Chronic cerebrospinal venous insufficiency: state of the art and research challenges
ABSTRACT
This review discusses a potential vascular basis for multiple sclerosis (MS). The idea that MS could be related to vascular disease is not new, with the first reports describing connections between veins and MS plaques dating from the 19th century. The venous abnormalities found in MS patients, the socalled chronic cerebrospinal venous insufficiency, are currently interpreted as congenital venous malformations. Catheter venography is regarded as the gold standard to assess these lesions, while other tests, such as Doppler sonography and magnetic resonance venography, are much less accurate. Importantly, a relationship between venous pathologies and MS has not yet been proven, especially regarding the causative role of compromised venous outflow in the pathogenesis of MS. Although the results of small open-label studies are promising and treatments have been found to be safe, welldesigned clinical trials will be needed to validate the efficacy of endovascular procedures of malformed veins for the treatment of MS.
MULTIPLE SCLEROSIS – A PARADIGM SHIFT?
This review discusses a potential vascular basis for multiple sclerosis (MS), an idea that has been hotly debated by both neurologists and vascular specialists.1-7 MS is a chronic neurological disease characterized by multifocal areas of inflammation, demyelination, and neurodegeneration within the central nervous system. The current ruling paradigm is that MS is an autoimmune disease, which means that it is caused by an autoimmune attack against nervous tissue, primarily against myelin antigens, a process carried out by reactive T cells. Therapeutic strategies predominantly target— unfortunately, not very effectively8-11—the inflammatory cascade or modify the immune response. However, a mechanism triggering this hypothetical autoimmune reaction remains elusive. For the time being, concepts regarding the pathophysiology of MS and mechanisms responsible for its initiation and progression, as well as the philosophy of its treatment, are all based on an animal model of the disease: experimental autoimmune encephalomyelitis (EAE). Yet, there are substantial differences in the size, timing, localization, and composition of the cellular infiltrate between MS and EAE. Although the EAE model has been widely accepted by neurologists, it actually poorly mirrors MS in humans. EAE, unlike MS, is an acute monophasic illness, and even the chronic subtypes of EAE contrast with human disease.12 The autoimmune origin of MS is undermined by the fact that no autoantigen responsible for MS immunity has been identified and by the lack of clonally expanded T cells.13-17 Moreover, although most of the current research on MS is focused on its autoimmune aspects, it should be stressed that during the last century, researchers had considered the roles of other potential factors (infectious, environmental, genetic and— importantly—vascular).
Italian vascular surgeon Paolo Zamboni has recently stridden into this murky milieu with his chronic cerebrospinal venous insufficiency (CCSVI) theory,18 which has managed to stoke the ongoing debate in the scientific community. The CCSVI paradigm claims that venous blockages have a primary role in initiating the immune reactions of MS. In addition, this hypothesis suggests that venous insufficiency may also contribute to MS pathology by causing chronic ischemia of the brain or iron toxicity.19,20
Zamboni insists that his CCSVI theory offers important insight into the pathophysiology of MS, a contention supported by some studies,21-25 while others vigorously challenge it.1,26,27 However, an hypothetical role for venous blockages in the pathogenesis of MS is not necessarily contrary to the currently accepted paradigm. It is possible that both vascular and immune mechanisms may play synergistic roles in pathology—similarly to chronic venous insufficiency of the lower extremities, where pathological venous flow is primarily responsible, but for which immune reactions seem to be an auxiliary mechanism leading to cellular infiltrates in the skin and, finally, to the development of venous ulcers.28,29
VASCULAR ETIOLOGY OF MS –
HISTORICAL BACKGROUND
The idea that MS could be related to vascular disease is not new. The first observations relating the presence of pathological blood vessels, or a connection between cerebral veins and the location of MS plaques were made in the 19th century. Plaques—the foci of pathological nervous tissue, a hallmark of MS—preferentially localize around small cerebral veins or extend along the axis of these vessels. This venocentric characteristic of MS plaques has been known since the first histopathological descriptions of MS. The vascular paradigm of MS has been extensively studied during the first part of the 20th century, when Dawson described periventricular MS plaques extending along the cerebral veins. Another neurologist, Putnam, tried to validate the idea of a venous etiology of MS. To prove this hypothesis, he carried out an experimental animal study in which he obstructed the intracranial veins of 14 dogs. On autopsy the majority of these animals exhibited demyelinated cerebral lesions that were very similar to those seen in MS.30 Unfortunately, Putnam’s research was not continued and has since largely been forgotten. With autoimmunity becoming a leading topic in MS research, and due to the difficulty in demonstrating occlusions in the intracranial veins, this first attempt to investigate a relationship between the venous system and MS was abandoned.
The next studies on this topic were not carried out until the 1970s. Schelling observed a widening of the main venous passages through the skull and hypothesized that these dilated veins could be a consequence of venous hypertension, with the cause of this venous pathology situated outside the cranium.31 Ogleznev et al described stenoses and occlusions in the internal jugular and brachiocephalic veins in patients with myelopathies of unknown origin (perhaps these patients actually presented with MS, since a definite diagnosis for MS was not made in this Russian study).32,33 Similar observations were also published by French authors.34 There were also some reports by neuroradiologists, who had measured abnormal cerebral flow with MR techniques.35-41 These flow disturbances could not be explained within the autoimmune paradigm of MS and their characteristics suggested a venous origin.
RESEARCH BY ZAMBONI’S GROUP
Much controversy has emerged since the first article published by Zamboni. In 2006, he published a review paper in which he hypothesized that there may be some common features between MS and venous leg ulcers.20 The following year, his team detected flow abnormalities in the intracranial veins of a group of MS patients. This type of pathological venous flow is not frequently seen in healthy subjects.42 Two years later, the same group published a study that demonstrated the presence of significant stenoses and occlusions in the main veins draining the central nervous system: the internal jugular veins (IJVs) and the azygous vein.18 Importantly, these blockages were situated outside the skull and spinal canal, while the abnormal intracerebral flow seemed to be a secondary effect resulting from these extracranial blockages. In another important study, Zamboni et al proposed the following set of sonographic criteria:
• Reflux. Constant reflux (>0.8 s) in a single IJV or the vertebral veins, in the sitting or supine position.
• Reflux in intracranial veins. Reflux >0.5 s in the deep cerebral veins in the sitting and supine position.
• Stenosis. A reduction of the cross-sectional area (CSA) of the IJV of less than 0.3 cm2 in both body positions, or the presence of intraluminal defects (such as webs, septa, or malformed valves).
• No flow. Absence of Doppler signal in the IJV or vertebral veins in both the supine and upright body positions.
• Negative ΛCSA. A cross-sectional area of the IJV that is greater in the sitting position than in the lying position, or that appears unchanged despite a change in posture.43-45
They found at least two positive criteria in all the MS patients they examined and found none in the healthy controls. Moreover, they described four distinct patterns of abnormal outflow: type A – obstruction of the proximal azygous vein accompanied by a stenosis of one of the IJVs; type B – obstruction of the proximal azygous vein together with bilateral stenoses of the IJVs; type C – stenoses of both IJVs; type D – numerous stenoses or occlusions in the azygous vein system. Interestingly, distinct flow patterns were seen in different clinical types of MS patients: types A, B, and C were seen primarily in patients with relapsing-remitting MS and secondary progressive MS, while type D was seen in patients with primary progressive MS.18,46 The following year, a study reporting the results of endovascular treatment of these venous blockages was published. The results of this small open-label trial were promising, especially in patients with relapsing-remitting MS. In addition, another study from this team showed a reduced number of new plaques and a trend toward a decreased number of new relapses.47,48
NATURE OF VASCULAR LESIONS IN CCSVI
Although long-term and pediatric observations are currently missing, it is suspected that CCSVI patients present with congenital venous malformations of the veins draining the brain or spinal cord.49,50 Taking into account the embryologic development of the IJVs and brachiocephalic veins, one should expect a higher prevalence of these pathologies on the left side. IJVs develop from the precardinal veins, which must join the common cardinal veins during embryological development. In some cases this process may go awry, especially on the left side, where part of the left common cardinal vein involutes. In addition, in some patients, the development of the jugular valves may not be perfect, resulting in structural abnormalities and stenoses. Indeed, some researchers have found a higher prevalence of CCSVI lesions in the left IJV.51-53 Similarly, most of the abnormalities in the azygous vein are found slightly distally from the arch, in the area where, during embryological development, the proximal part of the left postcardinal vein joins the left supracardinal vein. In some cases this fusion of the fetal veins may be imperfect, resulting in twisting or focal hypoplasia of the vein. For the time being, it remains controversial whether impaired outflow from other veins could influence the functioning of the central nervous system. Theoretically, in case of significant occlusion of the left iliac vein or the left renal vein, the azygous system could be overloaded by collateral flow coming from the lower part of the body. Yet, MS patients rarely present with plaques in the thoracic and lumbar segments of the spinal cord, which are drained by the azygous vein, thus making this potential iliac or renal source of neurological pathology unlikely.
CURRENT DIAGNOSTICS FOR CCSVI
CCSVI patients present with obstructive venous lesions, a unique vascular pathology (Figure 1). The most frequently found abnormalities are stenotic “overcompetent” jugular valves. Other vascular lesions— for example stenoses not related to the valves—are rarely encountered. Since this type of vascular pathology is not often seen in other vascular territories, a noninvasive method for their assessment has not been established yet. For the time being, only Zamboni’s CCSVI criteria have been validated. Still, the inconsistent results obtained in the studies that have used these criteria indicate that they are probably far from perfect and may need revising. Contrary to Zamboni, who found a 100% prevalence of CCSVI in MS patients,45 Zivadinov54 and Centonze,26 who used the same sonographic criteria, demonstrated a 50-60% prevalence of CCSVI. In addition, these authors found sonographic features of CCSVI in many healthy controls. Recently, revised sonographic criteria have been proposed,55 but their diagnostic accuracy was not found to be much better than that of the previous ones.56 Obviously, more research is needed to understand flow disturbances in this unique venous territory and, consequently, the sonographic features of such a disturbed flow. Until a reliable set of criteria is developed, the results of screening using Doppler sonography should be interpreted with caution. The localization of the azygous vein makes its assessment using traditional sonography virtually impossible. Some authors nevertheless believe that lesions in the azygous vein can be diagnosed indirectly by evaluating the flow in the vertebral veins.43 Yet, according to the anatomy of the venous system, it would be rather unlikely to detect a change in the flow pattern of the vertebral veins resulting from compromised azygous flow. The other diagnostic option is to evaluate the azygous vein using a transesophageal approach, since such an examination is currently performed in patients with portal hypertension. But, as yet, no published reports exist on transesophageal assessment of this vein in CCSVI patients.
Magnetic resonance venography (MRV) is another noninvasive diagnostic method (Figure 2), which seems to be less operator-dependent than Doppler sonography. It also enables the assessment of the intracranial veins, as well as the azygous vein. However, the currently available MR protocols are the source of significant imaging artifacts. Consequently, the diagnostic accuracy of MRV is even lower than that of Doppler sonography.7,54,57,58
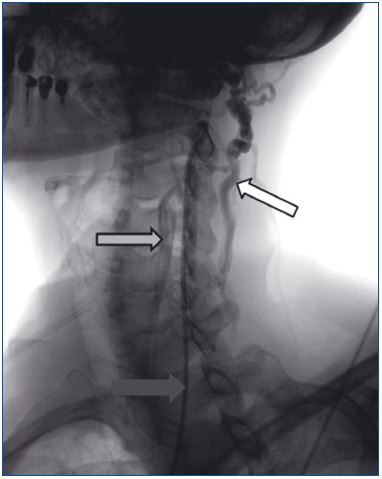
Figure 1. Severe stenosis of the left internal jugular vein. A
catheter has been placed in this vein (black arrow), but contrast is
flowing out through the vertebral vein (white arrow) and deep
cervical veins (grey arrow).
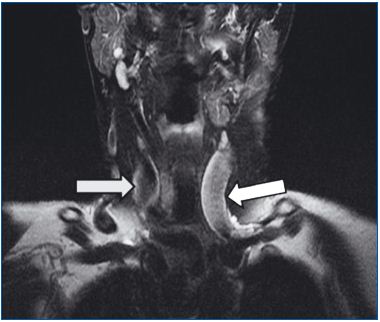
Figure 2. The flow in the left internal jugular vein (white arrow)
is slowed down, as demonstrated by fast-spin-echo-T2-weighted
sequences with fat-saturation MR imaging. With this imaging
modality, blood vessels with slow flow appear whitish, while those
with normal flow are black, as shown in the right internal jugular
vein (grey arrow). Please note that the left internal jugular vein is
of normal size, and therefore may appear unchanged on
conventional MR venography.
Catheter venography (CV) is currently regarded as the gold standard reference test for the assessment of CCSVI. Using CV, at least 90% of MS patients were found to have venous occlusive abnormalities.18,27,51-53 The main problem related to the use of CV is the technique used and the interpretation of venographic images (Figure 3). Since CCSVI is primarily a functional pathology, venographic tests should mirror blood flow in the examined veins. Thus, contrast should be injected under low pressure (as in the case of venous blood flow) and the injection of large volumes of contrast should be avoided (in order not to overload the vein and not to induce artifactual collateral outflow). Therefore, contrast should be injected by hand and not with an automatic syringe. Similarly to the generally accepted venographic signs of impaired venous outflow in other venous obstructive pathologies, when assessing the IJVs or the azygous vein, the following venographic patterns should be regarded as abnormal:
• slowed down venous outflow (retention of injected contrast in the examined vein);
• reversed flow direction;
• outflow through collaterals;
• complete occlusion or agenesia of the vein.
It remains a matter of debate if findings such as:
• intraluminal structures (webs, septa, membranes);
• hypoplasia or narrowing of the vein;
• prestenotic dilation of the vein should always be regarded as abnormal, or should be interpreted as a pathology only when associated with other signs of compromised outflow. Perhaps in most cases, CV should be accompanied by intravascular ultrasonography (IVUS) to resolve these uncertainties.
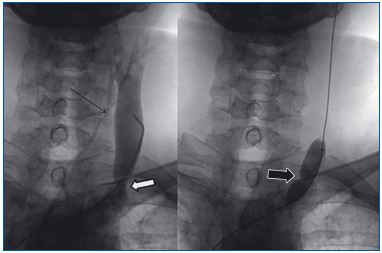
Figure 3. Stenotic valve of the left internal jugular vein. Contrast
is flowing out through the deep cervical veins (thin arrow);
although the valve (white arrow) does not seem too significantly
stenosed on venography, there is actually a tight stenosis, as
indicated by an indentation in the angioplastic balloon (black
arrow).
Since patients present with different degrees of venous pathology, a four-grade venographic classification of CCSVI has been proposed:
• Grade 1: slowed down venous outflow, no reflux detected;
• Grade 2: slowed down venous outflow, mild reflux and/or pre-stenotic dilation of the vein;
• Grade 3: slowed down venous outflow, with reversed flow direction and outflow through collaterals;
• Grade 4: no outflow through the vein, huge outflow through collaterals.51
Taking into account the low accuracy of a single test, many doctors opt for multimodal diagnostics, including: Doppler sonography, MR venography, catheter venography, and IVUS.
CCSVI: DOES THE SYNDROME ACTUALLY EXIST?
The CCSVI theory attracted vigorous criticism from neurologists.3 Some researchers tried to replicate Zamboni’s findings, using a modified sonographic protocol. While some of them demonstrated results similar to Zamboni’s,21-25 others were unable to demonstrate venous lesions.59-62 Other researchers looked for potential MRI markers of CCSVI and did not find any signs of pathology.63,64 Consequently, they claimed that CCSVI does not exist, and that the results of Zamboni are mere artifacts.
However, these “negative” studies were carried out either on a small group of MS patients—thus, with a high probability that non-CCSVI patients were assessedjust by chance—or using improper diagnostic tools. Nevertheless, only a minority of researchers now question the existence of venous abnormalities in MS patients (irrespective of the clinical meaning of the findings) and research performed with the use of reliable diagnostic tools (eg, catheter venography) has demonstrated the presence of lesions in about 90% of patients.27,51-53
However, it is important to emphasize that the actual prevalence of venous abnormalities in the IJVs and the azygous vein in a healthy population is not known. Reliable data should be obtained using CV, which—due to the invasive nature of this test—will not be an easy task. However, some already published data indicate that such flow disturbances could be found in as many as 30% of healthy people.26,54 Thus, the total number of the so-called “CCSVI” patients in the general population may be much higher than the number of MS patients. Consequently, these vascular abnormalities should probably not be regarded as the pathology per se, but rather as a permissive lesion. This would mean that MS may be more likely to evolve from its preclinical form into clinically overt disease in the presence of venous abnormalities, while the triggering factor (eg, genetic predisposition or viral infection) may not be related to the vascular system.65
The CCSVI hypothesis is a perfect example of the haphazard nature of science, which often produces progress ahead of our understanding. Our knowledge of the anatomy and physiology of the veins draining the central nervous system is rather scarce. Most previous research has focused on the arterial side of the cerebral and spinal circulation, and also on the competence of the jugular valves.66-70 Nevertheless, we have some data regarding the localization and diameter of the IJVs. For example, Troianos et al measured the diameter of IJVs in a group of 1136 non-MS patients. He did not observe occluded or severely narrowed veins.71 Similarly, Denys et al found patent and normal-sized right IJVs in 96.4% of 928 critically ill patients. In the remaining 3% of patients, the vein was occluded by iatrogenic thrombi resulting from many previous cannulations.72 Likewise, Lin et al found a 1.0% prevalence of occluded IJVs in a group of 104 uremic patients.73 In contrast, except for a few case reports, we have no information on malformed azygous veins.74 What is more, we do not know how often vascular malformations occur in “healthy” individuals nor what the clinical meaning of finding a “lesion” is.
The main problem related to the CCSVI concept is the difficulty in defining this clinical entity. According to Zamboni, CCSVI is a syndrome characterized by stenoses of the IJVs and/or the azygous vein, with opening of the collaterals and insufficient venous drainage, demonstrated by reduced cerebral blood flow and pathologic perfusion MR parameters (eg, prolonged cerebral mean transit time).44,75,76 Currently, CCSVI is primarily defined in terms of pathologic Doppler sonography parameters. But should CCSVI be exclusively seen as a state of impaired venous outflow? An alternative view is that, in order to diagnose the pathology, venous outflow impairment should be accompanied by abnormalities of the central nervous system. It would appear to be more than a mere semantic problem. If this dilemma were solved, it would help decide which anatomical or structural lesions should be managed, and which ones should be considered only as anatomic variants with no potential clinical impact.
IMPLICATIONS OF THE CCSVI HYPOTHESIS
According to the Consensus Document of the International Union of Phlebology on the diagnosis and treatment of venous malformations, CCSVI is interpreted as a truncular venous malformation.50 These lesions obstruct the main outflow routes from the central nervous system: the brain and spinal cord. Yet, it remains elusive whether these vascular abnormalities actually contribute to neurological pathology. Irrespective of these controversies, since CCSVI compromises the blood outflow from a vital organ, for many doctors it seems reasonable to unblock these obstructions. Others, however, argue that such interventions should only be accepted as a valid treatment option for MS on condition that:
• the impact of venous insufficiency on MS is demonstrated;
• procedures to alleviate these vascular pathologies have been proven to be technically feasible and safe;
• treatments have been shown to result in clinical benefit.
CAUSATIVE RELATIONSHIPS BETWEEN MS AND CCSVI
So far, an unquestionable relationship between CCSVI and MS has not been proven yet, especially regarding a causative role of venous abnormalities. Some data even support the idea of a secondary nature of CCSVI, ie, that these lesions develop due to the action of proinflammatory agents released by the diseased brain, or that they result from brain atrophy.27,60 However, taking into account the morphological characteristics of CCSVI lesions (primarily: malformed valves) and that these malformations are seen more frequently on the left side,51-53 it is very difficult to suggest a reasonable mechanism by which these vascular abnormalities could develop secondarily. It seems far more likely that the CCSVI lesions are of a congenital nature. Indeed, a recent study showed no correlation between the duration of MS and the severity of venous pathology, backing the idea of the primary nature of these vascular lesions.65
There are several theoretical mechanisms by which CCSVI could trigger or exacerbate MS-associated inflammation and neurodegeneration. In addition to a potential role for iron19,77,78 —although it is possible that an increased concentration of iron within the cerebral parenchyma is only an epiphenomenon and the sign of a weakened blood-brain barrier—it has been hypothesized that CCSVI augments neurological disability through chronic brain hypoxia resulting from the blockage of venous outflow.39 Current research favors this idea. Measurements of pO2 and pCO2 in blood samples obtained from the IJVs before and after angioplasty of the stenotic jugular valve have demonstrated dramatic improvements of these blood gas parameters, indicating a better oxygenation of the brain after the procedure.79
It is also possible that venous reflux in the cerebral circulation elicits disintegration of the blood-brain barrier, which in turn initiates an autoimmune attack against nervous tissue.80-82 Moreover, in the setting of chronic ischemia and a leaky blood-brain barrier, the axons could be injured via glutamate-mediated excitotoxicity. A potentially deleterious role of glutamate has long been suspected to be important in the pathogenesis of MS-related neurodegeneration, but it was difficult to find a factor that may be responsible for the increased susceptibility of axons to this amino acid.83-87 Perhaps CCSVI is the missing piece of the puzzle.
THERAPEUTIC OPTIONS FOR CCSVI AND THEIR SAFETY
Currently, balloon angioplasty remains the first-line option for the treatment of malformations of the veins draining the central nervous system (Figure 4). This procedure has been demonstrated to be safe.48,51,52,88 Only a few major complications have been observed. However, balloon angioplasties of IJVs are associated with a high rate of restenosis. Stenting of a stenotic vein was found more effective in the short term but— unfortunately—also associated with a higher risk of complications, which may even be life-threatening. Moreover, problems with maintaining long-term patency of the stents, which can be occluded by thrombi or intimal hyperplasia, have been reported (Figure 5).89 Therefore, stents should not be regarded as the preferredtreatment option. Perhaps, the use of cutting balloons (angioplastic balloons with small blades that cut the restricting annulus)—instead of stents—will be a better solution,90 but as yet no reports on the efficacy of this method have been published. Other endovascular techniques, such as drug-coated balloons, drug-eluting stents, or dissolvable stents, may become useful in the future, but they have not yet been tested in this new indication.
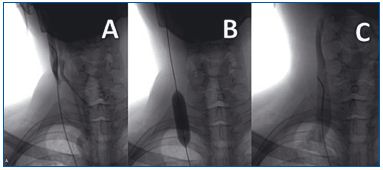
Figure 4. Severely narrowed right internal jugular vein. Injected
contrast flows out from this vein only through the collateral
network (A); balloon angioplasty of the malformed jugular valve
(B) resulting in physiologic outflow after the procedure (C).
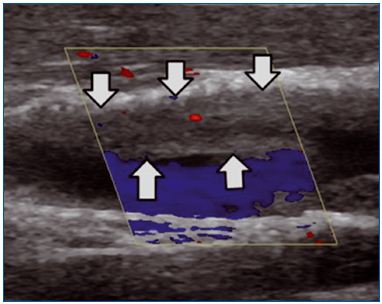
Figure 5. Intimal hyperplasia (arrows) inside the stent implanted
into the internal jugular vein. There is sufficient flow through the
free part of the stent, but sometimes the stent can be completely
blocked by ingrown tissue.
The other unsolved problem related to endovascular treatment for CCSVI is the choice of optimal postprocedural medication. All reported fatal complications following the treatment of CCSVI were not directly related to the procedure, but were associated with the use of antithrombotic drugs. On the other hand, insufficient antithrombotic or antiplatelet medication can potentially result in thrombotic occlusion of the treated vein.91,92 Thus, an optimal (ie, safe and effective) posttreatment medication scheme should be established. Taking this into account and until an optimal protocol is established, endovascular techniques that require more aggressive anticoagulation (primarily the use of stents) should be avoided whenever possible, since MS patients seem to be at higher risk of serious bleeding complications.
CLINICAL EFFICACY OF ENDOVASCULAR TREATMENTS
For the time being, only a few small open-label studies investigating the clinical efficacy of endovascular treatment for CCSVI in MS patients have been published. The results of these open-label studies were promising, especially in patients with relapsing-remitting clinical MS.47,48,93-96 It has also been found that only some MS-related symptoms (eg, chronic fatigue, bladder control, impaired balance) may improve after the treatment, while others, especially walking ability, are not very likely to get better. Therefore, in patients with Extended Disability Severity Scores above 5 points, one should not expect an improvement in this scale. Unfortunately, high rates of restenoses of the treated veins have been observed, which may explain the fact that some authors observed only temporary benefits following angioplasty.90,96 Still, only prospective randomized sham-surgery-arm or crossover trials, assessing objective and widely accepted neurological parameters (eg, the number of new plaques, number of relapses, or EDSS scores) would unequivocally prove or disprove the clinical efficacy of endovascular procedures for CCSVI.
ONGOING RESEARCH AND FUTURE PERSPECTIVES
The association of MS with impaired venous outflow from the central nervous system has shed new light on the cause and potential treatment options for this incurable neurological disease. There has been a shift away from viewing MS solely as an autoimmune disease, with no other potential treatment options. It is estimated that about 12 000 MS patients have already received endovascular treatment worldwide,97 with only a few serious complications reported. Even if only a subgroup of MS patients actually benefited from vascular treatment, these procedures could potentially be a breakthrough in the management of MS.
In addition, perhaps some “vascular” drugs may improve venous insufficiency and—in turn—modify the clinical course of MS. Therefore, these pharmaceutical agents should be tested for potential anti-MS effects. Moreover, the discovery of CCSVI has shown another possible research avenue. It is known that only a minority of MS patients improve after administration of immunomodulating drugs. It cannot be ruled out that these “responders” actually represent a unique subset of CCSVI. If it were the case, these drugs should be given only to this group of MS patients. This hypothesis may also be validated by ongoing research.

REFERENCES
1. Awad AM, Marder E, Milo R, et al. Multiple sclerosis and chronic cerebrospinal venous insufficiency: a critical review. Ther Adv Neurol Disord. 2011;4:231-235.
2. Haacke EM. Chronic cerebral spinal venous insufficiency in multiple sclerosis. Expert Rev Neurother. 2011;11:5-9.
3. Khan O, Filippi M, Freedman MS, et al. Chronic cerebrospinal venous insufficiency and multiple sclerosis. Ann Neurol. 2010;67:286-290.
4. Qiu J. Venous abnormalities and multiple sclerosis: another breakthrough claim? Lancet Neurol. 2010;9:464-465.
5. Waschbisch A, Manzel A, Linker RA, et al. Vascular pathology in multiple sclerosis; mind boosting or myth busting. Exp Translat Stroke Med. 2011;3:7.
6. Weir B. Multiple sclerosis – a vascular etiology? Can J Neurol Sci. 2010; 37:745- 757.
7. Zivadinov R, Ramanathan M, Dolic K, et al. Chronic cerebrospinal venous insufficiency in multiple sclerosis: diagnostic, pathogenetic, clinical and treatment perspective. Expert Rev Neurother. 2011;11:1277-1294.
8. Ciccone A, Beretta S, Brusaferri F, et al. Corticosteroids for the long-term treatment in multiple sclerosis. Cochrane Database Syst Rev. 2008;1:CD006264.
9. Munari LM., Lovati R, Boiko A. Therapy with glatiramer acetate for multiple sclerosis. Cochrane Database Syst Rev. 2004;4:CD004678.
10. Rice GP, Incorvaia B, Munari LM., et al. Interferon in relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2007;2:CD002002.
11. Rojas JI, Romano M, Ciapponi A, et al. Interferon beta for primary progressive multiple sclerosis. Cochrane Database Syst Rev. 2009;1:CD006643.
12. Sriram S, Steiner I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann Neurol. 2005;58:939-45.
13. Chaudhuri A, Behan PO. Multiple sclerosis: looking beyond autoimmunity. J Roy Soc Med. 2005;98:303-306.
14. Chaudhuri A, Behan PO. Multiple sclerosis is not an autoimmune disease. Arch Neurol. 2004;61:1610-1612.
15. Barnett MH, Henderson AP, Prineas JW. The macrophage in MS: just a scavenger after all? Pathology and pathogenesis of the acute MS lesion. Mult Scler. 2006;12:121-132.
16. Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65:32-46.
17. Behan PO. Futility of the autoimmune orthodoxy in multiple sclerosis research. Expert Rev Neurother. 2010;10:1023-1025.
18. Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple slerosis. J Neurol Neurosurg Psychiatry. 2009;80:392-399.
19. Singh AV, Zamboni P. Anomalous venous blood flow and iron deposition in multiple sclerosis. J Cereb Blood Flow Metab. 2009;29:1867-1878.
20. Zamboni, P. The big idea: irondependent inflammation in venous disease and proposed parallels in multiple sclerosis. J Roy Soc Med. 2006;99:589-593.
21. Al-Omari MH, Rousan LA. Jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol. 2010;29:115-120.
22. Radak D, Kolar J, Tanaskovic S et al. Morphological and haemodynamic abnormalities in the jugular veins of patients with multiple sclerosis. Phlebology. 2011Sep1. Epub ahead of print. 312 doi:10.1258/phleb.2011.011004.
23. Sclafani S. Chronic cerebrospinal venous insufficiency: a new paradigm and therapy for multiple sclerosis. Endovascular Today. 2010;July:41-6.
24. Simka M, Kostecki J, Zaniewski M, et al. Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis. Int Angiol. 2010;29:109-114.
25. Simka M, Kostecki J, Zaniewski M, et al. Preliminary report on pathologic flow patterns in the internal jugular and vertebral veins of patients with multiple sclerosis. Phlebol Rev. 2009;17:61-64.
26. Centonze D, Floris R, Stefani M, et al. Proposed chronic cerebrospinal venous insufficiency criteria do not predict MS risk or MS severity. Ann Neurol. 2011;70:51-58.
27. Yamout B, Herlopian A, Issa Z, et al. Extracranial venous stenosis is an unlikely cause of multiple sclerosis. Mult Scler. 2010;16:1341-1348.
28. Bergan JJ, Schmid-Schönbein GW, Smith PD, et al. Chronic venous disease. N Engl J Med. 2006;355:488- 498.
29. Simka M. Cellular and molecular mechanisms of venous leg ulcers development-the “puzzle” theory. Int Angiol. 2010;29:1-19.
30. Putnam T. Studies in multiple sclerosis: encephalitis and sclerotic plaques produced by venular obstruction. Arch Neurol Psychiatry. 1935;33:929-940.
31. Schelling F. Damaging venous reflux into the skull or spine: relevance to multiple sclerosis. Med Hypothes. 1986;21:141-148.
32. Ogleznev KO, Tsuladze II. Diagnosis of venous circulatory disorders in the cervical portion of the spine and cord by selective phlebography. Vestn Rentgenol Radiol. 1993:46-49.
33. Tsuladze II. The selective phlebography of the large tributaries of the vena cava system in the diagnosis of venous circulatory disorders in the spinal complex. Zh Vopr Neirokhir Im N N Burdenko. 1999;2:8-13.
34. Aboulker J, Bar D, Marsault C, et al. Intraspinal venous hypertension caused by multiple abnormalities of the caval system: a major cause of myelopathies. Acta Radiol. Suppl 1976;347:395-401.
35. Adhya S, Johnson G, Herbert J, et al. Pattern of hemodynamic impairment in MS: dynamic susceptibility contrast perfusion MR imaging at 3.0 T. Neuroimage. 2006;33:1029-1035.
36. De Keyser J Steen C, Mostert JP, et al. Hypoperfusion of the cerebral white matter in multiple sclerosis: possible mechanisms and pathophysiological significance. J Cereb Blood Flow Metab. 2008:28:164516-51.
37. Ge Y, Law M, Johnson G, et al. Dynamic susceptibility contrast perfusion MR imaging of MS lesions: characterizing hemodynamic impairment and inflammatory activity. AJNR Am J Neuroradiol. 2005;26:1539- 1547.
38. Law, M, Saindane, AM, Babb, JS, et al. Microvascular abnormality in relapsing-remitting multiple sclerosis: perfusion MR imaging findings in normal-appearing white matter. Radiology. 2004;231:645-652.
39. Simka M, Zaniewski M. Reinterpreting the magnetic resonance signs of hemodynamic impairment in the brains of multiple sclerosis patients from the perspective of a recent discovery of outflow block in the extracranial veins. J Neurosc Res. 2010;88:1841-1845.
40. Varga AW, Johnson G, Babb JS, et al. White matter hemodynamic abnormalities precede sub-cortical gray matter changes in MS. J Neurol Sci. 2009;282:28-33.
41. Wuerfel J, Bellmann-Strobl J, Brunecker P, et al. Changes in cerebral perfusion precede plaque formation in MS: a longitudinal perfusion MRI study. Brain. 2004;127:111-119.
42. Zamboni P, Menegatti E, Bartolomei I, et al. Intracranial venous haemodynamics in multiple sclerosis. Curr Neurovasc Res. 2007;4:252-258.
43. Menegatti E, Genova V, Tessari M, et al. The reproducibility of colour Doppler in chronic cerebrospinal venous insufficiency associated with multiple sclerosis. Int Angiol. 2010;29:121-126.
44. Zamboni P, Consorti G, Galeotti R, et al. Venous collateral circulation of the extracranial cerebrospinal outflow routes. Curr Neurovasc Res. 2009;6:204- 212.
45. Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci. 2009;282:21-27.
46. Bartolomei I, Salvi F, Galeotti R, et al. Hemodynamic pattern of chronic cerebrospinal venous insufficiency in multiple sclerosis. Correlation with symptoms at onset and clinical course. Int Angiol. 2010;29:183-188.
47. Zamboni P, Galeotti R, Weinstock- Guttman B, et al. Venous angioplasty in patients with multiple sclerosis: results of a pilot study. Eur J Vasc Endovasc Surg. 2011;43:116-122.
48. Zamboni P, Galeotti R, Menegatti E, et al. Endovascular treatment of chronic cerebrospinal venous insufficiency, A prospective open-label study. J Vasc Surg. 2009;50:1348-1358.
49. Lee BB, Laredo J, Neville R. Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebrospinal venous insufficiency. Int Angiol. 2010;29:95- 108.
50. Lee BB, Bergan J, Gloviczki P, et al. Diagnosis and treatment of venous malformations. Consensus Document of the International Union of Phlebology (IUP)-2009. Int Angiol. 2009;28:434-451.
51. Ludyga T, Kazibudzki M, Simka M, et al. Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe? Phlebology. 2010;25:286-295.
52. Petrov I, Grozdinski L, Kaninski G, et al. Safety profile of endovascular treatment for chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Endovasc Ther. 2011;18:314-323.
53. Simka M, Latacz P, Ludyga T, et al. Prevalence of extracranial venous abnormalities: results from a sample of 586 multiple sclerosis patients. Funct Neurol. 2011;26:197-203.
54. Zivadinov R, Marr K, Cutter G, et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS.
55. Nicolaides AN, Morovic S, Menegatti E, et al. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound. Recommendations for a protocol. Funct Neurol. 2011;4:229-248.
56. Simka M, Ludyga T, Latacz P et al. Diagnostic accuracy of current sonographic criteria for the detection of outflow abnormalities in the internal jugular veins. Phlebology. 2012; Apr 23. Epub ahead of print.
57. Ayanzen RH, Bird CR, Keller PJ, et al. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21:74-78.
58. Zivadinov R, Galeotti R, Hojnacki D, et al. Value of MR venography for detection of internal jugular vein anomalies in multiple sclerosis: a pilot longitudinal study. AJNR Am J Neuroradiol. 2011;32:938-946.
59. Auriel E, Kami A, Bornstein NM, et al. Extra-cranial flow in patients with multiple sclerosis. J Neurol Sci. 2011;309:102-104.
60. Baracchini C, Perini P, Calabrese M, et al. No evidence of chronic cerebrospinal venous insufficiency at multiple sclerosis onset. Ann Neurol. 2011;69:90-99.
61. Doepp F, Friedemann P, Valdueza JM, et al. No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol. 2010;68:173-183.
62. Marder E, Gupta P, Greenberg BM, et al. No cerebral or cervical venous insufficiency in US veterans with multiple sclerosis. Arch Neurol. 2011; 68:1521-1525.
63. Sundström P, Wåhlin A, Ambarki K, et al. Venous and cerebrospinal fluid flow in multiple sclerosis: A case-control study. Ann Neurol. 2010; 68:255-259.
64. Wattjes MP, van Oosten BW, de Graaf WL. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry. 2011;82:429-435.
65. Simka M, Ludyga T, Kazibudzki M, et al. Multiple sclerosis: an unlikely cause of chronic cerebrospinal venous insufficiency. J R Soc Med Sh Rep. 2011; doi 10.1258/shorts.2011.010146.
66. Agosti C, Borroni B, Akkawi NM, et al. Cerebrovascular risk factors and triggers in transient global amnesia patients with and without jugular valve incompetence: results from a sample of 243 patients. Eur Neurol. 2010;63:291-294.
67. Chung CP, Hu HH. Jugular venous reflux. J Med Ultrasound. 2008;16:210- 222.
68. Fisher J, Vaghaiwalla F, Tsitlik J, et al. Determinants and clinical significance of jugular venous valve competence. Circulation. 1982;65:188-196.
69. Nedelmann M, Eicke BM, Dietrich M. Functional and morphological criteria of internal jugular valve insufficiency as assessed by ultrasound. J Neuroimaging. 2005;15:70-75.
70. Velecchi D, Bacchi D, Gulisano M, et al. Internal jugular valves: an assessment of prevalence, morphology and competence by color Doppler echography in 240 healthy subjects. Ital J Anat Embryol. 2010;115:185-189.
71. Troianos CA, Kuwik RJ, Lim AJ, et al. Internal jugular vein and carotid artery anatomic relation as determined by ultrasonography. Anesthesiology. 1996;85:43-48.
72. Denys BG, Uretsky BF, Reddy PS. Ultrasound-assisted cannulation of the internal jugular vein: a perspective comparison to the external landmarkguided technique. Circulation. 1993;87:1557-1562.
73. Lin BS, Kong CW, Tarng DC, et al. Anatomical variation of the internal jugular vein and its impact on temporary haemodialysis vascular access: an ultrasonographic survey in uraemic patients. Nephrol Dial Transplant. 1998;13:134-138.
74. Chasen MH, Charnsangavej C. Venous chest anatomy: clinical implications. Eur J Radiol. 1998;27:2-14.
75. Zamboni P, Galeotti R. The chronic cerebrospinal venous insufficiency syndrome. Phlebology. 2010;25:269-79.
76. Zamboni P, Menegatti E, Weinstock- Guttman B, et al. Hypoperfusion of brain parenchyma is associated with the severity of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a cross-sectional preliminary report. BMC Med. 2011;9:22.
77. Adams CW. Perivascular iron deposition and other vascular damage in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1988;51:260-265.
78. Simka M, Rybak Z. Hypothetical molecular mechanisms by which local iron overload facilitates the development of venous leg ulcers and multiple sclerosis lesions. Med Hypothes. 2008;71:293-297.
79. Petrov I. Results of Endovascular treatment of chronic cerebrovascular insufficiency in patients with multiple sclerosis. Paper presented at: 14th Annual Meeting of the Australasian College of Phlebology. March 30-April 3, 2011; Melbourne, Australia.
80. Colgan OC, Ferguson G, Collins NT, et al. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am J Physiol Heart Circ Physiol. 2007;292:H3190- H3197.
81. Krizanac-Bengez L, Mayberg MR, Cunningham E, et al. Loss of shear stress induces leukocyte-mediated cytokine release and blood-brain barrier failure in dynamic in vitro blood-brain barrier model. J Cell Physiol. 2006;206:68-77.
82. Simka M. Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis. Curr Neurovasc Res. 2009;6:132-139.
83. Geurts JJ, Wolswijk G, Bö L, et al. Altered expression patterns of group I and II metabotropic glutamate receptors in multiple sclerosis. Brain. 2003;126:1755-1766.
84. Geurts JJ, Wolswijk G, Bö L, et al. Expression patterns of group III metabotropic glutamate receptors mGluR4 and mGluR8 in multiple sclerosis lesions. J Neuroimmunol. 2005;158:182-190.
85. Káradóttir R, Cavelier P, Bergersen LH, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162- 1166.
86. Newcombe J, Uddin A, Dove R, et al. Glutamate receptor expression in multiple sclerosis lesions. Brain Pathology. 2008;18:52-61.
87. Srinivasan R, Silasuta N, Hurd R, et al. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016-1025.
88. Mandato KD, Hegener PF, Siskin GP, et al. Safety of endovascular treatment of chronic cerebrospinal venous insufficiency: a report of 240 patients with multiple sclerosis. J Vasc Interv Radiol. 2012;23:55-9.
89. Kostecki J, Zaniewski M, Ziaja K, et al. An endovascular treatment of chronic cerebro-spinal venous insufficiency in multiple sclerosis patients – 6 month follow-up results. Neuro Endocrinol Lett. 2011;32:557-562.
90. Simka M. Safety of endovascular treatment for CCSVI and future perspectives. J Endovasc Ther. 2011;18:326-327.
91. Samson K. Experimental multiple sclerosis vascular shunting procedure halted at Stanford. Ann Neurol. 2010;67:A13-A15.
92. Pandey V, Shalhoub J, Malik O, et al. Internal jugular thrombosis post venoplasty for chronic cerebrospinal venous insufficiency. Phlebology. 2011;26:254-256.
93. Ludyga T, Kazibudzki M, Latacz P, et al. Early results of a prospective openlabel study on endovascular treatments for chronic cerebrospinal venous insufficiency in the patients with associated multiple sclerosis. Phlebol Rev. 2011;19:9-14.
94. Malagoni AM, Galeotti R, Menegatti E, et al. Is chronic fatigue the symptom of venous insufficiency associated with multiple sclerosis? A longitudinal pilot study. Int Angiol. 2010;29:176-182.
95. Denisliˇc M. Clinical disability and venous vessel pathology in multiple sclerosis. Paper presented at: 1st Annual Meeting of the International Society for Neurovascular Disease; March 14-15, 2011; Bologna, Italy.
96. Beelen R, Maene L, Castenmiller P. Evolution in quality of life and epidemiological impact after endovascular treatment of chronic cerebro-spinal venous insufficiency in patients with multiple sclerosis. Phlebology. 2012;27 (suppl 1):187–189.
97. Reid DB. Significance of the internal jugular vein in the treatment of cerebrovascular insufficiency. J Endovasc Ther. 2011;18:324-325.
