CLINICAL CASE 1. Diagnostic problems in a symptomatic patient with May-Thurner syndrome
George Geroulakos, MD, PhD
Aikaterini Poulou, MD, PhD
Efthymios Avgerinos, MD, PhD
Department of Vascular Surgery,
“Attikon” University Hospital, National
and Kapodistrian University of Athens,
Athens, Greece
A52-year-old male presented a 1-year history of heaviness, dull ache, and swelling from the calf down on both legs. Over the same period, he developed mild punctuated pigmentation on both legs that extended from the ankles to mid-calf, gradually getting worse. There was no previous or current history of deep venous thrombosis. On examination, no varicose veins existed. Circumferential bilateral punctuated pigmentation on the ankles was noticed, being worse on the left lower extremity than on the right lower extremity.
The duplex ultrasound scan (Figure 1) showed valvular insufficiency of the left anterior accessory saphenous vein. On the right limb, venous reflux of a perforator vein of the calf was found. The patient underwent endovenous laser ablation therapy (EVLT) of the left accessory saphenous vein under local anesthesia with ultrasound guidance and using a 1470-nm diode laser. The radial fiber was used, and the vein was ablated, applying a linear endovenous energy density (LEED) of 70.92 J/cm, and a total length of 39 cm of the vein was treated. No complications were reported intra- or postoperatively, and the patient was discharged.
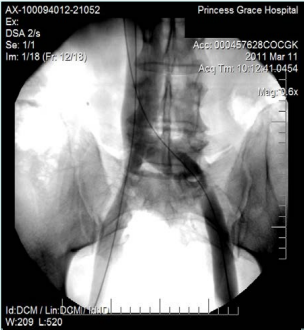
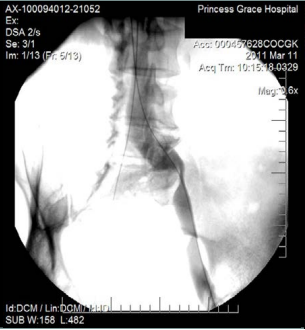
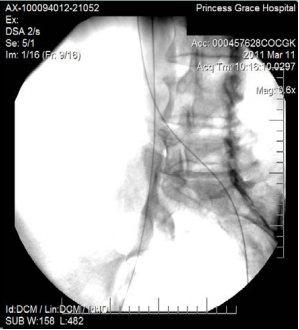
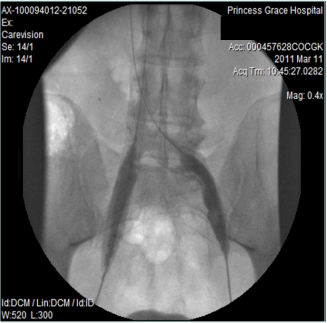
However, 8 months later, he returned, presenting deterioration of the pigmentation and stating that the EVLT procedure did not improve his symptoms. On clinical examination, no varicose veins were present, though pigmentation was more intense and extensive on the lower calf and ankles. A computed tomography (CT) contrast venography was performed with the direct puncture of both common femoral veins. Bilateral May-Thurner syndrome (MTS) was diagnosed. (Figures 2-4) The patient underwent balloon venoplasty and stenting. Self-expandable, open-cell stents, Wallstents, of 16-cm length and 9-mm diameter, were used (Figures 5 and 6). Cranially, both stents were extended in the inferior vena cava in a double-barrel configuration. The final venography revealed the elimination of the venous stenosis in the iliac veins. The patient was discharged on antiplatelet treatment for 6 weeks, and a 6-month follow-up was scheduled. After 6 months, the patient’s pain and swelling symptoms were resolved. On examination during the follow-up visit, it was noted that the right calf circumference had decreased from 48 to 46.5 cm. The left calf had decreased from 47 cm to 45 cm. The patient stated that his pain and swelling had completely resolved. A plain X-ray showed no displacement or stent stenosis. Skin pigmentation faded without signs of expansion.
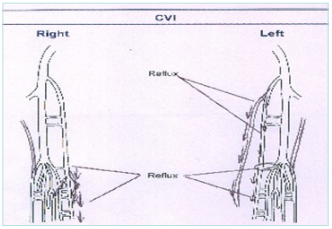
Figure 1. Schematic figure of ultrasound examination.
Abbreviation: CVI; chronic venous insufficiency.
Discussion
Dr Geroulakos. May-Thurner Syndrome (MTS) is also called iliac compression syndrome, where the right common iliac artery against the vertebral body compresses the left common iliac vein.1 It is a rare condition and may cause edema, varicose veins, deep venous thrombosis (DVT) and subsequent pulmonary embolism, and chronic venous stasis with ulcers.1,2 Right-sided MTS is also reported in the literature, even if unusual, usually coexisting with left sided inferior vena cava.3,4 The majority of MTS cases are left-sided; however, variants include right or bilateral iliac vein compression.5 More recent advancements in imaging technology reported that up to 20% of the general population has iliac vein obstructive lesions, which are present in patients of all ages and sexes.6 Bilateral MTS is extremely rare and can be primary, though present in the literature also in cases when iliac artery aneurysms coexist.7
The prevalence of MTS (iliac compression syndrome) was reported by Kibbe et al to reach a percentage of 24%, evaluated in an asymptomatic population using computed tomography. However, MTS can cause symptoms when severe and coexisted in 2% to 5% of patients with venous disease.6 Additionally, Cavalcante et al reported that the percentage of prevalence of MTS increases dramatically when DVT occurs, reaching 49%.5
Dr Nikolov. In 1851, Rudolf Virchow first proposed that the increased incidence of venous thrombosis in the left lower extremity resulted from the right common iliac artery compressing the left common iliac vein.8 In 1908, McMurrich reported that 32.7% of 107 cadavers from an unselected population exhibited obstructions, or adhesions, in the left common iliac vein.9 However, it was not until 1957 that May and Thurner reported the presence of intraluminal fibrous bands in the left common iliac vein secondary to compression from the right common iliac artery in 22% of the 430 cadavers they dissected and called this finding MTS.10 Cockett and Thomas were the first to report these findings in patients.11 Overall, MTS is estimated to cause 2% to 5% of all DVT. However, many retrospective studies have estimated the prevalence to be much higher. Multiple autopsy studies on unselected patients showed MTS prevalence to be between 14% and 32%.12
Dr Josnin. Left iliac vein compression syndrome—known as MTS in the United States and Cockett syndrome in Europe—can also be a postural phenomenon that can occur, particularly during pregnancy, when hyperlordosis occurs. The site of compression varies according to the level of the aortic bifurcation.
Apart from the consequences of a postthrombotic syndrome related to a proximal venous thrombosis generated by an MTS, its impact on varicose vein recurrence and chronic venous disease (CVD) predominance on the left limb remains debatable.
Dr Kan. MTS is often associated with symptoms of pelvic congestion and may be associated with varicose veins on the posterior surface of the thigh; it primarily affects young and middle-aged women with a history of multiple pregnancies, during the postpartum period, and those with oral contraceptive use, but many patients are completely asymptomatic.12 According to Zurkiya’s report, the incidence of MTS accounts for about 1.2% (10/763) of patients with venous reflux disorder.13
Most people with MTS never have a DVT symptom. They may develop left lower extremity venous hypertension unknowingly. However, a high degree of suspicion is warranted when a young woman develops a left lower extremity DVT in the presence of certain risk factors.
Dr Lobastov. According to the classical definition, MTS is a type of nonthrombotic iliac vein lesion (NIVL) represented by specific fibrotic changes in the vein lumen (spur, obliteration) that occur due to chronic compression and traumatization of the left common iliac vein.14 It may be found by autopsy in 20% to 30% of unselected cadavers.10,11,15 However, the prevalence of NIVL may be higher and depends on the screened population, imaging modality, and the diagnostic criteria of obstruction. In the general population without reported symptoms and signs of CVD, NIVLs of >50% may be found by computed tomography (CT) venography in 8.8% to 45%, whereas obstruction of >70% is found in only 0% to 31%.6,16-21 In one study, NIVL with obstruction of >50% was found in 80% of healthy volunteers by contrast venography.22 In patients with verified DVT (predominantly left sided) prevalence of NIVL of >50% to 70% is much higher, reaching 15.6% to 80% by CT venography.16,17,20,23 In individuals with CVD, iliac vein compression of >50% could be revealed by CT venography or intravascular ultrasound (IVUS) in 12% to 53%.24-28 They occupy an intermediate position between the general population and DVT patients.
Despite traditional notions on the left-side lesion, NIVL in patients with CVD could be found in only 38.7% at the typical point. In comparison, 29.2% of individuals have proximal compression of the right common iliac vein; 7.5%, of the right external iliac vein; and 2.5%, of the left external iliac vein, as revealed by IVUS.29
In contrast to classical MTS with morphological intraluminal changes, NIVL is a functional condition that depends on gravity and blood volume. Body position and hydration can affect the vein diameter and degree of obstruction measured by magnetic resonance venography (MRV) and IVUS.30,31
Dr Geroulakos. The pelvic outflow is rarely routinely investigated in clinical practice, and the findings on an infrainguinal ultrasound scan cannot exclude the presence of a significant proximal obstruction. Avgerinos and Geroulakos have reported the clinical scenarios that raise the index of suspicion and necessitate an investigation of the pelvic venous outflow. These include the presence of a history of DVT, persistent ulcer despite saphenous ablation, significant leg swelling or pain disproportionate to reflux and the extent and size of the varicose veins, deterioration of lipodermatosclerosis, and pigmentation in patients with adequate treatment of superficial venous reflux. These patients should be considered for iliac venous stenting if significant venous stenosis is shown. The improvement in hyperpigmentation after venous stenting for MTS is not adequately documented in the literature.32
Dr Josnin. In routine clinical practice, hyperpigmentation alone should not prompt a search for MTS. This may be discussed if it only affects a lower limb. However, in a subject who has never had a DVT and has no pelvic symptoms, if limited to lower-limb symptoms only, venous claudication is the symptom that could by itself justify this search.33
Dr Kan. MTS is best diagnosed using the following imaging modalities: duplex ultrasound (DUS), CT venography, MRV, and IVUS. Owing to the advantages of noninvasiveness and no radiation, DUS is the gold-standard diagnostic tool for vascular diseases. High-resolution and 3-dimensional CT venography provides a noninvasive and accurate technique for measuring the degree of left common iliac vein stenosis and has been successfully used to determine the caliber and length of stents needed. It remains the most useful diagnostic tool in our current clinical practice. Compared with CT, MRV requires more time to perform the examination and the time to reconstruct the image is currently longer, it can obtain comprehensive arterial and venous images without using contrast agents and radiation exposure, making it a promising tool for the future. Venography combined with IVUS, which allows for more precise stent placement in the iliac veins and minimizes the risk of developing a jailing effect, is a very informative tool in current practice.34,35
Although DUS is the gold-standard diagnostic tool for vascular disease, reliable imaging of iliac vein tributaries using ultrasound is impossible and, most important, completely unnecessary. According to Zurkiya’s report, the incidence of MTS accounts for only 1.2% of patients with venous reflux disorder by ultrasound survey.13 There is no point in wasting time and effort using ultrasound to identify regurgitation in iliac vein tributaries. However, for specific populations: young and middle-aged women, with significant differences in leg swelling, history of multiple pregnancies, those in postpartum, and those with use of oral contraceptives, further investigation may be required. Suppose a patient presents with hyperpigmentation and skin changes solely due to manifestations of venous reflux disease. Based on current evidence, I do not think routine workup for iliac vein stenosis is warranted.
Dr Nikolov. In everyday practice, NIVL is not routinely investigated. In most cases, we treat varicose veins first, and if there is no improvement, the next step will be to search for deep vein pathology.
Dr Lobastov. Outflow venous obstruction, including NIVL and MTS, has no specific symptoms and signs except for venous claudication.33 In the study of Raju S et al, among 4026 patients with CVD, examination with IVUS was performed in those with CEAP (clinical-etiological-anatomical pathophysiological classification) clinical class 3 or higher, significant limb edema, stasis skin changes, ulceration or lower CEAP clinical classes with severe limb pain (≥5 on a visual analog scale) or recurrent cellulitis. Iliac vein obstruction was found in 879 (22%) patients of the total sample, with NIVL in 319 (8%) individuals.24 A systematic review on venous stenting showed that across all CEAP clinical classes, most often, interventions are performed in limbs with C3 (42%), C4 (22%), and C6 (20%).36 According to these data, every fifth patient who underwent venous stenting had skin hyperpigmentation, induration, eczema, or atrophy. However, attempts to correlate venous symptoms with NIVL of >50% as detected by MRV in unselected patients who underwent medical imaging for other reasons failed.37 So, for today, the individual-based suspicion for NIVL and MTS in patients with CVD can be made in those who have venous symptoms and signs that are disproportionate to what would be explained by the DUS findings or in whom a standard conservative or interventional treatment failed.
Dr Geroulakos. DUS is the initial test in evaluating iliac vein obstruction because it is noninvasive, readily accessible, easy to perform, safe, and cost-effective. Labropoulos et al have reported that it is a sensitive method to identify clinically significant vein stenosis.38 A peak vein velocity ratio of >2.5 across the stenosis is the best criterion for a pressure gradient of ≥3 mm Hg. DUS can be used to select patients for intervention and monitor the treatment’s success during follow-up.
Dr Josnin. DUS remains limited in validation of vein stenosis. It is frequently reported in the literature that the figures are overestimated for one main reason: this examination is done in decubitus, and in some patients, the reduction in diameter is physiological.
Dr Kan. The ability of DUS to detect stenosis of the lilac vein is limited. Existing ultrasonographic diagnosis to verify MTS is via observing the shape and appearance of the vein and measuring the blood flow velocity of the iliac vein. However, it may still give us clues to verify iliac vein stenosis or obstruction.
Dr Nikolov. DUS is the most common technique used to diagnose a venous outflow obstruction. However, technical difficulties in assessing the inferior vena cava and iliac veins may limit its utility, especially in the case of compression and stenosis without full occlusion. DUS presented a high agreement with IVUS for detecting venous obstruction of ≥50%. The velocity ratio ≥2.5 is the best criterion for the detection of significant venous outflow obstructions in iliac veins.39
Dr Lobastov. Besides the well-known criterion of velocity ratio >2.5 over stenosis, the other ultrasound parameters of NIVL are as follows: the absence of blood flow phasicity (no synchronization with the breathing cycle) and the presence of reflux >2.5 seconds on a common femoral vein (CFV); flow index (the ratio of volume flow on the affected and contralateral CFV) <0.7; velocity index (the ratio of velocity on the affected and contralateral CFV) <0.9; obstruction ration ( the ratio of vein diameter) <0.5.38-40 All these criteria have high specificity and low sensitivity that depends on the degree of obstruction. For example, an easily noticeable lack of flow phasicity on CFV is typical for venous obstruction of >80% and rare in less-severe lesions. The presence of reversal flow at the saphenofemoral junction into the epigastric vein is a very specific sign of iliac vein occlusion but could be detected predominantly in postthrombotic vein lesions and not in NIVL.41-43 Generally, transabdominal DUS of iliac veins in expert hands can provide reliable results compared to IVUS in detecting NIVL and assessing the degree of stenosis.44 However, in most cases, it should be used to select patients with suspected venous obstruction for further investigation.
Dr Lobastov. Since NIVL is a radiological phenomenon that requires interventional treatment in some patients, contrast venography was long used for the final verification of obstruction. However, it appeared to underestimate the presence and severity of venous stenosis compared with IVUS.45 The last one is considered a reference standard that allows identifying up to 30% more patients with obstruction than multiplanar venography. The sensitivity of CT venography and MRV in comparison with IVUS reaches 97% to 100%, but specificity is lower (57%-86% for CT venography and 23% for MRV).45 Considering these data, IVUS is a gold standard for NIVL verification when available. If not, noninvasive CT venography and MRV could be used to select patients for venography. However, the extent of morphological lesions detected by IVUS does not always correlate with symptoms and signs of CVD, which raises a question about functional assessment to detect clinically significant venous outflow obstruction.46
Dr Nikolov. IVUS is the gold standard to verify MTS. It provides a real-time evaluation of the vessel lumen, the accurate size of the luminal diameter, and information regarding the vessel wall’s structural changes. It also provides information about the chronicity of the process, helps in correct implantation of venous stents, and also is contrast free.47
Dr Geroulakos. Zymvragoudakis et al prospectively studied 100 consecutive ambulatory outpatients without any history of DVT, presenting to the radiology department for prescheduled abdominal contrast CT for reasons unrelated to venous disease. Patients underwent thorough physical examination, while demographics and a clinical class of the CEAP classification were documented. The diameter and percentage compression of the common iliac vein, compared with the adjacent ipsilateral and the contralateral common iliac vein at the same level, were measured. More than half of the patients presented NIVL as a relatively common anatomic variant, seldom associated with signs and symptoms of CVD.48 As there is no standard in the prescription of the hemodynamic significance of venous stenosis and as the criterion for stenting is arbitrarily considered to be morphological obstructions higher than 50%, Jayarai et al—supporting that the criterion of 50% stenosis is not helpful for treatment decision-making—proposed a new score, the chronic venous insufficiency score (CCVIS). The CCVIS has a maximum score of 134 and uses a combination of the visual analog scale (VAS) for pain score (range, 0-10), venous clinical severity score (VCSS) (range, 0-24), and the 20-item CVD quality-of-life questionnaire (CIVIQ-20) (range, 0-100).46 Moreover, due to the uncertainty mentioned above, a large oversizing of 20% in venous stenting is usually chosen, leading to increased wall shear stress and neointimal hyperplasia formation.
Dr Josnin. First of all, it is necessary to standardize the pelvic venous disorders. There is a fundamental inter-relationship between the different pelvic syndromes, and considering them as separate entities often leads to treatment with suboptimal results. Under the aegis of the American Venous and Lymphatic Society, with a great deal of reflection, a consensus of international experts has resulted in a classification system called the Symptoms-Varices-Pathophysiology classification of pelvic venous disorders, thus allowing, like the CEAP that we use, a better understanding and adaptation of treatment for each patient according to what is appropriate for them and for follow-up over time.49
Concerning the diagnosis of MTS, CT venography, MRV, or IVUS will reveal intraluminal formations; however, the diagnosis of MTS itself will require the demonstration of a network of collaterals upstream from the left common iliac vein.
Dr Kan. For MTS verification, patients must have detailed history tracking, physical examination, and documentation of CEAP classification, as well as VAS and VCSS scorings. The best method of diagnosis depends on the hospital facilities and equipment available. My hospital can use CT venography and venography with recently added IVUS as our equipment. I prefer to use MRV as a better diagnostic tool, but our magnetic resonance tomography cannot be used for this purpose.
Dr Geroulakos. As regards MTS, there is no indication for treatment when asymptomatic. These lesions are permissive, and typically an additional event needs to occur to manifest clinically. The indication to treat should always be clinically driven. When symptomatic, the fibrotic nature of the disease, per se, is responsible for increased percentages of recoil, resulting in angioplasty alone not being sufficient for treatment.50
Therefore, iliac vein stenting is the treatment of choice, as it appears to have excellent long-term patency, minimal morbidity, and satisfactory durable clinical outcome. Wallstents were only available for venous stenting for several years, with excellent results reported by the group of Dr Raju in Jackson, Mississippi.51 Complications such as migration and compression of the stent’s upper end were reported, resulting in reintervention. Lack of radial force at the stent ends collapses the proximal end of the Wallstents and gives a coning configuration when deployed right across the stenosis with no extension in the inferior vena cava.51 Additionally, “jailing,” the impairment of the contralateral flow when a venous stent is extended into the inferior vena cava, increases the risk of contralateral DVT.52 Raju et al described a technique different from extension in the vena cava to protect the contralateral venous flow by using a Gianturco Z stent deployed on the upper part of the Wallstents. The cumulative primary and secondary patency reported at 24 months were 69% and 93%, respectively. Reinterventions were needed in 11% to fix a malfunction. However, the Z stent seemed to facilitate the bilateral stenting.53
The more recent introduction of nitinol venous stents improved some of the limitations of the Wallstents. Nitinol stents do not foreshorten, resulting in more accurate positioning during deployment compared with Wallstents. Moreover, they are more flexible, having a good compression radial force and crush resistance.
Dr Kan. In patients with symptomatic MTS and varicose veins, it is necessary to treat iliac vein disease first. Wallstents and nitinol stents are available with Taiwan Health Insurance. Some physicians have proposed the prophylactic use of bilateral iliac vein stents to prevent the contralateral flow jailing effects, but insufficient data support this view.
Dr Josnin. MTS without intraluminal formations, without venous thrombosis, is a matter of venous compression of the lower limbs.
Dr Lobastov. Venous stenting is a safe and effective procedure to treat chronic venous obstruction, resulting in ulcer healing in 70% of all patients.54,55 The other outcomes, like improvement in symptoms, disease severity, and quality of life (QOL), are being poorly reported and are not suitable for meta-analysis. The only available randomized controlled trial (RCT) found advantages of venous stenting compared with the best conservative care in patients with progressive CVD.56 New dedicated venous stents seem to be as effective as Wallstents, providing relief of venous claudication in 83% to 90% and healing of venous ulcers in 32% to 80% of all patients.55,57
In the absence of good evidence, the indications for venous stenting in patients with a combination of superficial reflux and NIVL are still debatable. One study showed that confirmed NIVL does not affect the results of radiofrequency ablation (RFA) of superficial veins, regarding technical efficacy, disease severity, and QOL.58 In contrast, 2 other trials showed that venous stenting, in addition to endovenous laser ablation therapy (EVLT), improves short-term and long-term outcomes, including ulcer healing and superficial reflux recurrence in patients with confirmed NIVL.59,60 From another point of view, ablation of superficial reflux in addition to venous stenting of NIVL may be beneficial only in patients with progressive CVD (CEAP clinical classes of C4-6),61 or may not provide any advantage in persons with postthrombotic iliac vein lesions.62,63
Considering all these data, venous stenting in patients with symptomatic NIVL should be suggested in progressive CVD (C3-6) when conservative treatment and ablation of superficial veins does not result in sufficient clinical improvement. In noncomplicated CVD (C0-2), ablation of superficial reflux should be considered only irrespective of NIVL, which may be a variation of individual anatomy.
Dr Geroulakos. With regard to anticoagulation and antiplatelet therapy after venous stenting for nonocclusive disease, there is no evidence-based strategy, and such treatment is still under debate. Mahnken et al recommended continuous anticoagulation with warfarin to a target international normalized ratio (INR) range of 2.5-3.0, though there are no evidence-based studies about this.64 However, that target mainly takes into consideration postthrombotic lesions more vulnerable to restenosis. Long-term warfarin is recommended when extended occlusions, thrombophilia, suprarenal occlusions, and poor outflow in angiogram exist.65,66 Meissner reported that antiplatelets seem most appropriate for primary nonocclusive iliac vein lesions, likewise for venous grafts when used in the arterial system; however, anticoagulants play a better role in postthrombotic disease.67 The latter seems to concur with our strategy of antiplatelet therapy for at least 6 weeks post venous stenting for nonocclusive venous disease. Still, the role of antiplatelets has been highly debated in recent literature. Tran et al, in their recent retrospective study, subcategorized the cases of stented NIVL according to the type of anticoagulation treatment received postoperatively for 90 days. The 3 regimens were as follows: i) double antiplatelet (aspirin and clopidogrel); ii) clopidogrel alone; and iii) apixaban/rivaroxaban. In-stent stenosis by DUS was observed, and freedom from in-stent stenosis in 52 weeks was 80.03%, 80.95%, and 83.18%, respectively, with no statistically significant difference between therapy groups.68
On the other hand, in the international Delphi Consensus, with accepting the absence of controlled trials for the use of anticoagulants and antiplatelets following venous stenting, Milinis et al stated that anticoagulation is preferable to antiplatelets for the first 6 to 12 months after stenting an NIVL.69 Of all experts, 72% preferred anticoagulation to antiplatelet therapy following venous stent placement for NIVLs. The recommendation for life-long antiplatelet therapy after anticoagulation is stopped did not achieve consensus. Also, low molecular weight heparins (LMWH) were stated as the anticoagulant of choice for the first 2 to 6 weeks after stenting.
Dr Josnin. Since the 1990s, endovenous treatments have largely supplanted surgery, which is now reserved for use when previous techniques fail. In the 6 months following treatment, patients remain at risk of thrombosis, justifying an antiplatelet or anticoagulant therapy. There is no validated consensus to date, but direct oral anticoagulants (DOACs) are increasingly used. Long-term patency rates remain satisfactory at around 80% to 90%.70
Dr Kan. Venous stenting has become a standard treatment for central deep venous outflow obstructions and postthrombotic syndrome. After a venous stent is placed, maintaining a healthy diet and exercise regimen, taking medications to prevent blood clots, avoiding strenuous activity for some time, and regular follow-up are essential to keep the venous stent patent. After successful recanalization and stenting, stent patency is endangered by in-stent thrombosis and recurrent venous thromboembolism (VTE). Antithrombotic therapy might reduce stent patency loss. The mean primary patency rate of venous stenting with antithrombotic drugs is 82.3% at 1 year and 73.3% 2 years after intervention. Still, there are no specific recommendations on the optimal drug-combination strategy after venous stent placement.71
The value of peri-interventional antithrombotic therapy for optimal long-term outcomes can be inferred from Virchow’s triad. Most previous studies used vitamin K antagonists (VKA) concomitant with LMWH, but recent trials increasingly used DOAC for the treatment. However, as well known, the recurrence rates are significantly lower in patients with nonthrombotic lesions than in patients with previous thrombosis. Therefore, strategies for anticoagulation and antiplatelet therapy after venous stenting for nonocclusive diseases should take into account the essence of the disease. Treating venous disease should differ from coronary or peripheral artery disease based on blood flow velocity and endothelial properties. To prolong patency, I would prefer triple therapy of aspirin and clopidogrel (dual antiplatelet) with apixaban or rivaroxaban for at least 1 month, even if there is no clear evidence yet.
Dr Nikolov. The patency rates, disease prognosis, and need for antithrombotic therapy primarily depend on the nature of iliac vein lesions. In NIVL, there is no evidence to justify prolonged anticoagulation because the risk of thrombosis is very low.72
Dr Tazi Mezalek. In recent years, there has been a growing interest in endovascular stenting of the iliofemoral vein to improve symptoms related to proximal venous obstruction. Maintaining the long-term patency of the stent is one of the main challenges. Published data on the safety and efficacy of the procedure come primarily from cohort studies that focused mainly on mechanical aspects related to stent placement and flow. The impact of the choice and duration of antithrombotic treatment has not been specifically studied. Although antiplatelets have been shown to be beneficial in preventing restenosis of arterial stents, these effects cannot necessarily be extrapolated to venous stents since the generation of thrombin drives venous stent thrombosis.69 Additionally, in an experimental porcine model, McBane et al demonstrated that aspirin and clopidogrel did not prevent stent vein thrombosis, unlike the inhibitor of factor Xa, which completely inhibited venous stent thrombosis.73 Dual antiplatelet plus DOAC exposes patients to a high risk of bleeding without evidence of benefit. Our opinion is to maintain a full dose of oral anticoagulant (preferably DOAC) for long-term treatment.
Dr Lobastov. According to a recent systematic review, stent patency depends on the type of primary lesion (nonthrombotic, postthrombotic, acute DVT) but is not affected by the type and duration of antithrombotic therapy.71 Undoubtedly, stenting in the settings of DVT and postthrombotic syndrome requires prolonged anticoagulation, predominantly with DOACs. In contrast, NIVL seems to be a benign disease with the lowest risk of in-stent thrombosis and stenosis, so prolonged anticoagulation is not obligatory. The recent systematic review suggests that 3 to 6 months of antiplatelet treatment may be enough after stenting of an NIVL.74
Dr Kan. Micronized purified flavonoid fraction (MPFF) can improve venous tone and capillary permeability, but the exact mechanism of action of the drug remains unclear. MPFF has anti-inflammatory, antioxidant, and powerful free-radical scavenging properties. MPFF decreases the expression of adhesion molecules by neutrophils and monocytes in patients with CVD. Based on the experimental results of MPFF usage in chronic venous hypertension, MPFF treatment was found to significantly prevent capillary rarefaction and initiation of the venous inflammatory cascade.75 Summarized results of cohort studies show the hemodynamic and clinical benefits of MPFF. It can normalize the diameter of the great saphenous vein and abolish afternoon reflux, night cramps, evening heaviness, and pain, decreasing the intensity of leg pain (measured using a VAS) and improving QOL.76
MTS is also a CVD, a complex condition characterized by chronic inflammation and remodeling of the venous wall, resulting in valve damage, reflux, and venous hypertension. Chronic inflammation eventually affects microcirculation, producing skin changes and ulceration. MPFF improves venous tone and increases lymphatic drainage and can be used alone in the early stages or as an adjunct to surgery, sclerotherapy, endovenous thermal ablation, or compression. After venous stenting, the use of MPFF is not contraindicated. Therefore, I would still use MPFF as an adjunct therapy for patients with May-Thurner syndrome.
Dr Josnin. By analogy with the validated and recommended indications for CVD, in particular by the latest recommendations of the European Society for Vascular Surgery, the use of MPFF in symptomatic patients is indicated.77 The pharmacokinetics and mode of action of the molecule have been well described and its efficacy demonstrated. To the best of my knowledge, there is no study in the literature specifically about its use in MTS, but there are many articles on chronic pelvic pain, particularly in pelvic congestion syndrome. Although the continuum between the different pathophysiological entities is sometimes difficult to define, an improvement in QOL and in severity of pathology was recently shown in women with pelvic congestion syndrome.78
Dr Lobastov. MPFF is a well-studied drug that demonstrated high efficacy in CVD of all clinical classes.79 It significantly improves individual symptoms, signs, and QOL, reduces edema, redness, and skin changes, and accelerates the healing of leg ulcers.80,81 It can be used in adjunct to open surgery and endovenous treatment to improve functional and aesthetic outcomes.82 Despite the absence of direct evidence of MPFF use in NIVL or MTS, the drug is still indicated to treat venous symptoms and signs of CVD before, after, or instead of stenting. Considering the prevalence of NIVL in a population of patients with CVD, it can be assumed that many individuals with venous obstruction participated in the trials with MPFF and achieved positive results. Moreover, in the only RCT with stenting, pain at 6 months after intervention reduced from a score of 9 to 2.5, and VCSS decreased from 18.5 to 11.0.56 So, even after interventional treatment, patients still had indications for MPFF due to the persistence of venous-specific symptoms and signs. Of course, the role of adjunctive pharmacological therapy in obstructive venous disease should be evaluated in specific trials.
Conclusion
• NIVL is a widespread condition in the general population and among patients with CVD that not always needs to be confirmed and treated. MTS is a type of NIVL with intraluminal fibrotic changes (spurs) in the common iliac vein, and it may be responsible for the development of CVD with skin changes.
• Investigation for venous outflow obstruction is not necessary for all CVD patients. The individual-based suspicion for NIVL and MTS should be made in those who have venous symptoms and signs that are disproportionate to what is explained by DUS findings or in whom a standard conservative or interventional treatment failed. This particularly includes patients with persistent ulcers despite saphenous ablation; significant leg swelling or pain disproportionate to reflux, the extent and size of the varicose veins; deterioration of lipodermatosclerosis; and pigmentation with adequate treatment of superficial venous reflux.
• DUS of femoral and iliac veins can be used as a first approach to detect patients with suspected NIVL. CT venography, MRV, and IVUS should verify the obstruction. IVUS is the reference standard to confirm NIVL, assess its degree, and assist with stenting.
• In patients with combined NIVL and superficial reflux, the efficacy of isolated superficial vein ablation is controversial. Adjunct venous stenting should be considered in those with progressive CVD (C3-6), especially when conservative treatment and superficial ablation are not effective.
• The type and duration of antithrombotic therapy after stenting of NIVL is under debate. Emerging evidence suggests that single antiplatelet therapy may be sufficient for 3 to 6 months. However, many experts and practitioners still prefer treatment with VKA and DOACs for 6 to 12 months.
• MPFF in NIVL and MTS is indicated to treat symptoms and signs of CVD before, after, or instead of venous stenting.

CORRESPONDING AUTHOR
George Geroulakos
Attikon University Hospital of the National and Kapodistrian University of Athens, 1 Rimini Street, Haidari, 124 62 Athens, Greece
email: ggeroulakos@med.uoa.gr
References
1. Duran C, Rohatgi S, Wake N, Rybicki FJ, Steigner M. May-Thurner syndrome: a case report. Eurasian J Med. 2011;43(2):129-131.
2. Peters M, Syed RK, Katz M, et al. May Thurner syndrome: a not so uncommon cause of a common condition. Proc (Bayl Univ Med Cent). 2012;25(3):231-233.
3. Abboud G, Midulla M, Lions C, et al. “Right sided” May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33(5):1056-1059.
4. Burke RM, Rayan SS, Kasirajan K, Chaikof EL, Milner R. Unusual case of right-sided May-Thurner syndrome and review of its management. Vascular. 2006;14(1):47-50.
5. Cavalcante LP, dos Santos Souza JE, Pereira RM, et al. Iliac vein compression syndrome: literature review. J Vasc Bras. 2015;14:78-83.
6. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937-943.
7. Png CYM, Nakazawa KR, Lau IH, Tadros RO, Faries PL, Ting W. Bilateral May-Thurner syndrome refractory to iliac aneurysm repair. J Vasc Surg Venous Lymphat Disord. 2018;6(5):657-660.
8. Virchow R. Thrombose und Embolie: Gefässenzýndung und Septische Infektion. Gesammelte Abhandlungen zur wissen schaftlichen Medicin. Meidinger, Sohn and Co; 1856.
9. Mcmurrich JP. The occurrence of congenital adhesions in the common iliac veins, and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1908;135(3):342.
10. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(5):419-427.
11. Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg. 1965;52(10):816-821.
12. Harbin MM, Lutsey PL. May-Thurner syndrome: history of understanding and need for defining population prevalence. J Thromb Haemost. 2020;18(3):534-542.
13. Zurkiya O, Ganguli S, Irani Z, et al. Incidence of May-Thurner syndrome (MTS) in patients under evaluation of lower extremity venous reflux: implications for treatment. J Vasc Interv Radiol. 2015;2(26):S139-S140.
14. Birn J, Vedantham S. May-Thurner syndrome and other obstructive iliac vein lesions: meaning, myth, and mystery. Vasc Med. 2015;20(1):74-83.
15. Virchow R. Ueber die erweiterung kleinerer gefäfse. Arch Pathol Anat Phyiol Klin Med. 1851;3(3):427-462.
16. Zhu Q, Yang L, Zhu H, et al. Prevalence of left iliac vein compression in an asymptomatic population and patients with left iliofemoral deep vein thrombosis: a multicenter cross-sectional study in southern China. Phlebology. 2022;37(8):602-609.
17. Oguzkurt L, Ozkan U, Ulusan S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19(3):366-370;quiz 71.
18. Nazzal M, El-Fedaly M, Kazan V, et al. Incidence and clinical significance of iliac vein compression. Vascular. 2015;23(4):337-343.
19. Wu MK, Luo XY, Zhang FX. Incidence and Risk Factors of Deep Venous Thrombosis in Asymptomatic Iliac Vein Compression: a prospective cohort study. Chin Med J (Engl). 2016;129(18):2149-2152.
20. Chen F, Deng J, Hu XM, Zhou WM. Compression of the right iliac vein in asymptomatic subjects and patients with iliofemoral deep vein thrombosis. Phlebology. 2016;31(7):471-480.
21. Cheng L, Zhao H, Zhang FX. Iliac Vein Compression Syndrome in an Asymptomatic Patient Population: a prospective study. Chin Med J (Engl). 2017;130(11):1269-1275.
22. van Vuuren T, Kurstjens RLM, Wittens CHA, van Laanen JHH, de Graaf R. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg. 2018;56(6):874-879.
23. Chung JW, Yoon CJ, Jung SI, et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15(3):249-256.
24. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143; discussion 44.
25. Marston W, Fish D, Unger J, Keagy B. Incidence of and risk factors for iliocaval venous obstruction in patients with active or healed venous leg ulcers. J Vasc Surg. 2011;53(5):1303-1308.
26. Liu Z, Gao N, Shen L, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg. 2014;28(3):695-704.
27. Dzieciuchowicz L, Krzyzanski R, Kruszyna L, Krasinski Z, Gabriel M, Oszkinis G. Prevalence of non-thrombotic iliac vein lesions in patients with unilateral primary varicose veins. Eur J Vasc Endovasc Surg. 2016;51(3):429-433.
28. Liu P, Peng J, Zheng L, et al. Application of computed tomography venography in the diagnosis and severity assessment of iliac vein compression syndrome: a retrospective study. Medicine (Baltimore). 2018;97(34):e12002.
29. Aurshina A, Kheyson B, Eisenberg J, et al. Clinical correlation of anatomical location of non-thrombotic iliac vein lesion. Vascular. 2017;25(4):359-363.
30. Behzadi AH, Khilnani NM, Zhang W, et al. Pelvic cardiovascular magnetic resonance venography: venous changes with patient position and hydration status. J Cardiovasc Magn Reson. 2019;21(1):3.
31. Krzanowski M, Partyka L, Drelicharz L, et al. Posture commonly and considerably modifies stenosis of left common iliac and left renal veins in women diagnosed with pelvic venous disorder. J Vasc Surg Venous Lymphat Disord. 2019;7(6):845-852.e2.
32. Avgerinos ED, Geroulakos G. Ablate early the superficial reflux but don’t neglect deep reflux or obstruction. J Vasc Surg Venous Lymphat Disord. 2019;7(3):315- 316.
33. Tsouknidas I, Charisis N, Eklof B, Labropoulos N. Venous claudication: a scoping review of the pathophysiology and clinical importance. Eur J Vasc Endovasc Surg. 2022;64(5):535-543.
34. Chen CW, Ting H, Chen PY, et al. Usefulness of triggered non-contrast-enhanced magnetic resonance angiography in assessing lower extremity venous disease. Medicine (Baltimore). 2021;100(20):e25809.
35. Chen CW, Tseng YH, Wong MY, Wu CM, Lin BS, Huang YK. Stasis leg ulcers: venous system revises by triggered angiography non-contrast-enhanced sequence magnetic resonance imaging. Diagnostics (Basel). 2020;10(9).
36. Seager MJ, Busuttil A, Dharmarajah B, Davies AH. Editor’s choice– a systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51(1):100-120.
37. Aurshina A, Huber S, Deng Y, et al. Correlation of venous symptoms with iliac vein stenosis on magnetic resonance imaging. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1291-1296.e1.
38. Labropoulos N, Borge M, Pierce K, Pappas PJ. Criteria for defining significant central vein stenosis with duplex ultrasound. J Vasc Surg. 2007;46(1):101-107.
39. Metzger PB, Rossi FH, Kambara AM, et al. Criteria for detecting significant chronic iliac venous obstructions with duplex ultrasound. J Vasc Surg Venous Lymphat Disord. 2016;4(1):18-27.
40. 4Mousa AY, Broce M, Yacoub M, et al. Validation of venous duplex ultrasound imaging in determining iliac vein stenosis after standard treatment of active chronic venous ulcers. J Vasc Surg Venous Lymphat Disord. 2016;4(3):307-312.
41. Kolluri R, Fowler B, Ansel G, Silver M. A novel duplex finding of superficial epigastric vein flow reversal to diagnose iliocaval occlusion. J Vasc Surg Venous Lymphat Disord. 2017;5(3):358-362.
42. Sermsathanasawadi N, Pruekprasert K, Pitaksantayothin W, et al. Prevalence, risk factors, and evaluation of iliocaval obstruction in advanced chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2019;7(3):441-447.
43. Chinchalongporn W, Tanmit P, Pruekprasert K, et al. Prevalence and predictors of combined >50% iliocaval venous obstruction and superficial venous reflux in chronic venous insufficiency patients with healed or active venous leg ulcer. J Vasc Surg Venous Lymphat Disord. 2023;11(3):502-509.
44. Villalba L, Larkin TA. Transabdominal duplex ultrasound and intravascular ultrasound planimetry measures of common iliac vein stenosis are significantly correlated in a symptomatic population. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1273-1281.
45. Saleem T, Raju S. Comparison of intravascular ultrasound and multidimensional contrast imaging modalities for characterization of chronic occlusive iliofemoral venous disease: a systematic review. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1545-1556.e2.
46. Jayaraj A, Powell T, Raju S. Utility of the 50% stenosis criterion for patients undergoing stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1408-1415.
47. Knuttinen M, Naidu S, Oklu R, et al. May Thurner: diagnosis and endovascular management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S159-S164.
48. Zymvragoudakis V, Spiliopoulos S, Moulakakis K, Lattimer C, Geroulakos G. Incidence and clinical significance of non thrombotic iliac vein lesions. Eur J Vasc Endovasc Surg. 2019;58(6):e125.
49. Meissner MH, Khilnani NM, Labropoulos N, et al. The Symptoms-Varices Pathophysiology classification of pelvic venous disorders: a report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord. 2021;9(3):568-584.
50. Park JY, Ahn JH, Jeon YS, Cho SG, Kim JY, Hong KC. Iliac vein stenting as a durable option for residual stenosis after catheter directed thrombolysis and angioplasty of iliofemoral deep vein thrombosis secondary to May-Thurner syndrome. Phlebology. 2014;29(7):461-470.
51. Raju S. Best management options for chronic iliac vein stenosis and occlusion. J Vasc Surg. 2013;57(4):1163-1169.
52. Le TB, Lee TK, Park KM, Jeon YS, Hong KC, Cho SG. Contralateral deep vein thrombosis after iliac vein stent placement in patients with May-Thurner Syndrome. J Vasc Interv Radiol. 2018;29(6):774-780.
53. Raju S, Ward M Jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014;28(6):1485-192.
54. Wen-da W, Yu Z, Yue-Xin C. Stenting for chronic obstructive venous disease: a current comprehensive meta-analysis and systematic review. Phlebology. 2016;31(6):376-389.
55. Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and venacaval occlusive disease. J Vasc Surg Venous Lymphat Disord. 2020;8(1):145- 153.
56. Rossi FH, Kambara AM, Izukawa NM, et al. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6(2):183-191.
57. Majeed GM, Lodhia K, Carter J, et al. A systematic review and meta-analysis of 12-month patency after intervention for iliofemoral obstruction using dedicated or non-dedicated venous stents. J Endovasc Ther. 2022;29(3):478-492.
58. Li X, Zhang H, Niu L, et al. Clinical outcomes of radiofrequency ablation for patients with varicose veins of the lower extremities combined with grade II iliac vein compression. J Vasc Surg Venous Lymphat Disord. 2021;9(3):676-682.e2.
59. Yang X, Wu X, Peng Z, Yin M, Lu X, Ye K. Outcomes of endovenous laser ablation with additional iliac vein stenting of nonthrombotic lesions in patients presenting with active venous ulcers. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1517-1525.
60. Yin M, Huang X, Cui C, et al. The effect of stent placement for May-Thurner syndrome combined with symptomatic superficial venous reflux disease. J Vasc Surg Venous Lymphat Disord. 2015;3(2):168-172.
61. Guo Z, Li X, Wang T, Liu J, Chen B, Fan L. Effectiveness of iliac vein stenting combined with high ligation/endovenous laser treatment of the great saphenous veins in patients with clinical, etiology, anatomy, pathophysiology class 4 to 6 chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2020;8(1):74-83.
62. Nayak L, Hildebolt CF, Vedantham S. Postthrombotic syndrome: feasibility of a strategy of imaging-guided endovascular intervention. J Vasc Interv Radiol. 2012;23(9):1165-1173.
63. Lawrence PF, Hager ES, Harlander-Locke MP, et al. Treatment of superficial and perforator reflux and deep venous stenosis improves healing of chronic venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020;8(4):601-609.
64. Mahnken AH, Thomson K, de Haan M, O’Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37(4):889-897.
65. Kurklinsky AK, Bjarnason H, Friese JL, et al. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single center experience. J Vasc Interv Radiol. 2012;23(8):1009-1015.
66. Raju S, Neglen P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009;50(2):360- 368.
67. Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology. 2013;28(suppl 1):91-98.
68. Tran MA, Lakhanpal P, Lakhanpal S, Satwah VK, Lakhanpal G, Pappas PJ. Type of antithrombotic therapy for venous stenting in patients with non-thrombotic iliac vein lesions does not influence the development of in-stent restenosis. Phlebology. 2020;35(10):805-813.
69. Milinis K, Thapar A, Shalhoub J, Davies AH. Antithrombotic therapy following venous stenting: international Delphi consensus. Eur J Vasc Endovasc Surg. 2018;55(4):537-544.
70. Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio-caval obstructive lesions: long-term results. Eur J Vasc Endovasc Surg. 2009;38(1):118- 124.
71. Notten P, Ten Cate H, Ten Cate-Hoek AJ. Postinterventional antithrombotic management after venous stenting of the iliofemoral tract in acute and chronic thrombosis: a systematic review. J Thromb Haemost. 2021;19(3):753-796.
72. Pappas PJ, Lakhanpal G, Lakhanpal S, et al. Immediate postprocedure anticoagulation with factor Xa inhibitors of venous stents for nonthrombotic venous lesions does not increase stent patency. J Vasc Surg Venous Lymphat Disord. 2022;10(3):633-639.e1.
73. McBane RD 2nd, Leadley RJ Jr, Baxi SM, Karnicki K, Wysokinski W. Iliac venous stenting: antithrombotic efficacy of PD0348292, an oral direct factor Xa inhibitor, compared with antiplatelet agents in pigs. Arterioscler Thromb Vasc Biol. 2008;28(3):413-418.
74. Veyg D, Alam M, Yelkin H, Dovlatyan R, DiBenedetto L, Ting W. A systematic review of current trends in pharmacologic management after stent placement in nonthrombotic iliac vein lesions. Phlebology. 2022;37(3):157-164.
75. das Graças C de Souza M, Cyrino FZ, de Carvalho JJ, Blanc-Guillemaud V, Bouskela E. Protective effects of micronized purified flavonoid fraction (MPFF) on a novel experimental model of chronic venous hypertension. Eur J Vasc Endovasc Surg. 2018;55(5):694-702.
76. Bouskela E, Lugli M, Nicolaides A. New perspectives on micronised purified flavonoid fraction in chronic venous disease: from microvalves to clinical effectiveness. Adv Ther. 2022;39(10):4413-4122.
77. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s choice – European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
78. Akhmetzianov RV, Bredikhin RA. Clinical efficacy of conservative treatment with micronized purified flavonoid fraction in female patients with pelvic congestion syndrome. Pain Ther. 2021;10(2):1567- 1578.
79. Mansilha A, Gianesini S, Ulloa JH, et al. Pharmacological treatment for chronic venous disease: an umbrella review of systematic reviews. Int Angiol. 2022;41(3):249-257. 80.
80. Kakkos S, Nicolaides A. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and meta analysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(2):143-154.
81. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30(2):198-208.
82. Mansilha A, Sousa J. Benefits of venoactive drug therapy in surgical or endovenous treatment for varicose veins: a systematic review. Int Angiol. 2019;38(4):291-298.