Combination of May-Thurner syndrome and pelvic congestion syndrome: terra incognita
MD, PhD
Professor of Surgery, Savelyev University
Surgical Clinic, Pirogov Russian National
Research Medical University, Russia
Abstract
This review presents up-to-date data on the pathophysiology, epidemiology, classification, diagnosis, and treatment of the combination of May-Thurner syndrome (MTS) and pelvic congestion syndrome (PCS). It includes hypotheses to explain the predominant lesion of the pelvic veins in these patients and describes in detail the clinical symptoms of combined lesions of the iliac and pelvic veins. The article discusses modern methods of diagnosis of MTS and PCS, as well as advantages and disadvantages of ultrasound and radiological methods of investigation. It goes further to discuss the issues of choosing a method of treatment for combination of MTS and PCS and highlights the optimal sequence of using endovascular methods of treatment. It presents current data on the efficacy of iliac vein stenting in relieving PCS symptoms and discusses rational use of endovascular treatment methods to avoid unnecessary interventions on the gonadal veins. Altogether, these data indicate the lack of our knowledge both in regard to pathogenesis of the MTS and PCS combination and in determining the optimal set of diagnostic tests for verifying the diagnosis and choosing a treatment method. Multicenter randomized trials are needed to address many of the controversial issues in the diagnosis and treatment of MTS and PCS.
Introduction
Pelvic congestion syndrome (PCS) and May-Thurner syndrome (MTS) are two nosologies that have long been considered separately from each other. This is understandable because PCS is the common cause of chronic pelvic pain (CPP)1-3 and MTS is a factor in the development of deep-vein thrombosis (DVT), venous claudication, and edema of the left lower extremity.4,5 The situation changed within the last decade with the publication of studies6-8 reporting case series of the combination of PCS and MTS; at that time, the main clinical end point was pelvic pain, not the symptoms of chronic venous disease (CVD) or venous thrombosis. The paradigm shift was reflected both in the updated Clinical, Etiology, Anatomic, Pathophysiology (CEAP) classification9 and in changes in terminology, as well as in the direction of research.10,11 The authors proposed use of the umbrella term “pelvic venous disorder” (PeVD) to describe various disorders of the pelvic veins (PCS, MTS, nutcracker syndrome, and ovarian vein syndrome). Despite a significant number of studies on the diagnosis and treatment of MTS and PCS, more and more questions arise about the optimal method for assessing the degree of stenosis of the left common iliac vein (CIV) and the approaches to treatment of patients with a combination of MTS and PCS. This article aims to provide an overview of current trends in the diagnosis and treatment of the combination of MTS and PCS.
Epidemiology
The reported prevalence of MTS varies widely from 4% to 60% depending on the degree of CIV stenosis and the size of the studied population.12,13 In the study of Kibbe et al, a narrowing of the left CIV of greater than 50% was identified in 24% of patients, and stenosis greater than 25% was diagnosed in 66% of the examined asymptomatic patients.14 Liu et al reported the presence of MTS in 11% of symptomatic patients.15 PCS is diagnosed in 15% of women of reproductive age and in 30% of women seeking medical help from a gynecologist for CPP.16 According to different authors, one in every 10 women has a dilation of the gonadal veins (GV), and 60% of them eventually develop PCS.17,18
However, the real prevalence of the MTS and PCS combination is difficult to determine, as the screening for pelvic venous disease in a large population requires reliable imaging techniques. Pelvic vein dilation or left CIV stenosis does not necessarily indicate the presence of PCS or CVD.19,20 As such, the prevalence of combined MTS and PCS is mostly reported for certain groups of patients, most often with CPP or DVT. Thus, in a study of 277 patients with pelvic venous insufficiency, Santoshi et al reported an 80% prevalence of CIV stenosis greater than 50%.8 According to data from our clinic, the prevalence of combined MTS and PCS in patients with CPP is 23%, and 8% of patients have a left CIV stenosis greater than 50% as assessed by multiplanar pelvic venography (MPPV).21
Pathophysiology
The morphological substrate of MTS is the narrowing of the left CIV lumen due to its pulsatile compression between the overlying right iliac artery and the fifth lumbar vertebrae. To date, it is unclear why some patients with MTS present with CVD symptoms and signs while others develop PCS. Probably, in patients with MTS, the lesion of the left internal iliac veins (IIV), parametrial veins (PV), uterine veins (UV – uterine veins), and GV is one of the variants of the disease course, which is genetically determined (Figure 1). It can be speculated that, in such patients, the valves in IIV, PV, GV are absent or underdeveloped, and with worsening stenosis, blood reflux initially occurs in the left IIV and triggers a cascade of abnormalities in other veins.
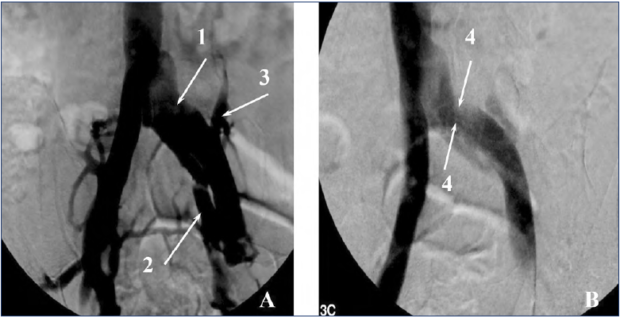
Figure 1. Pelvic venography. (A) Direct projection. (B) Lateral projection. 1, dilated left common iliac vein; 2, dilated left internal iliac vein; 3, reflux of contrast media in the left iliolumbar vein; 4, compression of the left common iliac vein by the right common iliac artery.
Nevertheless, MTS is an obvious morphological substrate for the development of pelvic varicose veins and occurrence of reflux and venous congestion in the pelvic organs. Disruption of normal blood flow in the left CIV is accompanied by active collateralization of venous outflow from the pelvis. This is manifested by the left IIV dilation and occurrence of reflux in its trunk and tributaries, PV, UV, and in the left iliolumbar vein. GV, same as collaterals, are probably affected at the late stage, as dilation of these veins and reflux in them are caused by an increase in hemodynamic load and in the volume of blood flowing through these thin-walled vessels, which are not adapted to such intense blood flow. As a result, the dilation of PV and reflux in these veins lead to the development of PCS. Combined lesions of the pelvic and lower-extremity veins can be a variant of MTS. In such patients, the clinical picture also includes symptoms and signs of CVD.
Classification
The symptomatic and asymptomatic course of MTS is distinguishable, which largely determines treatment strategy. Conventionally, left CIV stenosis is considered hemodynamically significant or insignificant if a reduction in lumen diameter is greater or lesser than 50%, respectively.8,12 Significant CIV stenosis requires intervention, whereas insignificant CIV does not affect blood outflow from the pelvis and lower extremities. Patients with a combination of MTS and PCS should be classified using the updated revision of the CEAP classification.9 Although the clinical part of that classification section does not include symptoms and signs of PCS, the other three parts are quite applicable for such patients. Meissner et al proposed a new classification of pelvic venous disorders (PeVDs),11 which includes the following three domains: Symptoms (S), Varices (V), and Pathophysiology (P), with the pathophysiology domain encompassing the Anatomic (A), Hemodynamic (H), and Etiologic (E) features of the patient’s disease. An individual patient’s classification is designated as SVP A, H, E. For patients with pelvic origin of the lower-extremity signs or symptoms, the SVP classification is complementary to and should be used in conjunction with CEAP classification. Indeed, the development of the SVP classification is an important step forward in the implementation of new terminology and stratification of patients with PeVD. Whereas it is the first that is focused on pelvic venous insufficiency; it does contain controversial statements and generalizations that require further clarification. It is appropriate here to recall the well-known quote from Albert Einstein: “Everything should be made as simple as possible, but no simpler.” Nevertheless, it should be recognized that the foundation for further research has been laid and clinicians now have something to work on.
Clinical manifestations
Regarding clinical manifestations, it’s better to start not with the description of venous-specific symptoms and signs, but with a presentation of the general status of patients with a combination of MTS and PCS. Typically, they are young women aged 25 to 40 years who have given birth several times, who have a low body mass, bad mood, and are presenting numerous emotional complaints. Exaggerating, I would designate three signs of such a woman: young, thin, and angry (or emotionally labile). This is supported by studies that indicate that patients with PCS have a reduced body mass index.22 Behavioral changes are understandable, as long-term pelvic pain affects the personality.23
Patients with a combination of MTS and PCS most often have symptoms and signs of venous congestion of the pelvic organs, such as pelvic pain, feeling of heaviness or discomfort in the hypogastric region, coital and postcoital pain, urination disorders, vulvar varicose veins, and varicose veins on the posterior surface of the thigh.24,25 Pelvic venous pain (PVP) is typically described as a constant, dull, and aching pain localized in the hypogastric, left or right iliac regions, which increases in the second phase of the menstrual cycle, after static load or physical exertion, and with intake of gestagenic drugs, and decreases when the patient is in the horizontal position, after a night’s rest, and with use of venoactive drugs. The PVP is characterized most often as a dull and aching feeling; however, a number of patients describe pain as stabbing, burning, or cramping.26-28 PVP is noncyclic pain lasting more than 6 months and arising with dilation of the pelvic veins, which is localized in the lesser pelvis. It worsens the patient’s quality of life and requires medical or surgical treatment.
A feature of dyspareunia in PCS is the persistence of pain after intercourse for a period of time lasting from 15 minutes to a day.29 The superficial dyspareunia, which occurs in patients with vulvar varicose veins, is distinguished from deep dyspareunia characteristic of venous congestion of the uterus. The presence of deep dyspareunia is an unfavorable prognostic sign indicating significant pelvic venous congestion.26,29,30 Vulvar (gluteal, perineal) varicose veins and varicose veins of the posterior surface of the thigh are pathognomonic signs of pelvic vein abnormalities (Figure 2).
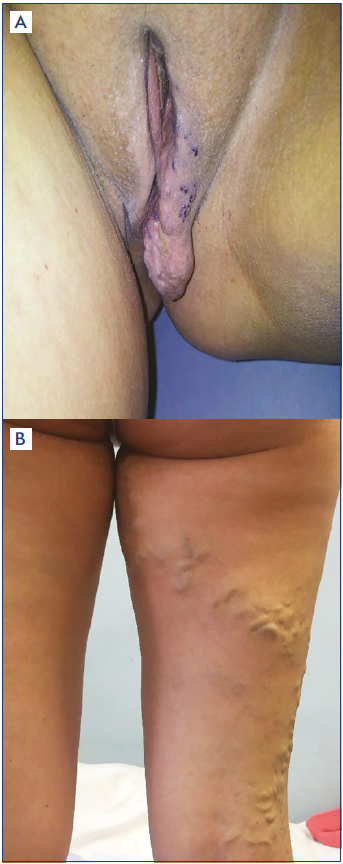
Figure 2. Photos of patients with (A) vulvar varicosities and (B) varicose veins on the posterior surface of the thigh at the confluence with the pelvic veins.
It is notable that a number of patients with a combination of MTS and PCS have symptoms and signs of CVD, including leg pain, edema, telangiectasias, and varicose veins of the lower limbs.31 One of the signs of iliocaval obstruction is venous claudication.32 Thors et al revealed symptoms and signs of CVD in 75% of patients with PCS, and 6% of them had MTS.33 Left CIV stenosis is also considered one of the factors in the development of DVT. In a study by Larkin et al, iliac vein obstruction was considered more important in the development of CPP than valvular incompetence of GV.34 Although the authors point to a high prevalence of CVD symptoms and signs, it should be understood that they are related to post-thrombotic syndrome and not MTS. This emphasizes the significance of differentiation between thrombotic and nonthrombotic obstructions of the left CIV, as clinical manifestations and treatment approach in such patients are cardinally different. Esposito et al presented an analysis of 27 publications focused on MTS, but for some reason there is not a single mention of MTS as the reason for the development of PCS.35
Based on experience from our clinic, where, annually, we treat more than 5000 patients with various venous disorders, it can be argued that symptoms and signs of CVD in patients with a combination of MTS and PCS are rare and in 80% of cases are limited to CEAP class C1.21
Other clinical manifestations of the combination of MTS and PCS include urination disorders characterized by frequent urge to urinate.26 This is due to the venous congestion of the bladder wall. Women with MTS and PCS often report various irregularities in the menstrual cycle, including irregular heavy and prolonged periods. Venbrux et al have demonstrated the absence of the effect of GV embolization (GVE) on the menstrual cycle in such patients.36 Dysmenorrhea is likely due more to hormonal disorders than PeVD.
Thus, the clinical manifestations of the combination of MTS and PCS are highly variable and nonspecific. Perhaps only vulvar varicose veins can be used as a pathognomonic sign of such combined lesions. Additional diagnostic tests are required for the timely and precise diagnosis of this combined pathology.
Diagnosis
Detection and assessment of pelvic veins is impossible without the use of ultrasound and radiological techniques. This is determined by the deep location of pelvic veins, the nonspecific clinical picture, and the need to obtain accurate information about the diameters of the pelvic, iliac, and renal veins and to establish the presence and duration of pelvic venous reflux in numerous pelvic veins. This is especially true for patients with a combination of MTS and PCS because the disorders of pelvic vein hemodynamics are the most complex in this category of patients.
Duplex ultrasound scanning (DUS)
Duplex ultrasound scanning (DUS) of the pelvic veins is considered the gold standard method for the diagnosis of pelvic varicose veins.37,38 With DUS it is possible to measure diameters of pelvic (parametrial, uterine, gonadal) veins, establish the presence of reflux and its duration, as well as objectively assess the state of the left renal vein (nutcracker syndrome) and the patency and diameters of the iliac veins.39 Other advantages of DUS include its noninvasiveness, the reproducibility of results, and the absence of radiation exposure. Disadvantages include operator dependence and the need for bowel preparation and following of a diet before the examination for better imaging of the pelvic veins. Whiteley et al report a high informative value of DUS in detecting pelvic-perineal reflux (PPR) and valvular incompetence of the internal pudendal, obturator, and inferior gluteal veins.37 Our experience with DUS in patients with MTS and PCS suggests the opposite: the reliable imaging of the IIV tributaries with DUS is unlikely and, most importantly, is completely unnecessary.40 If the treatment of vulvar varicose veins consists of sclerotherapy or excision of the vulvar veins, the presence of reflux in the IIV tributaries makes no difference. But if the task is localization diagnosis of PPR and embolization of IIV tributaries, pelvic venography is essential. Therefore, there is no point in wasting time and effort to identify reflux in the IIV tributaries using DUS. The main purpose of performing DUS in PeVD is to detect pelvic varicose veins and pelvic venous reflux and to assess the left renal vein (Figure 3).
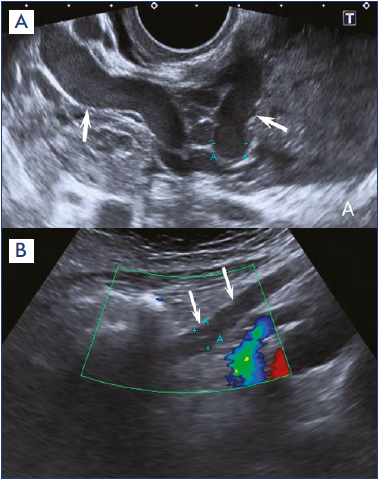
Figure 3. Duplex ultrasound scanning. (A) Arrows indicate dilated parametric veins. (B) Arrows indicate a dilated left gonadal vein.
The ability of DUS to detect stenosis of the left CIV is limited. A number of authors use various functional tests to diagnose compression of the iliac veins and to measure the blood flow velocity in the iliac veins in order to verify MTS.41 This can only indirectly indicate a narrowing of the left CIV. Additional methods should be used to accurately assess the iliac veins.
Multispiral computed venography (MSCV)
Multispiral computed venography (MSCV) has been successfully used in the diagnosis of venous pelvic disorders. MSCV provides reliable visualization of GV, PV, UV, inferior vena cava, iliac, and renal veins.30,42-44 MSCV does not allow detection of venous reflux and measuring blood flow velocity; however, it shows venous anatomy very well (Figure 4). The possibility of obtaining frontal, sagittal, transverse, and three-dimensional images also contributes to a qualitative assessment of the status of pelvic veins and to the accurate assessment of the degree of left CIV stenosis. Many authors report a high accuracy of MSCV in the verification of MTS and PCS, as well as the correlation between the results of computed venography and intravascular ultrasound (IVUS).45 According to Jayaraj and Raju, three-dimensional computed venography represents a noninvasive and accurate technique for measuring the degree of left CIV stenosis and can be successfully used to determine the required caliber and length of the stent.46 It should be noted that the results of MSCV are very indicative in the nutcracker syndrome, and native computed tomography can reveal the pathology of organs and tissues of the abdominal cavity and pelvis.47,48 This is important for differential diagnosis of the causes of CPP. The disadvantages of the techniques are radiation exposure and the need to use contrast media.
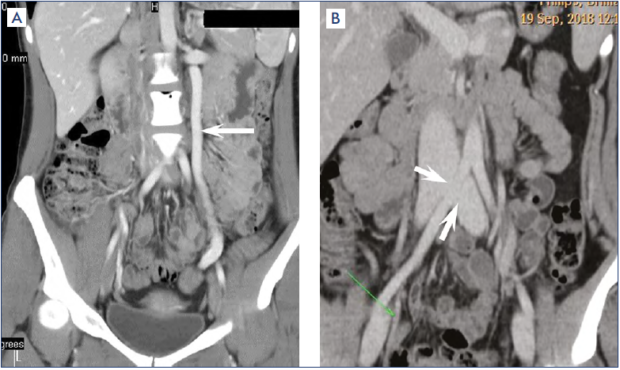
Figure 4. Computed venography. (A) Arrow indicates a dilated left gonadal vein. (B) Arrows indicate compression of the left common iliac vein.
Magnetic resonance venography (MRV)
Magnetic resonance venography (MRV) provides the same information about the pelvic veins as MSCV.49-51 However, unlike computed tomography, it is possible to obtain images of the veins without using contrast media, and there is no radiation exposure. The use of contrast media increases the sensitivity of the test, and the dynamic scanning mode allows visualization of pelvic venous reflux. MRV detects pelvic varicose veins, and compression of the left CIV and left renal vein. According to McDermott et al, MRV does not always allow an objective assessment of the degree of left CIV stenosis.52 The study is contraindicated in patients with pacemakers or metal implants.
Ovarian venography (OV) and multiplanar pelvic venography (MPPV)
Despite the availability of such high-tech modalities as MSCV, MRI, and IVUS, conventional venography has not lost its relevance to date.53,54 Until embolization and stenting of veins are used, it will not be possible to refuse venography because it is a part of these procedures. The classic venographic sign of pelvic venous congestion is reflux of the contrast media in the left or right GV with the cross flow of contrast agent to the opposite side through the incompetent PV and UV. Renal venography always precedes left GV imaging in order to detect or exclude stenosis of the left renal vein. Pelvic venography in patients with MTS should be performed in frontal and lateral views. This provides the most accurate assessment of the degree of left CIV stenosis. Signs of hemodynamically significant MTS are narrowing of the left CIV in combination with its prestenotic dilation, substantial reflux of contrast media into the left IIV and visualization of pelvic collaterals, and reflux of the contrast media into the dilated left iliolumbar vein (Figure 5). In addition, during venography, it is possible to measure the pressure in pre- and post-stenotic parts of the left CIV. A pressure gradient greater than 2-4 mm Hg is considered a sign of hemodynamically significant stenosis of the left CIV.55
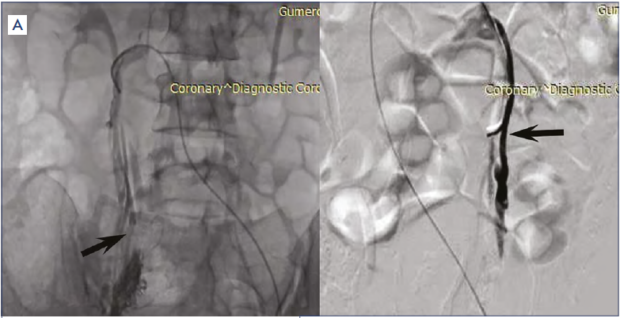
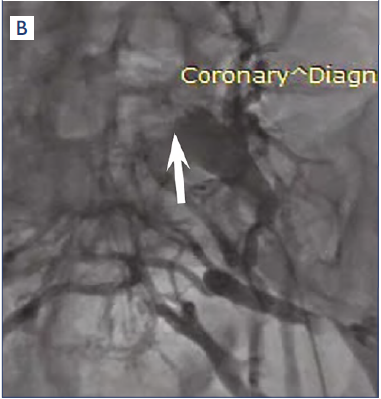
Figure 5. Ovarian and pelvic venography. (A) Arrows indicate dilated left and right gonadal veins. (B) Arrow indicates compression stenosis of the left common iliac vein.
Intravascular ultrasound (IVUS)
IVUS is a reliable method for assessing the status of iliac veins. According to many authors, the possibility to assess the status of the venous wall and to determine the diameter and area of the CIV stenosis significantly increases the accuracy of the technique.56,57 Knuttinen et al suggest that IVUS in combination with conventional venography is the standard for diagnosingMTS.58 According to van Vuuren et al, pelvic venography often results in overdiagnosis of MTS, as the classic venographic features of MTS may be present in healthy individuals.59 There is no contradiction in this statement, since the treatment strategy is determined by clinical manifestations of the disease and not the degree of stenosis. Venography or IVUS findings are useful in the presence of symptoms and signs of PCS or chronic venous insufficiency.
In other studies, the authors report a high correlation between data, obtained by MPPV, MSCV, and IVUS, and indicate the need for the combined use of venography and IVUS.46,55,60 Forauer et al state that IVUS provides a more precise placement of the stent into the iliac veins, minimizing the risk of developing a jailing effect (overlapping of the contralateral CIV by the stent).61 However, IVUS also has drawbacks. In particular, the IVUS findings of a significant iliac vein lesion are sometimes not accompanied by any clinical manifestations. Birn and Vedantham propose to match ultrasound and clinical data in order to determine the optimal treatment for a patient with iliac vein lesion.55
Therefore, a variety of diagnostic tests including DUS, MSCV, and venography with or without IVUS should be used in patients with concurrent MTS and PCS. IVUS is not a panacea. Our experience suggests that successful diagnosis and treatment of patients with a combination of MTS and PCS is possible without the use of IVUS. Severe symptoms of venous congestion of the pelvic organs and disturbances of pelvic venous blood flow, according to DUS, MSCV/MRV, and venography, substantiate the need to restore blood flow in the pelvic and iliac veins.
Treatment
Regarding a combination of MTS and PCS, we a priori consider symptomatic patients, as PCS cannot exist without pelvic pain, dyspareunia, etc. Furthermore, in such patients, the disturbances in venous outflow from the pelvis are significant, consistently confirmed by the results of ultrasound and radiopaque methods. When choosing a technique for restoring venous outflow from the pelvis in patients with MTS and PCS, the main factors are: (i) the degree of left CIV stenosis; (ii) involvement of GV, as PCS may be a result of an isolated PV and UV dilation without ovarian venous insufficiency; and (iii) the presence of vulvar/gluteal varicose veins, varicose veins on the posterior surface of the thigh, and detection of PPR by imaging.
The indication for endovascular intervention on the left CIV depends on the severity of its stenosis. Carr et al argue that narrowing the left CIV to 4 mm increases the risk of DVT and requires CIV stenting.62 Ahmed et al expressed the same opinion about stenting in MTS.7 Daugherty and Gillespie considered the following indication for stenting: reduction in CIV lumen diameter to 2–6 mm and stenosis area from 65% to 99% by IVUS.31 For patients in our clinic with a combination of MTS and PCS, we consider compression stenosis of the left CIV greater than 50%, as assessed by venography, to be an absolute indication for stenting.21
GV reflux is a significant factor in the development of severe venous congestion of the pelvic organs. Therefore, reduction in blood flow through GV is one of the necessary steps in PeVD treatment. However, in patients with combined MTS and PCS, the role of ovarian venous insufficiency may be secondary. It was shown that isolated excision of varicose tributaries of the great saphenous vein or crossectomy in combination with excision of tributaries (ASVAL [Ambulatory Selective Varicose vein Ablation under Local anesthesia] and CHIVA [Conservatrice Hémodynamique de l’Insuffisance Veineuse en Ambulatoire] methods) is accompanied by restoration of great saphenous vein valvular function in 60% to 70% of cases.63,64 It is likely that the restoration of normal blood flow in the left CIV would reduce reflux in the left GV. In patients with PCS, we observed the elimination of the dilation of PV and UV and the restoration of their valvular function after GVE.65
In patients with PeVD, the occurrence of vulvar varicosities (VV) and varicose veins on the posterior surface of the thigh is associated with reflux in the IIV tributaries (internal pudendal, obturator, and inferior gluteal veins).66,67 Vulvar vein dilation most often occurs during pregnancy; the rate of its detection in pregnant women ranges from 10% to 20%.68 However, in two-thirds of these women, VV disappears after childbirth without any treatment. This suggests that IIV reflux is reversible. Khan et al presented the case of a woman with combined MTS, PCS, and VV69 and who had no detectable images of IIV tributaries or PPR on venography despite a severe VV. Our experience shows that the reflux in the IIV trunk, internal pudendal vein, and obturator vein is found in only 8%, 8%, and 6% of patients with VV, respectively.40 These data should be taken into account when choosing a method for eliminating VV.
Stenting or embolization?
What is the first intervention to perform in patients with a combination of MTS and PCS? Is it really necessary to perform GVE? Should the IIV inflows be embolized in patients with a combination of MTS, PCS, and VV? These and a number of other questions arise for surgeons when taking into account clinical, ultrasound, and radiological data in this cohort of patients.
Findings from recent studies suggest that CIV stenting is the first intervention on the pelvic veins to perform in patients with concomitant MTS and PCS. Daugherty and Gillespie reported elimination of PCS symptoms in 79% of patients after CIV stenting.31 Santoshi et al successfully used isolated CIV stenting in 22% of patients with combined MTS and PCS.8 Ahmed et al managed to relieve symptoms of venous congestion of the pelvic organs in 68% of patients with MTS and PCS via stenting and without GVE.7 A study by Gavrilov et al showed complete relief of PCS symptoms in 17% of patients with left CIV stenosis.21 Lakhanpal et al noted a significant reduction or complete relief of PCS symptoms in 76% of patients.70 These data indicate the possibility of curing patients who have a combination of MTS and PCS through CIV stenting alone, without GVE.
In the presence of hemodynamically significant CIV stenosis in these patients, the treatment of choice is stenting of the iliac veins (Figure 6). At the same time, there is a certain proportion of patients (from 30% to 70%) in whom stenting does not significantly improve PCS symptoms. Various explanations include a long history of the disease (more than 5-7 years), incorrectly chosen stent size, and erroneous stent implantation in patients with hemodynamically insignificant stenosis of the iliac veins. Persistence of PCS symptoms after CIV stenting is an indication for GVE.
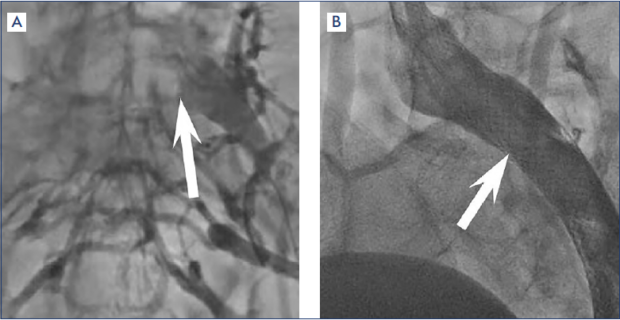
Figure 6. Pelvic venography (A) before and (B) after stenting of the left common iliac vein. The arrows indicate a stenosis of the left common iliac vein and stents in the lumen of the left iliac veins.
It is inappropriate to perform GVE without elimination of hemodynamically significant CIV stenosis. Patients report an increase in pelvic pain, and some of them develop VV.71 Left GV occlusion in persisting MTS will increase pelvic venous congestion (Figure 7). Our clinical experience indicates the need to use a staged approach to the endovascular treatment in patients with a combination of MTS and PCS: stenting in the first stage and then, depending on its clinical effect, deciding whether GVE is needed.21,71 In the case of CIV stenosis less than 50%, GVE can be performed as a primary intervention. It may be used as the sole treatment in patients with MTS and PCS when symptoms are resolved. To date, there is no indication for iliac-vein stenting in patients with PCS and hemodynamically insignificant (<50%) stenosis of the left CIV. If clinical signs persist, stent placement should probably be considered after careful reassessment of the iliac veins and exclusion of any other cause of persistent pelvic pain. GVE is a widely accepted treatment for PCS. Most often, it is performed using coils (nitinol, platinum, fibered or not fibered). However, published data and our own clinical experience indicate that coils are not the optimal agent for treating GV occlusion. Frequent complications of GVE are postembolization syndrome (10%-53%), protrusion of the coils (4%-8%), and nickel allergy.72-74 This indicates the need to use other embolizing agents for GVE (Amplatzer devices, cyanoacrylate glue).75,76
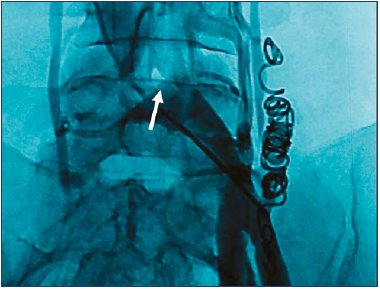
Figure 7. Pelvic venography of a patient with unresolved compression stenosis of the left common iliac vein (indicated by an arrow) after embolization of the left gonadal vein. Marked pelvic collaterals and reflux of the contrast media into the dilated left internal iliac vein are visualized.
Choice of a stent
The stenting procedure is well developed and adequately described in the literature.5,77,78 An important point is choice of stent. Raju et al proposed to use stents that have a diameter of 16-18 mm to restore the normal lumen of the CIV.79 Furthermore, these authors prefer to install stents having a diameter 2 mm greater than the recommended size. This makes it possible to perform aggressive post-dilation to eliminate residual stenosis and to achieve better fixation of the stent in the vein lumen.
Another issue in choosing an optimal stent is braiding and length. A wide range of venous stents have been developed to date. They are distinguished by flexibility, strength, and radial resistance force, and they have various braiding, etc.80 The authors recommend using Z-stents. The length of iliac vein stents should be at least 90 mm. The experience of our clinic is based on the use of Wallstent stents (Boston Scientific). These devices have been used in the treatment of MTS for 15 years, and there have been no cases of reported breakage, migration, or thrombosis of these stents.71
Placement of the stent in the CIV lumen should also be touched on. The ideal position is placement within the immediate area of confluence of the iliac veins. However, this is almost impossible to achieve in real practice because stent placement within the same plane as the CIV orifice is associated with a risk of proximal residual stenosis after stenting. Therefore, most authors recommend placing a stent in a vein so that it protrudes 0.5-1 cm into the lumen of the inferior vena cava.78 Other authors point out that even 2-cm displacement of the stent in the inferior vena cava should not be regarded as a serious defect in stenting technique.78 The “jailing” effect leads to thrombosis of the contralateral OPV in no more than 1% of cases.81 This is demonstrated by tomograms of a patient with MTS and PCS who underwent CIV stenting with two stents in our clinic 15 years ago (Figure 8). PCS symptoms were completely eliminated and are absent to date; stents remain completely patent; and there have been no episodes of thrombosis of the iliocaval segment.
Venoactive drugs (VAD) in the treatment of patients with a combination of MTS and PCS
When using endovascular methods of treatment, it should be understood that CIV stenting and/or GVE does not result immediately in a decrease in the diameter of the pelvic veins, elimination of reflux in them, and restoration of venous outflow from the pelvic organs. Given this fact, patients should be advised to take venoactive drugs (VADs); these have a beneficial effect on the walls of the pelvic veins and improve microcirculation in the pelvic organs. The only VAD studied in PCS patients is the micronized purified flavonoid fraction (MPFF). Several studies have shown a positive effect of MPFF on venous outflow from the pelvis, pelvic pain, and other symptoms of the disease.82-85 This drug is characterized by high efficacy and safety in the treatment of patients with PCS. The use of MPFF in the pre- and postoperative period provides early relief of symptoms of the disease and facilitates rehabilitation of patients with MTS and PCS after endovascular treatment.
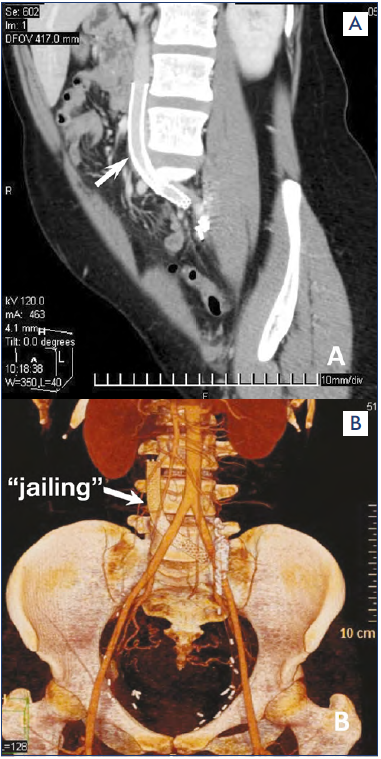
Figure 8. Computed tomography in a patient who underwent stenting of the left iliac veins with two Wallstent stents and embolization of the left gonadal vein with coils. (A) Sagittal projection. (B) Frontal projection. The stents are patent. The arrow indicates the “jailing” effect.
Conclusion
It is difficult to address all the issues and nuances of diagnosis and treatment of patients with a combination of MTS and PCS in one review; there are many anatomical and clinical variants of the course of this combined pathology. MTS and PCS are the most common causes of pelvic vein disease. The main directions of research are clear, focusing on the study of pathophysiological processes in PeVD, adequate and reliable diagnosis of disease, and determination of optimal approaches and methods for correction of venous outflow disturbances in MTS and PCS. It is also obvious that these issues are impossible to resolve without international consolidation of the efforts of surgeons, phlebologists, vascular, and interventional surgeons. Large multicenter studies are needed, sharing international experience in the diagnosis and treatment of PeVDs.

REFERENCES
1. Stones RW. Chronic pain in women: new perspectives on pathophysiology and management. Reprod Med Rev. 2000;8(3):229-240.
2. Hobbs J.T. The pelvic congestion syndrome. Practitioner. 1976;216:529-540.
3. Daniels JP, Khan KS. Chronic pelvic pain in women. BMJ. 2010;341:c4834.
4. May R, Thurner J. The cause of the predominately sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
5. Oguzkurt L, Tercan F, Ozkan U, Gulcan O. Iliac vein compression syndrome: outcome of endovascular treatment with long-term follow-up. Eur J Radiol. 2008;68:487-492.
6. Rastogi N, Kabutey NK, Kim D. Incapacitating pelvic congestion syndrome in a patient with a history of May-Thurner syndrome and left ovarian vein embolization. Ann Vasc Surg. 2012;26(5):732.e7-e11.
7. Ahmed O, Ng J, Patel M, et al. Endovascular stent placement for May- Thurner Syndrome in the absence of acute deep vein thrombosis. J Vasc Interv Radiol. 2016;27(2):167-173.
8. Santoshi RKN, Lakhanpal S, Satwah V, Lakhanpal G, Malone M, Pappas PJ. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 201;6(2):202-211.
9. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-352.
10. Khilnani NM, Meissner MH, Learman LA, et al. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30(6):781-789.
11. Meissner MH, Khilnani NM, Labropoulos N, et al. The Symptoms-Varices- Pathophysiology (SVP) Classification of pelvic venous disorders: a report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord. 2021;9(3):568-584.
12. Shammas NW, Jones-Miller S, Kovach T, et al. Predicting significant iliac vein compression using a probability scoring system derived from minimal luminal area on computed tomography angiography in patients 65 years of age or younger. J Invasive Cardiol. 2021;33(1):E16-E18.
13. Cheng L, Zhao H, Zhang FX. Iliac vein compression syndrome in an asymptomatic patient population: a prospective study. Chin Med J (Engl). 2017;130(11):1269-1275.
14. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937- 943.
15. Liu Z, Gao N, Shen L, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg. 2014;28(3):695-704.
16. Ignacio EA, Dua R, Sarin S, et al. Pelvic congestion syndrome: diagnosis and treatment. Semin Intervent Radiol. 2008;25:361-368.
17. Belenky A, Bartal G, Atar E, Bachar GN. Ovarian varices in healthy female kidney donors: incidence, morbidity and clinical outcome. Am J Roentgenol. 2002;179:625-627.
18. Meneses LQ, Uribe S, Tejos C, Andıa ME, Fava M, Irarrazaval P. Using magnetic resonance phase-contrast velocity mapping for diagnosing pelvic congestion syndrome. Phlebology. 2011;26(4):157- 161.
19. Dos Santos SJ, Holdstock JM, Harrison CC, Lopez AJ, Whiteley MS. Ovarian vein diameter cannot be used as an indicator of ovarian venous reflux. Eur J Vasc Endovasc Surg. 2015;49(1):90-94.
20. Gavrilov SG, Moskalenko EP, Karalkin AV, Lebedev IS, Son DA, Turischeva OO. Can the diameter of pelvic veins be a predictor of pelvic congestion syndrome? Flebologiia. 2017;11(1):28-31.
21. Gavrilov SG, Vasilyev AV, Krasavin GV, Moskalenko YP, Mishakina NY. Endovascular interventions in the treatment of pelvic congestion syndrome caused by May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8(6):1049- 1057.
22. Nanavati R, Jasinski P, Adrahtas D, Gasparis A, Labropoulos N. Correlation between pelvic congestion syndrome and body mass index. J Vasc Surg. 2018;67(2):536-541.
23. Siqueira-Campos VME, Da Luz RA, de Deus JM, Martinez EZ, Conde DM. Anxiety and depression in women with and without chronic pelvic pain: prevalence and associated factors. J Pain Res. 2019;12:1223-1233.
24. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 suppl):2S-48S.
25. Antignani PL, Lazarashvili Z, Monedero JL, et al. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol. 2019;38(4):265- 283.
26. Herrera-Betancourt AL, Villegas-Echeverri JD, López-Jaramillo JD, López-Isanoa JD, Estrada-Alvarez JM. Sensitivity and specificity of clinical findings for the diagnosis of pelvic congestion syndrome in women with chronic pelvic pain. Phlebology. 2018;33(5):303-308.
27. Beard RW, Reginald PW, Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95:153-161.
28. Gavrilov SG, Moskalenko YP. Does pelvic congestion syndrome influence symptoms of chronic venous disease of the lower extremities? Eur J Obstet Gynecol Reprod Biol. 2019;243:83-86.
29. Champaneria R, Shah L, Moss J, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess. 2016;20(5):1-108.
30. Knuttinen MG, Xie K, Jani A, Palumbo A, Carrillo T, Mar W. Pelvic venous insufficiency: imaging diagnosis, treatment approaches, and therapeutic issues. AJR Am J Roentgenol. 2015;204(2):448-458.
31. Daugherty SF, Gillespie DL. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2015;3(3):283-289.
32. Perrin M, Eklöf B, Van Rij A, et al. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. Int Angiol. 2016;35(4):374-398.
33. Thors A, Haurani MJ, Gregio TK, Go MR. Endovascular intervention for pelvic congestion syndrome is justified for chronic pelvic pain relief and patient satisfaction. J Vasc Surg Venous Lymphat Disord. 2014;2(3):268-373.
34. Larkin TA, Hovav O, Dwight K, Villalba L. Common iliac vein obstruction in a symptomatic population is associated with previous deep venous thrombosis, and with chronic pelvic pain in females. J Vasc Surg Venous Lymphat Disord. 2020;8(6):961-969.
35. Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60(1):118-125.
36. Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol. 2002;13(2 pt 1):171- 178.
37. Whiteley MS, Dos Santos SJ, Harrison CC. Holdstock JM, Lopez AJ. Transvaginal duplex ultrasonography appears to be the gold standard investigation for the haemodynamic evaluation of pelvic venous reflux in the ovarian and internal iliac veins in women. Phlebology. 2015;30(10):706-713.
38. White AM, Holdstock JM. Ultrasound assessment of pelvic venous reflux. Indian J Vasc Endovasc Surg. 2018;5:234-243.
39. Malgor RD, Adrahtas D, Spentzouris G, Gasparis AP, Tassiopoulos AK, Labropoulos N. The role of duplex ultrasound in the workup of pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2014;2:34-38.
40. Gavrilov SG, Vasiliev A, Moskalenko Y, Mishakina N. Diagnostic value of pelvic venography in female patients with pelvic varicose veins and vulvar varicosities. Int Angiol. 2020;39(6):452-460.
41. Jones TM, Cassada DC, Heidel RE, et al. Maximal venous outflow velocity: an index for iliac vein obstruction. Ann Vasc Surg. 2012;26(8):1106-1113.
42. Karaosmanoglu D, Karcaaltincaba M, Karcaaltincaba D, Akata D, Ozmen M. MDCT of the ovarian vein: normal anatomy and pathology. AJR Am J Roentgenol. 2009;192(1):295-299.
43. Lamba R, Tanner DT, Sekhon S, McGahan JP, Corwin MT, Lall CG. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34(1):93-115.
44. Lugo-Fagundo C, Nance JW, Johnson PT, Fishman EK. May-Thurner syndrome: MDCT findings and clinical correlates. Abdom Radiol (NY). 2016;41(10):2026- 2030.
45. Krzanowski M, Partyka L, Drelicharz L, et al. Posture commonly and considerably modifies stenosis of left common iliac and left renal veins in women diagnosed with pelvic venous disorder. J Vasc Surg Venous Lymphat Disord. 2019;7(6):845-852.
46. Jayaraj A, Raju S. Three-dimensional computed tomography venogram enables accurate diagnosis and treatment of patients presenting with symptomatic chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(1):73-80.
47. Hartung O, Grisoli D, Boufi M, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42(2):275-280.
48. Steenbeek MP, van der Vleuten CJM, Schultze Kool LJ, Nieboer TE. Noninvasive diagnostic tools for pelvic congestion syndrome: a systematic review. Acta Obstet Gynecol Scand. 2018;97(7):776-786.
49. Nascimento AB, Mitchell DG, Holland G. Ovarian veins: magnetic resonance imaging findings in an asymptomatic population. J Magn Reson Imaging. 2002;15(5):551-556.
50. Costa LMG, Tachibana A, Magao FDS, Wolosker N, Baroni RH. Magnetic resonance imaging evaluation of left common iliac vein compression in patients with and without symptoms of venous disease. Circ J. 2020;84(5):763-768.
51. Osman AM, Mordi A, Khattab R. Female pelvic congestion syndrome: how can CT and MRI help in the management decision? Br J Radiol. 2021;94(1118):20200881.
52. McDermott S, Oliveira G, Ergul E, et al. May-Thurner syndrome: can it be diagnosed by a single MR venography study? Diagn Interv Radiol. 2013;19:44-48.
53. Borghi C, Dell’Atto L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293(2):291- 301.
54. Bendek B, Afuape N, Banks E, Desai NA. Comprehensive review of pelvic congestion syndrome: causes, symptoms, treatment options. Curr Opin Obstet Gynecol. 2020;32(4):237-242.
55. Birn J, Vedantham S. May-Thurner syndrome and other obstructive iliac vein lesions: meaning, myth, and mystery. Vasc Med. 2015;20(1):74-83.
56. Radaideh Q, Patel NM, Shammas NW. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. 2019;15:115-122.
57. Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord. 2019;(6):801-807.
58. Knuttinen MG, Naidu S, Oklu R, et al. May-Thurner: diagnosis and endovascular management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S159-S164.
59. van Vuuren TMAJ, Kurstjens RLM, Wittens CHA, van Laanen JHH, de Graaf R. Illusory angiographic signs of significant iliac vein compression in healthy volunteers. Eur J Vasc Endovasc Surg. 2018;56(6):874-879.
60. Raju S, Davis M. Anomalous features of iliac vein stenosis that affect diagnosis and treatment. J Vasc Surg Venous Lymphat Disord. 2014;2(3):260-267.
61. Forauer AR, Gemmete JJ, Dasika NL, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13(5):523-527.
62. Carr S, Chan K, Rosenberg J, et al. Correlation of the diameter of the left common iliac vein with the risk of lowerextremity deep venous thrombosis. J Vasc Interv Radiol. 2012;23(11):1467-1472.
63. Pittaluga P, Chastanet S, Rea B, Barbe R. Midterm results of the surgical treatment of varices by phlebectomy with conservation of a refluxing saphenous vein. J Vasc Surg. 2009;50(1):107-118.
64. Franceschi C, Cappelli M, Ermini S, et al. CHIVA: hemodynamic concept, strategy and results. Int Angiol. 2016;35(1):8-30.
65. Shipovskiĭ VN, Kapranov SA, Gavrilov SG. Endovascular embolization of ovarian veins in varicose disease of the pelvic veins [in Russian]. Angiol Sosud Khir. 2008;14(4):69-72.
66. Lasry JL, Coppe G, Balian E, Borie H. Pelviperineal venous insufficiency and varicose veins of the lower limbs: duplex Doppler diagnosis and endoluminal treatment in thirty females. J Mal Vasc. 2007;32(1):23- 31.
67. Dos Santos SJ, Holdstock JM, Harrison CC, Whiteley MS. Long-term results of transjugular coil embolisation for pelvic vein reflux – Results of the abolition of venous reflux at 6-8 years. Phlebology. 2016;31(7):456-462.
68. Gavrilov SG. Vulvar varicosities: diagnosis, treatment, and prevention. Int J Womens Health. 2017;9:463-475.
69. Khan TA, Rudolph KP, Huber TS, Fatima J. May-Thurner syndrome presenting as pelvic congestion syndrome and vulvar varicosities in a nonpregnant adolescent. J Vasc Surg Cases Innov Tech. 2019;5(3):252-254.
70. Lakhanpal G, Kennedy R, Lakhanpal S, Sulakvelidze L, Pappas PI. Pelvic venous insufficiency secondary to iliac vein stenosis and ovarian vein reflux treated with iliac vein stenting alone. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1193- 1198. doi:10.1016/j.jvsv.2021.03.006.
71. Gavrilov SG, Shipovskiĭ VN, Karalkin AV, Maksimova MA, Beliaeva ES. A case of successful treatment of pelvic venous congestion caused by May-Thurner syndrome [in Russian]. Flebologiia. 2010;4(1):68-71.
72. Monedero JL, Zubicoa Ezpeleta S, Castro Castro J, Calderon Ortiz M, Sellers Fernandez G. Embolization treatment of recurrent varices of pelvic origin. Phlebology. 2006;21(1):3-11.
73. Fahrni J, Gloviczki P, Friese JL, Bakkum- Gamez JN. Hypersensitivity to nickel in a patient treated with coil embolization for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2015;3(3):319- 321.
74. Gavrilov SG, Krasavin GV, Mishakina NY, Kirsanov KV. Postembolization syndrome in endovascular interventions on the gonadal veins. J Vasc Surg Venous Lymphat Disord. 2021;9(3):697-702.
75. Basile A, Marletta G, Tsetis D, Patti MT. The Amplatzer vascular plug also for ovarian vein embolization. Cardiovasc Intervent Radiol. 2008;31(2):446-447.
76. Sze DY, Kao JS, Frisoli JK, McCallum SW, Kennedy WA, Razavi MK. Persistent and recurrent postsurgical varicoceles: venographic anatomy and treatment with N-butyl cyanoacrylate embolization. J Vasc Interv Radiol. 2008;19(4):539-545.
77. O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11(7):823-836.
78. Raju S, Oglesbee M, Neglen P. Iliac vein stenting in postmenopausal leg swelling. J Vasc Surg. 2011;53(1):123-130.
79. Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology. 2018;33(7):451-457.
80. Shamimi-Noori SM, Clark TWI. Venous stents: current status and future directions. Tech Vasc Interv Radiol. 2018;21(2):113- 116.
81. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143.
82. Simsek M, Burak F, Taskin O. Effects of micronized purified flavonoid fraction on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol. 2007;34(2):96-98.
83. Tsukanov YT, Tsukanov AY, Levdanskiy EG. Secondary varicose small pelvic veins and their treatment with micronized purified flavonoid fraction. Int J Angiol. 2016;25(2):121-127.
84. Gavrilov SG, Moskalenko YP, Karalkin AV. Effectiveness and safety of micronized purified flavonoid fraction for the treatment of concomitant varicose veins of the pelvis and lower extremities. Curr Med Res Opin. 2019;35(6):1019-1026.
85. Gavrilov SG, Karalkin AV, Moskalenko YP, Grishenkova AS. Efficacy of two micronized purified flavonoid fraction dosing regimens in the pelvic venous pain relief. Int Angiol. 2021;40(3):180-186.
