participate in the social life. This is reflected in high satisfaction scores of MPFF treatment from both patients and physicians. Similar results were seen by Croatian angiologists.<sup>20</sup>
Conclusion
The intake of 2 tablets of MPFF per day for 2 months significantly reduces the frequency of many CVD symptoms. Simplicity of usage, universal nature of the standard dose, and good tolerance allow us to recommend MPFF for widespread usage in the everyday practice of physicians and general practitioners.
1. Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Mechanisms of disease, Chronic Venous Disease. N Engl J Med. 2006; 355:488- 498.
2. Nicolaides ANN, Allegra C, Bergan J, et al. Management of Chronic Venous Disorders of the Lower Limbs. Guidelines According to Scientific Evidence. Int Angiol. 2008; 27:1-59.
3. Eklof B, Perrin M, Delis KT, Rutherford RB, Gloviczki P; American Venous Forum; European Venous Forum; International Union of Phlebology; American College of Phlebology; International Union of Angiology. Updated terminology of chronic venous disorders: the vein term Transatlantic Interdisciplinary consensus document. J Vasc Surg. 2009;49:498-501.
4. Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175-184. 5. Robertson L, Evans C, Fowkes FGR. Epidemiology of chronic venous disease. Phlebology. 2008;23:103-111.
6. Fowkes FGR, Evans CJ, Lee AJ. Prevalence and risk factors of chronic venous insufficiency. Angiology. 2001;52:S5-S15.
7. McLafferty RB, Lohr JM, Caprini JA, et al. Results of the national pilot screening program for venous disease by the American Venous Forum. J Vasc Surg. 2007;45(1):142-148.
8. Rabe E, Pannier F. What have we learned from the Bonn Vein Study? Phlebolymphology. 2006;13:188-194.
9. Jawien A, Grzela T, Ochwat A. Prevalence of chronic venous insufficiency in men and women in Poland: multicenter cross-sectional study in 40 095 patients. Phlebology. 2003;18:110-121.
10. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP Coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31(2):105-115.
11. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of life improvEment with micronized Flavonoids. Angiology. 2002;53:245-256.
12. Andreozzi GM, Cordova RM, Scomparin A, Martini R, D’Eri A, Andreozzi F; Quality of Life Working Group on Vascular Medicine of SIAPAV . Quality of life life in chronic venous insufficiency. An Italian pilot study of the Triveneto Region. Int Angiol. 2005;24:272-277.
13. Katzenis K. Micronized Purified Flavonoid Fraction (MPFF)*: A review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol. 2005;3:1-9.
14. Paysant J, Sansilvestri-Morel P, Bouskela E, Verbeuren TJ. Different flavonoids present in the micronized purified flavonoid fraction (MPFF at a dose of 500 mg) contribute to its antihyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81-85.
15. Nicolaides AN. From symptoms to leg edema: efficacy of MPFF at a dose of 500 mg. Angiology. 2003;54:S33-S44. 16. Lyseng-Williamson A, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
17. Garner RC, Garner JV, Gregory S, Whattam M, Calam A, Leong D. Comparison of the absorption of micronized MPFF at a dose of 500 mg by accelerator mass spectrometry and liquid scintillation counting. J Pharm Sci. 2002;91:32-40.
18. Yanushko VA , Bayeshko AA , Sushkov SA, et al. Chronic venous diseases in outpatient population: risk factors and symptoms according to CEAP classification. Meditsinskaya panorama. 2010;4:40-43.
19. Launois R, Mansilha A, Jantet G. International psychometric validation of the chronic venous disease quality of life questionnaire CIVIQ-20. Eur J Vasc Endovasc Surg. 2010;40:783-789.
20. Lenkovic M, Stanic–Zgombic Z, Manestar– Blazic T, Brajac I, Perisa D. Benefit of MPFF at a dose of 500 mg in the reduction of chronic venous disease-related symptoms. Phlebolymphology. 2012;19(2):79-83.