Congenital arteriovenous malformations: what are the perspectives?
Claude LAURIAN
Paris, France
SUMMARY
Arteriovenous malformations (AVMs), irrespective of their localization, are a therapeutic challenge. Available imaging modalities permit complete identification of lesions and definition of their relation to adjacent tissues. Conventional arteriography is now replaced by noninvasive techniques, mainly the CT scan. Duplex scan provides a variety of information and confirms the diagnosis of AVM, pointing to the AV shunts by localizing the zones of shunts within the tissues, and by showing the main feeding arteries and draining veins. Flow measurement and its comparison between the limbs can also help in the determination and follow-up of hemodynamic changes. CT scan with contrast enhancement can identify precisely the zone of shunts within the tissue (intramuscular localization, intra-articular, or in the cellular space, most often around a joint). The 3D reconstruction visualizes the feeding arteries, the zones of shunt, and the draining veins better than arteriography.
Therapeutic protocols require a multidisciplinary approach involving interventional radiologists, vascular surgeons, and plastic or orthopedic surgeons. Therapeutic approaches may differ a lot depending on the diversity of the lesions that must be treated and also to the diverse backgrounds of treating specialists. Procedures have improved, thanks to embolization techniques and surgical approaches combined with sclerotherapy procedures. Arterial selective embolization may be used to control the incapacitating symptoms, but is always palliative and is usually reserved for hemorrhagic complications. Nowadays, it is rarely indicated before surgery because it provides few benefits in controlling bleeding. Retrograde percutaneous venous embolization may be complementary to the arterial approach with a bigger risk of pulmonary embolism. Some forms of vascular architecture may be more indicated for such procedures (Rose’s vascular architecture, pelvic AVM). Percutaneous sclerotherapy with direct puncture has not been much used for these lesions. Intraoperative sclerotherapy is applicable for the venous compartment. It controls perioperative bleeding or is the complementary treatment of incomplete surgical excision.
The difficulty in assessing the results stems from variability in the therapeutic aims that may be adopted. These aims may include complete excision, partial excision supplemented by reconstructive surgery, or treatment of vascular complications of the AVM, leaving the malformation itself aside.
INTRODUCTION
Irrespective of their localization, AVMs are a rare and serious condition. Therapeutic approaches may greatly differ and this can be related to the diversity of the lesions and also the different backgrounds of treating specialists.
In this review, we consider malformations of the limbs and, of the thoracoabdominal wall and pelvic AVM.
ANATOMY – ANGIOARCHITECTURE
The arteriovenous shunts (or nidus) of the AVM are well individualized within a tissue or vascular region.
Proximal to the shunts, the high arterial flow may cause aneurysms or heart failure (proximal AVM of the limbs or pelvic AVM)
The nidus may present in two ways: either as multiple arteriovenous shunts well identified within the tissues (often around the knee joint in the lower limb)
(Figure 1) or as arteriovenous shunts within the wall of a congenital venous ectasia. The latter is currently better recognized and has been reported intracranially (arteriovenous fistulas of the lateral sinus or the vein of Galen) and extracranially in the parametrium. These arteriovenous fistulas constitute what we call the rose angioarchitecture (Figure 2).
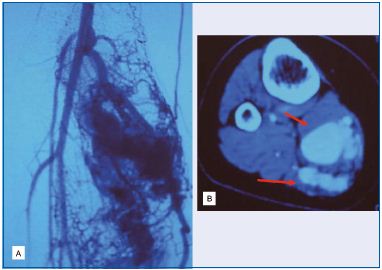
B. CT scan localization of the AVM in the medial gastrocnemius
muscle.
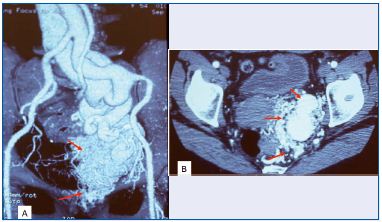
A. 3D CT scan of AVM in the parametrium.
B. Axial view of arteriovenous shunts around an ectasia of the
iliac internal vein (rose architecture).
The draining veins, downstream of the AVM, form pulsatile ectasias responsible for the swelling. The superficial localization of the AVM (in the distal part of the limbs) may be because of skin manifestations and hemorrhagic complications.
Imaging studies (CT scan) distinguish the various anatomic details.
Soft tissue AVMs can be separated into three categories:
• Localized AVMs within a well-defined structure (subcutaneous cellular tissue, intramuscular, intraarticular, or cellular spaces). The consequences of surgical excisions are relevant to the localization of the lesion.
• Regional AVMs involve a larger area of tissue. Treatment is palliative and is indicated only for complications.
• Diffuse or extensive AVMs where current modalities are not successful.
Truncal AVMs with a high flow are exceptional and difficult to identify.
CLINICAL PRESENTATION
Symptoms may vary according to the subject and the localization. We will limit our presentation to AVMs of the limbs, thoracoabdominal region and pelvic region. The most frequent localization is the limbs where 4 clinical features may lead to the diagnosis:
• a pulsatile tumor of some part of the limb responsible for functional limitation or pain
• pulsatile veins, mainly in the extremities (hand, foot), may be the weakness of an underlying AVM
• a flat warm angioma corresponding to a superficial AVM
• complications of the AVM may necessitate some form of treatment (management of trophic problems such painful ulcer, or bleeding complications requiring emergency hemostasis procedures) (Figure 3).
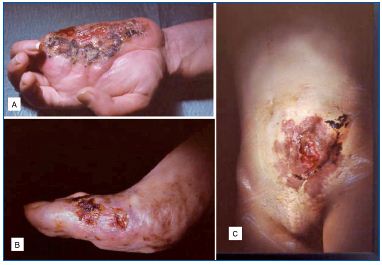
A. Hand.
B. Forefoot.
C. Abdominal wall.
Thoracoabdominal AVMs are more often limited in parietal structures, while the localization of pelvic AVMs may be visceral (uterine) or extravisceral within the parametrium.
NONINVASIVE IMAGING
Many advances in the understanding of arteriovenous malformations have been possible by progress in imaging techniques, which are now sufficient for making the diagnosis.
Bone radiography of the region of concern, in the search for osseous or periosteal reaction or a cortical fracture often indicative of bone destruction by draining veins.
Duplex scanning is involved at several stages of the diagnosis:
• it helps locate arteriovenous shunts within the tissues,
• it can identify the main feeding pedicles and draining veins preoperatively (in well-defined AVMs),
• it permits a skin marking of the lesion in order to accomplish a well-targeted surgical approach
• it can identify residual shunts and assess the comparative flow rates in the limbs in postoperative monitoring and follow-up.
CT scanning. With axial acquisitions, CT scanning is the most efficient exploration of AVMs. Without contrast it gives an image of bone lesions. With contrast it defines the topography of the malformation in relation to surrounding tissues. Three-dimensional reconstruction defines the location of the nidus better than arteriography.
Arteriography has restricted indications. There is a place for it before attempts at embolization or at control of bleeding.
Coagulation control is usually normal: AVMs are not associated with hemolytic anemia. There is no need for coagulation factor therapy.
Cardiac flow measurement may be restricted to highflow AVMs (proximal part of limb, or pelvis)
THERAPEUTIC PROCEDURES
There has been significant progress thanks to new pretreatment investigations.
Embolization and sclerotherapy
Few embolization agents are applicable to AVMs:
• Ethanol is not often used as there is a risk of migration or tissue ischemia. Combination of ethanol with coils has reduced this risk. Gelified ethanol, which is not yet commercially available, might also reduce this risk.
• Cyanocrylate agents (Glubran) or ethylene alcohol copolymer (Onyx) are most often used during arteriography. Ethiboc is no longer available: its poor resorption can create a mass effect.
• Cement can be injected percutaneously in areas of bone destruction. Its efficacy is often incomplete because of technical problems in controlling the draining veins.
• Ethylene glycol (bone wax) may be used exclusively perioperatively. It helps achieve hemostasis in difficult cases or occludes the venous compartment in some forms of AVM.
The injection site of these products can be discussed according to the surgical approaches used and the type of lesions: arterial or venous retrograde embolization, percutaneous or perioperative sclerotherapy.
Surgical procedures must be adapted to lesion localization. There are three strategies:
• Complete surgical excision for well-localized AVMs (seen on CT scan), in well-defined tissue (cellular space or subcutaneous, muscle or joint tissues). It is a complex procedure that often requires associated reconstructive procedures (Figure 4).
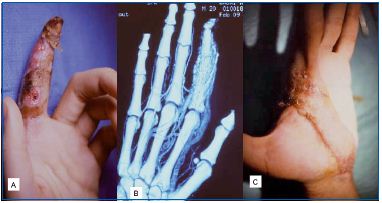
A. Extensive trophic lesions.
B. CT scan of AVM in P1-P2 segments.
C. Amputation with muscle free flap.
• Partial excision may be the only feasible approach in order to preserve function (hand, foot). Hybrid methods are possible combining excision with reconstructive procedures or sclerotherapy.
• The treatment of complications of AVMs may require specific skills: vascular surgery for proximal arterial aneurysms, orthopedic surgery for bone or joint lesions (arthroplasty combined with sclero therapy) (Figure 5), reconstructive surgery (tissue expansion, tendon transfer, free tissue flap).
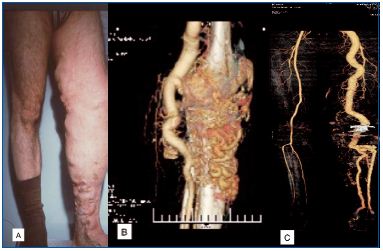
A. AVM of the knee and ankle.
B. Preoperative 3D CT scan.
C. Postoperative CT scan of arthroplasty of the knee and
perioperative sclerotherapy.
THERAPEUTIC INDICATIONS
The main thing is to avoid proposing treatment for an asymptomatic and uncomplicated AVM and in the absence of functional or significant cosmetic problems. Any mistake in the treatment may lead to detrimental complications. The indications are based on the anatomic and clinical data of each case.
– AVMs in the limbs
The main problems occur in the extremities (hands and fingers, ankles and feet). Intra-arterial embolization is not effective. Hybrid surgical procedures are used for hemorrhagic complications or trophic lesions.
– AVMs in the thoracoabdominal wall
Parietal lesions (subcutaneous, intramuscular) may be treated with extensive resection as reconstructive surgery has extensive possibilities in these cases.
– Pelvic AVMs
Visceral (uterine) or extravisceral (parametrium) AVMs pose complex therapeutic problems. Intra-arterial embolization is most often palliative. Surgery combined with intra-operative embolization of the venous compartment (ethylene glycol) may permit a definitive treatment.
CONCLUSION
Good information from the patient and on his or her environment is needed to determine the significance of an AVM. Advances in noninvasive imaging techniques (CT scan) have led to new treatment strategies. The complex nature of the lesions requires the involvement of multidisciplinary teams including radiologists, surgeons, in particular vascular surgeons, and reconstructive and orthopedic surgeons.

READING MORE
Kim JY, Kim DJ, Do YD, et al. Surgical treatment for congenital arteriovenous malformation: 10 years’ experience. Eur J Vasc Endovasc Surg. 2006;32:101-106. Laurian C, Mallios A, Gigou F, Marteau V. Are there surgical indications in the treatment of arteriovenous malformations? In: Becquemin JP, ed. Controversies and updates in vascular surgery. Paris, France: Minerva Medica; 2010:101-108.
