Course duration for venoactive-drug treatment in chronic venous disease
DMedSc; Igor SUCHKOV, MD, PhD,
DMedSc; Aleksey KAMAEV, MD, PhD;
Nina MZHAVANADZE, MD, PhD. MD
Department of Cardiovascular,
Endovascular, Operative Surgery and
Topographic Anatomy, Ryazan State
Medical University, Ryazan, Russia
Abstract
Chronic venous disease (CVD) is currently the most common vascular disorder of the lower extremities. The gradual progression of CVD has a negative impact on patients and also leads to high economic costs of medical care, especially for the advanced forms of chronic venous insufficiency (CVI). Conservative management of CVD includes the use of compression stockings and pharmacological therapy. Despite the efficacy of compression therapy, patients may often experience difficulties with the daily use of medical hosiery, which leads to lower adherence than pharmacotherapy. Venoactive drugs (VADs) exert a systemic effect on various components of the pathogenesis of CVD, leading to improvement in patient quality of life and slowing disease progression. This article presents a literature review addressing the issues of prescribing pharmacotherapy to patients with CVD. It includes an analysis of the published data on the efficacy and safety of VADs and discusses the aspects of timing and duration of a course of treatment with VADs in accordance with clinical class of CVD and their use in combination with surgical treatment or sclerotherapy. It describes the effects of VADs on pathophysiological mechanisms of the development and progression of CVD and reviews clinical studies assessing the effects of VADs on various components of CVD pathogenesis (endothelial dysfunction, activation of leukocytes, vein specific inflammation, and activation of proteolytic enzymes that contribute to the degradation of the extracellular matrix). In this regard, it focuses particularly on micronized purified flavonoid fraction as having the largest evidence base for proven efficacy, safety, and long-term use. This article proposes, based on the data discussed here, several approaches to determine course duration of VAD treatment according to clinical class of CVD. It also emphasizes the importance and necessity of a personalized approach when choosing the optimal duration of VAD therapy.
Introduction
Chronic venous disease (CVD) is an important medical and social problem, holding a leading place among the most common peripheral vascular diseases, as shown in a number of epidemiological studies.1,2 The pathogenesis of CVD is a complex and multifactorial process and has been studied in detail in patients with varicose veins of the lower extremities (VVLE). It is a cascade of changes triggered by venous stasis that occurs at molecular and cellular levels. In this cascade, certain changes develop in endothelium due to a change in the shear stress, inducing inflammatory and thrombogenic phenotypes of endotheliocytes.3,4
Leukocyte activation, with their subsequent interaction with endothelial cells, leads to the production of cell adhesion molecules and proteolytic enzymes, in particular matrix metalloproteinases (MMPs; synthesized by endothelial cells and macrophages), and cause degradation of the extracellular matrix of the venous wall and its varicose transformation.3,5-9
One of the important factors in the pathogenesis of varicose transformation of superficial veins in VVLE is dysregulation of collagen synthesis. Studies have identified various changes in the content of different types of collagen in the venous wall. Most attention has been given to the content of types I and III interstitial collagens, which form large fibrils in the venous wall.8,10,11
In this regard, it seems relevant to select optimal treatment regimens with venoactive drugs (VADs; ie, venotonics, vein-protective agents) that will be effective on these components of pathogenesis. VADs are a large group of biologically active substances produced from plant materials or by chemical synthesis, characterized by the ability to reduce the severity of vein-specific symptoms and syndromes and to increase venous tone. VADs for systemic use are produced in various forms (tablets, suspensions, powders for oral administration, etc).12 The classification of the main VADs is presented in Table I.12
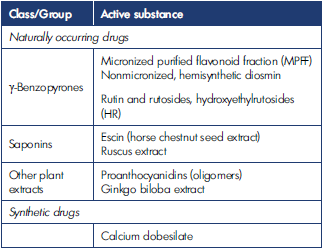
Table I. Classification of venoactive drugs. Based on reference 12: Kirienko et al. Flebologiya. 2018;12(3):146. doi:10.17116/flebo20187031146.
In addition to VADs, other pharmacological agents (eg, antiplatelets, heparin-like compounds, enzymes, prostaglandins) are also used for systemic pharmacotherapy.
Issues in prescribing VAD therapy
When prescribing these drugs, differences in the mechanisms of action and in therapeutic effect should be taken into consideration. The main principle in the prescription of VADs is the effect on a particular clinical outcome as shown in randomized controlled clinical trials confirming the efficacy and safety of a certain drug. The main indications for prescribing VADs are the combination of patient complaints and well-known CVD symptoms and signs.
Although indications for VAD use and their therapeutic effects have been determined in numerous studies, many questions remain, including those on duration of use and frequency of treatment courses.13
Aspects of timing and duration of VAD treatment courses
For many VADs, recommendations for duration of treatment remain vague: it may not be specified in the instructions for use; if it is, instructions may note that the treatment course can last several months, and that in case of a recurrence of symptoms, the treatment course can be repeated according to physician recommendations. Thus, there are currently no specific guidelines on the duration of VAD therapy in accordance with the disease severity and its class according to the CEAP (Clinical-Etiological- Anatomical-Pathophysiological) classification.
In clinical practice, VADs are prescribed for periods of continual treatment, ie, courses, the frequency and duration of which are most often selected based on empirical evidence, taking into account the experience of a particular phlebologist. The specialist makes a decision based on the course of disease and a combination of symptoms, considering possible adverse events. At the initial stages of CVD, the standard VAD course is usually 2 to 3 months, and in classes C3 to C6 (CEAP), it is possible to prescribe treatment up to 6 months, preferably twice a year. Moreover, in severe chronic venous insufficiency (CVI) or obesity, if patients have difficulties using compression hosiery, VADs may be prescribed for an indefinite time. Regarding the safety of long-term use (up to 12 months), the largest evidence base has been obtained for micronized purified flavonoid fraction (MPFF).12,13 Table II presents the results of studies on the duration of VAD treatment in CVD patients.14-34
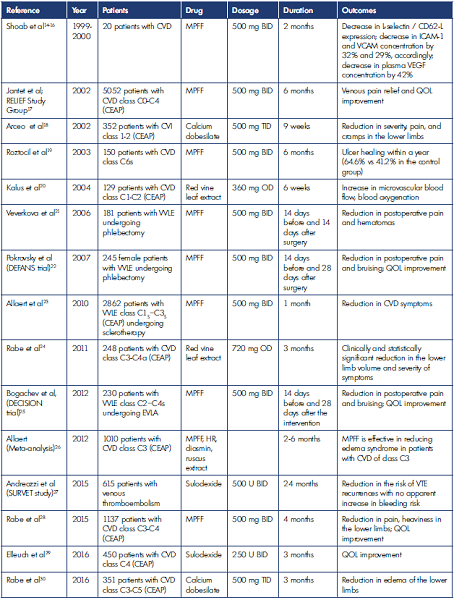
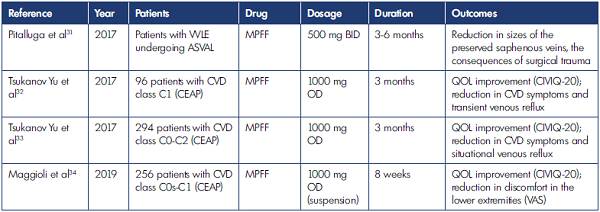
ASVAL, ambulatory selective varicose vein ablation under local anesthesia; BID, twice daily; CEAP, Clinical, Etiological, Anatomical and Pathophysiological classification; CIVIQ-20, Chronic Lower Limb Venous Insufficiency Questionnaire, 20 items; CVD, chronic venous disease; CVI, chronic venous insufficiency; EVLA, endovenous laser ablation; HR, hydroxyethylrutosides; ICAM-1, intercellular adhesion molecule 1; MPFF, micronized purified flavonoid fraction; OD, once daily; QOL, quality of life; TID, thrice daily; U, units; VAS, visual analog scale; VCAM, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; VTE, venous thromboembolism; VVLE, varicose veins of the lower extremities. Table II. Results of the main studies on the duration of CVD treatment.
Effect of VADs on CVD development and progression
The initiation of conservative therapy at early stages of varicose veins, ie, before onset of pathomorphological transformation of the venous wall and its valves, can lead to a reduction in the number of patients with severe CVD. The main determinants in CVD pathogenesis are leukocyte endothelial reactions, followed by an inflammatory process in the venous wall. Therefore, systemic pharmacotherapy in patients with VVLE should be based on the elimination of these changes. Considering this fact, the most promising are pharmacological agents than can improve endothelial function.7,35
At present, the main drug with proven efficacy in suppressing leukocyte-endothelial interaction is MPFF. A number of clinical trials have been conducted with various durations of treatment with this VAD, and their results have demonstrated efficacy and safety of MPFF in patients with various forms of CVD.
MPFF relieves signs and symptoms of CVD and improves QOL
The RELIEF study (Reflux assEssment and quaLity of life ImprovEment with micronized Flavonoids), one of the largest international trials for VAD treatment, was conducted in 23 countries for 2 years and included 5052 patients with CVD classes C0 to C4 (CEAP). The study showed that 6-month treatment courses with MPFF significantly reduced venous pain in patients with VVLE.17
The study of Rabe et al included 1137 CVD patients with classes C3 to C4 (CEAP) who were randomized to 4-month treatment with MPFF or placebo. MPFF use was associated with a reduction in pain and heaviness in the lower extremities, an improvement in quality of life (QOL).28
As for the effect of VADs on venous edema, a meta-analysis of 10 studies that included 1010 patients with CVD class C3 was carried out in 2012. MPFF demonstrated the highest efficacy in the treatment of venous edema.26
In several clinical studies assessing the efficacy of MPFF in patients with early stages of CVD (C0-C2 classes), treatment lasted for 2 to 3 months and was associated with QOL improvement (20-item Chronic Venous Insufficiency Questionnaire [CIVIQ-20]) and a reduction in CVD symptoms with the drug used in either tablet form32,33 or suspension.34
MPFF mediates components of VVLE pathogenesis
Randomized clinical trials have also been carried out to evaluate the effect of MPFF on various components of VVLE pathogenesis. A few studies showed that a 2-month treatment course with MPFF was associated with a reduction in L-selectin/CD62-L expression by monocytes and neutrophils, a reduction in intercellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule (VCAM) plasma levels by 32% and 29%, respectively, and in vascular endothelial growth factor (VEGF) plasma levels by 42%.14-16
These data demonstrate the benefits of MPFF in terms of improvement in venous endothelial function, as well as prevention of activation and adhesion of leukocytes. In experimental studies, MPFF has been proven to be effective in reducing the inflammatory response in venous valves and slowing down the development of venous reflux.36
In severe forms of CVD, a 6-month treatment course with MPFF has been studied. In patients with venous ulcers, MPFF was associated with better outcomes in terms of ulcer healing after 1 year (64.6% vs 41.2% in the control group with compression therapy only).19
VADs in combination with surgical intervention
The duration of conservative therapy in patients undergoing surgery or sclerotherapy is of special interest. In a pioneer study of perioperative use of venotonics, Veverkova et al assessed 181 patients aged 18 to 60 years who underwent crossectomy or stripping of the great saphenous vein. Patients were allocated to treatment with MPFF at a dose of 1000 mg daily for 14 days before and 14 days after surgery (n=92) or to the control group (n=89). Treatment with MPFF was associated with a reduction in the intensity of postoperative pain and, consequently, in the use of analgesics, as well as smaller size of postoperative hematoma, compared with the control group.21
The Russian trial DEFANS (Detralex – Assessment of Efficacy and Safety for Combined Phlebectomy) also evaluated the efficacy and safety of MPFF in patients undergoing phlebectomy. The main group consisted of 200 female patients with VVLE class C2s (CEAP) who received MPFF. The control group included 45 females who did not receive VADs. In this study, the duration of MPFF treatment was different: 500 mg twice daily (BID) for 2 weeks before surgery and 4 weeks after surgery.22 The study showed similar QOL parameters at baseline (CIVIQ); postoperative hematomas in the main group on days 7, 14, and 30 after phlebectomy were significantly smaller (P<0.05), and the greatest differences (over 70%) were observed 4 weeks after surgery. Moreover, the rates of symptoms (pain) were significantly lower in the MPFF group than in the control group (2.9 and 3.5 visual analog scale [VAS] scores, respectively, at day 7 after the procedure). The DEFANS trial proved the feasibility of prescribing VADs in the preoperative period and for 1 month after surgery.
VADs in combination with minimally invasive surgical techniques
In addition to studies of the adjuvant treatment with VADs after open surgery, there are data on the duration of conservative therapy after minimally invasive surgical procedures such as endovenous laser ablation (EVLA). Thus, the DECISION trial (Observational Study Among Patients with Varicose veins of the Lower Limbs Undergoing Endovenous Ablation Alone or in Association with Venoactive Drugs), which was conducted in eight Russian clinical centers, included 230 patients with VVLE classes C2 to C4s (CEAP) and indications for endovascular treatment. The study group received MPFF 1000 mg daily for 2 weeks before and 4 weeks after endovascular treatment. Venous clinical severity score (VCSS) scales and the CIVIQ-14 questionnaire were used. Patients’ satisfaction with MPFF treatment was significantly higher than in the control group.25
In addition, VADs were evaluated in patients who underwent sclerotherapy. Allaert et al conducted a study involving 2862 patients with VVLE class C1S through C3S (CEAP). The 1-month treatment course with MPFF at a dose of 1000 mg daily after sclerotherapy was associated with improvement of the main CVD symptoms.23
There are alternative (vein sparing), minimally invasive techniques for which it is also advisable to provide treatment with VADs. For example, in the studies performed by Pittaluga, VAD treatment (MPFF 1000 mg daily) was prescribed for a period of 3 to 6 months after surgery. According to Pittaluga, due to certain pleiotropic effects, MPFF not only reduces the consequences of surgical trauma, but also leads to a decrease in the diameter and to restoration of the working capacity of superficial veins spared with the Ambulatory Selective Varices Ablation Under Local Anaesthesia (ASVAL) technique.31
According to the Russian National Guidelines, sulodexide may be used for the treatment of advanced stages of CVD.12 Although not a venotonic, sulodexide may be beneficial in affecting CVD pathogenesis and attenuating the inflammatory cascade. Sulodexide inhibits the release of interleukin (IL)-2, IL-12 (p70), IL-10, and VEGF from wound fluid–stimulated THP-1 monocytes in subjects with venous trophic ulcers, and also inhibits the synthesis of MMP-9, which slows down the degradation of the extracellular matrix.37,38
In a study that included 450 patients with CVD, participants received sulodexide at a dose of 250 units BID for 3 months. The results showed an improvement in the QOL of patients with CVD class C4 (CEAP). Adverse events were registered in only 2 patients (epigastric pain in one patient and abdominal pain with vomiting in another).29 In addition, sulodexide can be used for treating post-thrombotic syndrome (PTS). In the SURVET study (Multicenter, Randomized, Double Blind, Placebo Controlled Study on Long-term Treatment with Sulodexide for Prevention of Recurrent Deep Vein Thrombosis in Patients with Venous Thromboembolism), sulodexide demonstrated a reduction in the risk of venous thromboembolism (VTE) recurrences without an apparent increase in bleeding risk.27
In patients with severe CVI and venous ulcers, combination therapy with sulodexide and MPFF has been shown to be more effective than MPFF monotherapy.39
Approaches to determine course duration of VAD treatment according to clinical class of CVD
The analysis of various studies of VADs has demonstrated that in patients with early manifestations of CVD, the treatment benefits become apparent within 1 to 2 months of conservative therapy.18,20 In patients with severe forms of CVD, treatment duration should be extended up to 3 to 6 months.24,30
The Society for Vascular Surgery (SVS) and American Venous Forum (AVF) guidelines do not provide clear recommendations on duration of the VAD treatment course. On the basis of data provided by American colleagues, VADs (diosmin, hesperidin, rutosides, MPFF, escin) in combination with compression therapy are indicated for patients with CVD-related pain and edema in countries where the above-mentioned drugs are available (class 2, level C), and the duration of the course of therapy should be up to 6 months.40
According to the European Venous Forum (EVF) guidelines, the treatment with MPFF for 3 to 6 months is effective in the reduction in CVD symptoms, including pain, feelings of heaviness or tightness (level of evidence A), cramps (level B), functional discomfort (grade A), skin changes (grade A), and severity of ankle edema (grade B).41
Conclusion
CVD is a gradually progressive disorder, and given the pathogenetic mechanisms of its development, it is the vein specific inflammation that leads to the occurrence and worsening of symptoms and severe forms of the disease complicated by trophic changes. Through results of many studies, VADs have proven their efficacy and safety in the treatment of CVD in terms of their influence on the various components of pathogenesis. At present, MPFF is the VAD with the largest evidence base for safety and long-term use and the main one with proven efficacy in suppressing leukocyte-endothelial interactions, in treatment of venous edema and reducing venous pain and heaviness in VVLE, and improving QOL. Duration of VAD treatment course and frequency, as well as the optimal dosing schedule, depends on many factors, mainly on the current status of the patient’s venous system. At the early stages of the disease, it is possible to prescribe short courses of VADs (up to 2 months), but with the worsening of CVD symptoms and development of CVI, it is necessary to prescribe prolonged VAD treatment courses: for at least 3 months in case of edema and at least 6 months for trophic skin changes. VADs are also advisable to be used in the perioperative period, before the invasive treatment of CVD and in the early postinterventional period. A personalized approach to the prescription of VADs is required, taking into account the stage of the disease and the status of the venous system, in order to improve treatment outcomes and QOL in patients with CVD.

REFERENCES
1. Robertson L, Evans C, Fowkes FGR. Epidemiology of chronic venous disease. Phlebol J Venous Dis. 2008;23(3):103- 111. doi:10.1258/phleb.2007.007061.
2. Saveliev VS, Kirienko AI, Zolotukhin IA, Seliverstov EI. A prospective observational study SPECTRUM: a registry of patients with chronic lower limb venous disease [in Russian]. Flebologiya. 2012;6(1):4-9.
3. Bergan J. Molecular mechanisms in chronic venous insufficiency. Ann Vasc Surg. 2007;21(3):260-266. doi:10.1016/j.avsg.2007.03.011.
4. Kalinin RE, Suchkov IA, Pshennikov AS, Rudakova IN, Isakov SA. The level of nitric oxide in postthrombotic syndrome. IPPavlov Russ Med Biol Her. 2016;24(2):79. doi:10.17816/ PAVLOVJ2016279-85.
5. Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96(11):1231-1242. doi:10.1002/ bjs.6798.
6. Pocock ES, Alsaigh T, Mazor R, Schmid- Schönbein GW. Cellular and molecular basis of venous insufficiency. Vasc Cell. 2014;6(1):24. doi:10.1186/s13221-014- 0024-5.
7. Kalinin RE, Suchkov IA, Pshennikov AS, Kamaev AA. The Influence of the magnesium level on the concentration of matrix metalloproteinases in the patients presenting with primary varicose veins. Flebologiia. 2016;10(4):171. doi:10.17116/flebo2016104171-175.
8. Kalinin RE, Suchkov IA, Pshennikov AS, Kamaev AA, Isakov SA, Ryabkov AN. Application of magnesium drugs and their influence on the indicators of connective tissue dysplasia in patients with varicose veins. Nov Khirurgii. 2018;26(1):51-59. doi:10.18484/2305- 0047.2018.1.51.
9. Shanaev IN. Modern theories of pathogenesis of trophic ulcer of venous etiology. Nauk molodykh (Eruditio Juvenium). 2019;7(4):600-611. doi:10.23888/HMJ201974600-611.
10. Haviarova Z, Janega P, Durdik S, Kovac P, Mraz P, Stvrtinova V. Comparison of collagen subtype I and III presence in varicose and non-varicose vein walls. Bratisl Lek Listy. 2008;109(3):102- 105. http://www.ncbi.nlm.nih.gov/ pubmed/18517131.
11. Sansilvestri-Morel P, Fioretti F, Rupin A, et al. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci. 2007;112(3-4):229-239. doi:10.1042/ CS20060170.
12. Kirienko AI, Zatevakhin II, Pokrovsky AV, et al. Russian clinical guidelines for the diagnostics and treatment of chronic venous diseases [in Russian]. Flebologiya. 2018;12(3):146. doi:10.17116/ flebo20187031146.
13. Kalinin RE, Suchkov IA, Kamaev AA, Mzhavanadze ND. Duration of treatment with phlebotonics in patients with chronic venous disease. Angiol Vasc Surg. 2020;26(3):60. doi:10.33529/ ANGI02020301.
14. Shoab SS, Porter JB, Scurr JH, Coleridge- Smith PD. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg. 2000;31(3):456-461. http://www.ncbi. nlm.nih.gov/pubmed/10709057.
15. Shoab S, Porter J, Scurr J, Coleridge- Smith P. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease – a prospective study. Eur J Vasc Endovasc Surg. 1999;17(4):313-318. doi:10.1053/ ejvs.1998.0751.
16. Shoab SS, Scurr JH, Coleridge-Smith PD. Plasma VEGF as a marker of therapy in patients with chronic venous disease treated with oral micronised flavonoid fraction – a pilot study. Eur J Vasc Endovasc Surg. 1999;18(4):334-338. doi:10.1053/ejvs.1999.0890.
17. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF Study. Angiology. 2002;53:245-256. doi:10.1177/000331970205300301.
18. Arceo A, Berber A, Treviño C. Clinical evaluation of the efficacy and safety of calcium dobesilate in patients with chronic venous insufficiency of the lower limbs. Angiology. 2002;53(5):539-544. doi:10.1177/000331970205300506.
19. Roztocil K, Stvrtinová V, Strejcek J. Efficacy of a 6-month treatment with MPFF at a dose of 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol. 2003;22(1):24- 31. http://www.ncbi.nlm.nih.gov/ pubmed/12771852.
20. Kalus U, Koscielny J, Grigorov A, Schaefer E, Peil H, Kiesewetter H. Improvement of cutaneous microcirculation and oxygen supply in patients with chronic venous insufficiency by orally administered extract of red vine leaves AS195: a randomised, doubleblind, placebo-controlled, crossover study. Drugs R D. 2004;5(2):63-71. doi:10.2165/00126839-200405020- 00001.
21. Veverkova L, Jedlicka V, Wechsler J, Kalac J. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of MPFF at a dose of 500 mg to postoperative symptoms. Phlebolymphology. 2006;13(4):195.
22. Pokrovsky A V, Saveljev VS, Kirienko AI, et al. Surgical correction of varicose vein disease under micronized diosmin protection (results of the Russian multicenter controlled trial DEFANS). Angiol Sosud Khir. 2007;13(2):47- 55. http://www.ncbi.nlm.nih.gov/ pubmed/18004259.
23. Allaert FGJ. Observation study on the synergy of action of sclerotherapy and Grade A phlebotonic agents in chronic venous disease of the lower limbs. Angiology. 2010;29(2):2.
24. Rabe E, Stücker M, Esperester A, Schäfer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency – results of a double-blind placebo-controlled study. Eur J Vasc Endovasc Surg. 2011;41(4):540-547. doi:10.1016/j.ejvs.2010.12.003.
25. Bogachev VY, Golovanova OV, Kuznetsov AN, Shekoyan AO; Investigator group. On the rationale of perioperational venous protection in the endocvascualr treatment of the varicose veins on the lower extremities: the first results of the DECISION trial [in Russian]. Angiol Sosud Khir. 2012;18(2):90-95.
26. Allaert FA. Meta-analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31(4):310-315. http://www.ncbi. nlm.nih.gov/pubmed/22801396.
27. Andreozzi GM, Bignamini AA, Davì G, et al. Sulodexide for the prevention of recurrent venous thromboembolism. Circulation. 2015;132(20):1891-1897. doi:10.1161/ CIRCULATIONAHA.115.016930.
28. Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomized trial. Int Angiol. 2015;34(5):428- 436. http://www.ncbi.nlm.nih.gov/ pubmed/25972136.
29. Elleuch N, Zidi H, Bellamine Z, Hamdane A, Guerchi M, Jellazi N. Sulodexide in patients with chronic venous disease of the lower limbs: clinical efficacy and impact on quality of life. Adv Ther. 2016;33(9):1536-1549. doi:10.1007/s12325-016-0359-9.
30. Rabe E, Ballarini S, Lehr L. A randomized, double-blind, placebo-controlled, clinical study on the efficacy and safety of calcium dobesilate in the treatment of chronic venous insufficiency. Phlebol J Venous Dis. 2016;31(4):264-274. doi:10.1177/0268355515586097.
31. Pittaluga P, Chastanet S. Treatment of varicose veins by ASVAL: results at 10 years. Ann Vasc Surg. 2017;38:e10. doi:10.1016/j.avsg.2016.07.021.
32. Tsukanov YT, Nikolaichuk AI. Orthostaticloading- induced transient venous refluxes (day orthostatic loading test), and remedial effect of micronized purified flavonoid fraction in patients with telangiectasia and reticular vein. Int Angiol. 2017;36(2):189-196. doi:10.23736/S0392-9590.16.03708-1.
33. Tsukanov YT, Tsukanov AY. Diagnosis and treatment of situational great saphenous vein reflux in daily medical practice. Phlebolymphology. 2017;24(3):144-151.
34. Maggioli A, Carpentier P. Efficacy of MPFF 1000 mg oral suspension on CVD C0s-C1-related symptoms and quality of life. Int Angiol. 2019;38(2):83-89. doi:10.23736/S0392-9590.18.04054-3.
35. Howlader M, Coleridge Smith P. Relationship of plasma vascular endothelial growth factor to CEAP clinical stage and symptoms in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 2004;27(1):89-93. doi:10.1016/j.ejvs.2003.10.002.
36. Pascarella L, Lulic D, Penn AH, et al. Mechanisms in experimental venous valve failure and their modification by MPFF at a dose of 500 mg. Eur J Vasc Endovasc Surg. 2008;35(1):102-110. doi:10.1016/j. ejvs.2007.08.011.
37. Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease – Part I: Inflammatory biomarkers in wound healing. Biochim Biophys Acta. 2016;1862(10):1964-1974. doi:10.1016/j.bbadis.2016.07.018.
38. Mannello F, Medda V, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide inhibition of MMP-9 gelatinase secretion and activity: possible pharmacological role against collagen degradation in vascular chronic diseases. Curr Vasc Pharmacol. 2013;11(3):354-365. doi:10. 2174/1570161111311030010.
39. González Ochoa A. Sulodexide and phlebotonics in the treatment of venous ulcer. Int Angiol. 2017;36(1):82-87. doi:10.23736/S0392-9590.16.03718-4.
40. O’Donnell TF, Passman MA, Marston WA, et al. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J Vasc Surg. 2014;60(2):3S-59S. doi:10.1016/j. jvs.2014.04.049.
41. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-254. doi:10.23736/S0392-9590.18.03999-8.
