Critical role of the vasa venarum in the pathogenesis of chronic venous disease. Part I: malperfused venules as pathogenetic hot spots
This is the first of the 2 chapters that make up the “critical role of the vasa venarum in the pathogenesis of CVD.” These chapters will be published consecutively in Phlebolymphology.
Dominik R. WEISS2*,
Gerd JUCHEM3,
Hugo PARTSCH4
2 Department of Transfusion Medicine and Hemostaseology, University of Erlangen-Nuremberg (FAU), 91054 Erlangen, Germany
3 Department of Cardiac Surgery, University of Munich (LMU), 81377 Munich, Germany
4 Emeritus Professor of Dermatology, Medical University of Vienna, Austria
ABSTRACT
A new concept for the pathogenesis of venous disease is presented in which the numerous postcapillary venules of the “vasa venarum” play a key pathogenetic role. These tiny veins consist of a highly specialized endothelium, reinforced by an adventitial network of pericytes. Local accumulation of inflammatory mediators induces contraction of the endothelial cells, rapidly leading to an increase in vessel permeability with subsequent efflux of plasma into the interstitium. This triggers the prothrombogenic and proinflammatory potential of the adventitial pericytes, which rapidly express high concentrations of tissue factor. Local accumulation of thrombin results in the formation of fibrin cuffs around neighboring blood and lymph vessels and thrombosis in collecting veins downstream. The subsequent draining of the vasa venarum results in luminal blood in larger downstream veins also becoming involved in the pathogenetic processes. Early systematic prophylaxis is important to prevent disease progression and limit restrictions to patient quality of life (see part II).
INTRODUCTION
Until recently, a convincing concept to explain the pathogenesis of the primary, idiopathic form of venous disease associated with acute thrombosis and the postthrombotic syndrome, and frequently leading to secondary chronic venous insufficiency (CVI) was missing. In particular, the link between gravity-dependent, adverse perfusion conditions in the legs during upright posture and the induction of the chronic inflammatory and microthrombotic processes associated with venous disease and CVI1,2 was still unclear. However, a consistent pathogenetic model of this disorder, based on progress in basic medical research, must be viewed as a prime prerequisite for rational therapeutic measures.
The circulatory system finds its greatest histological and functional manifestation and specialization in the microcirculatory beds within organs. This truism also applies to the vein walls, which are characterized by a very well developed nutritive system, the “vasa venarum.”3 Venules by far outnumber arterioles in these microvascular networks.4 They drain via small collecting veins into the lumen of the appropriate saphenous vein, so that the perfusion of the vasa venarum in all large leg veins depends not least on the prevailing transmural pressure differences, which, depending on posture, eg standing or sitting, can be very high in humans.
Reduced perfusion in microvascular systems may trigger acute inflammatory processes,5 in which the postcapillary venules play a seminal role by virtue of their recruitment of blood immune defense mechanisms.6-8 A highly specific thrombotic occlusion of these tiny veins has been regarded for some time as the first reliable sign of impending organ rejection.9-11 Apparently, and for reasons not yet understood, the postcapillary venules also play a central role in the induction of thrombotic processes.
The aim of the present review is to summarize recent relevant findings on the cellular and hemostatic properties of postcapillary venules, explaining the key elements in the pathogenesis of primary and secondary venous disease, with a particular emphasis on the unique and newly recognized functional features of the constituent venule cell types (endothelial cells and pericytes). Current therapeutic approaches will be critically reviewed and relevant perspectives will be outlined in a separate publication (Part II).
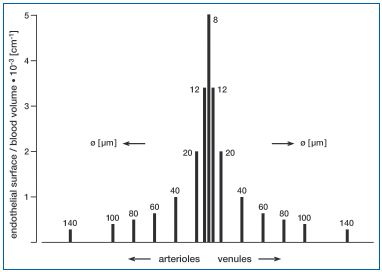
Figure 1: Blood endothelial contact in the various vascular regions of the circulation (ratio of endothelial surface area to blood volume). The contact between blood and endothelium is 5000 times higher in the capillaries than in the large arterioles (arteries) or venules (veins). Diameters are plotted from published data,48 assuming that the blood vessels are cylindric. The values in italics above/beside the columns indicate the diameters (in micrometers) of each vessel type.
THE COMPLEX VENOUS SYSTEM OF THE LEGS IN HEALTH AND IN CVI
When standing still, blood accumulates in the thin walled leg veins and the abdominal vena cava under the influence of the hydrostatic pressure exerted by the vertical fluid column. Blood flow velocity is slow, and slows even further if venous tone decreases, as during warm weather, resulting in further venous pooling in the lower extremities. The ratio between endothelial surface area and blood volume, a parameter characterizing the contact of blood with the embracing endothelium, is also correspondingly low (Figure 1). The increased hydrostatic pressure, highest in the feet, increases plasma water filtration from capillaries into the surrounding interstitium, causing edematous expansion of the dependent extravascular space (hydrostatic edema), impairment of substance exchange. Manifest edema in the lower extremities may result. Under these conditions, “auxiliary” mechanisms provide important support for the return of blood to the heart. These include the pulse wave in the arteries accompanying the large veins, and rhythmic pressure changes in the abdomen accompanying respiration. As soon as locomotion starts, the contraction of the leg muscles, particularly those of the calves, together with the vein valves, constitute the “muscle pump” (Figure 2). This plays a major role in supporting circulatory performance during physical effort, especially in the upright position.
When the delicate mechanisms transporting blood in the veins back to the heart against gravity fail, elevated transmural pressure in the leg veins may result. This is even more likely when the vein valves become incompetent, and reflux of venous blood and hence ambulatory venous hypertension occur. Although the above-mentioned hydrostatic and hydrodynamic consequences of upright posture12 are generally believed to be an important prerequisite in the pathogenesis of venous disease, the mechanisms leading to valve incompetence, vein wall sclerosis, thrombosis, severe inflammation and ulcers are not clear. The following new insights into cell and tissue architecture of the complex venous wall, in association with the hemostatic and inflammatory consequences of the upright position, may contribute to a better understanding.
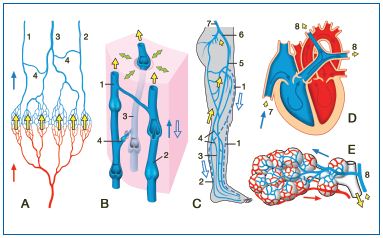
Figure 2: The venous vascular bed from the leg to the lung: consequences for the plasma concentrations of shortlived antithrombogenic mediators of endothelial origin (heavily-outlined yellow arrows). A) The skin and the various leg organs (muscle, bone etc) all possess their own microcirculatory systems, with an endothelium producing large amounts of short-lived inhibitors of platelets and coagulation factors that are released into the venous blood (large yellow arrows). 1, 2: Superficial veins (1: greater and 2: lesser saphenous veins). 3: Deep leg vein (eg peroneal vein). 4: perforating veins. Red or blue arrow: arterial or venous blood flow, respectively.
B) Morphological details of the veins and their valves at the level of the calf (1, 2: superficial veins; 3: deep vein: also shown: perforating veins). In the case of valve insufficiency, the direction of venous flow can be reversed (open blue arrow). The green double-ended arrows indicate the effect of the (calf) muscle pump, the smaller yellow arrows indicate the now lower autacoid oncentrations. C) The concentration of the endothelial inhibitors decreases further in the course of the long flow path to the lung via the deep and superficial leg veins, the common femoral vein (5), the internal iliac vein (6), the inferior vena cava (7) and the right side of the heart (D) and the pulmonary artery (8). Once in the arterial limb of the pulmonary microcirculation (E) the concentration of the inhibitors is restituted.
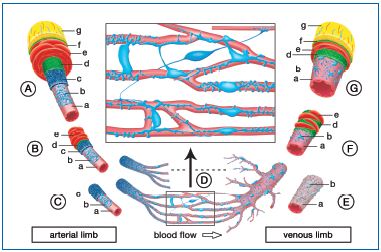
Figure 3: Bizarre shaped and interconnected pericytes as companion cells in all blood vessels. In the capillary beds the pericytes (blue) lie immediately at the endothelial tube (pink) on its adventitial side. Their extensions make contact with neighboring and even more distant terminal vascular beds (see enlargement in the inset). Each capillary bed is supplied by a precapillary arteriole, the endothelial tube of which is enveloped by a particularly dense layer of pericytes embedded in the self-synthesized extracellular matrix (ECM, dark blue). The larger, feed arterioles consist of the intimal double layer of endothelium and pericytes plus an outer layer of vascular smooth muscle (red). The smooth musculature continues upstream in a large artery, where it is much thicker and constitutes the media of these vessels. Big arteries also have an adventitia (yellow) containing a well-developed network of nutritive microvessels (vasa vasorum) that penetrate deep into the media. The right-hand diagram shows that the venous limb of the circulation is constructed similarly, although the pericytes of the venules and larger veins here form a looser, larger meshed
net and do not synthesize a continuous extracellular matrix. A large artery, B feed arteriole, C precapillary arterioles, D capillary bed, E postcapillary venules, F collecting venule, G large vein; a endothelial tube, b pericytes, c extracellular matrix (the “cocoon”of the pericytes), d internal elastic membrane, e smooth muscle cells (media), f external elastic membrane, g adventitia with vasa vasorum or vasa venarum, respectively.
Flowing blood is only able to fulfill its numerous transport and distribution functions as long as it is fluid. On the other hand, at sites of vessel injury it must rapidly solidify to prevent blood loss. The solution to this apparent functional paradox is found in the complex structure of the blood vessel intima that generally consists of two types of tissue with contrasting functions.13-15
Vascular endothelium covers the intimal surface of all blood vessels (Figure 3) and is thus the actual blood container of the body. This contact surface with the blood is normally characterized not only by plateletand coagulation-inhibiting activities (anti-aggregatory and anticoagulant activities), but can also develop profibrinolytic activity and thus even dissolve already formed fibrin thrombi (Figure 4).13,15-19 The corresponding biochemical processes are bolstered by typical release products of activated platelets, and also depend on the availability of certain plasma proteins of hepatic origin such as protein C, antithrombin III, and heparin cofactor II. Healthy endothelium — acting as a complex boundary catalyst continuously inhibiting or removing proaggregatory and procoagulant molecules and lysing fibrin — thus counteracts the formation of thrombi in the circulation. For geometrical reasons, the smaller the vessel, the higher the ratio between intimal surface area and blood volume. This contact between endothelial cells and the blood, and thus the antithrombotic and anti-inflammatory potency of the endothelium, will therefore be most intimate in the terminal vessels of organ microcirculatory systems (Figures 1 and 2).
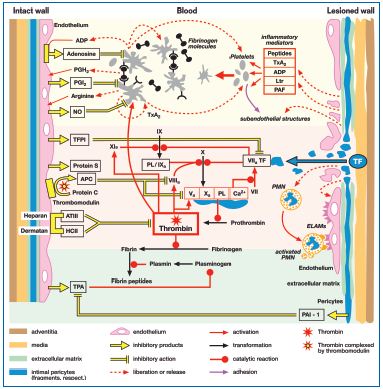
Figure 4: A survey of the most important antithrombogenic and prothrombogenic activities at the interface between the blood and the vessel wall. The thrombotic potential of blood is mainly determined by the behavior of the platelets (shown in the uppermost segment of the diagram on a pale yellow background), the coagulation system (middle segment on a pale pink background), and the fibrinolysis system (lower segment, pale green background). Healthy endothelium (left-hand side of the diagram) is consistently antithrombotic and, in the context of its anti-aggregatory activity, synthesizes platelet-inhibiting autacoids such as adenosine, prostacyclin (PGI2) and nitric oxide (NO), especially when activated platelets release substances that can serve as precursors, eg adenosine diphosphate (ADP), prostaglandin H2 (PGH2) and arginine, respectively. In the context of its anti-coagulatory activity the endothelium releases tissue factor pathway inhibitor (TFPI) and protein S. In addition, endothelial thrombomodulin can bind
thrombin and, in so doing, causes the latter to refold.
Thrombin now expresses antocoagulant activity and activates protein C (APC). The binding of antithrombin III (ATIII) to proteoglycans in the glycocalyx, together with participation of heparin cofactor II (HCII), enhances ATIII’s anticoagulant potency 1000-fold. Finally, healthy endothelium has a profibrinolytic effect by releasing tissue-type plasminogen activator (TPA). Under inflammatory conditions plasminogen activator inhibitor 1 (PAI-1) is released by the subendothelial pericytes.49
Damage to the endothelium (right-hand side of the diagram) results in the release of numerous inflammatory mediators—diverse peptides, thromboxane A2 (TxA2), ADP, leukotrienes (Ltr), and platelet activating factor (PAF) from the activated pericytes or necrotic tissue—and the blood makes contact with tissue factor (TF) expressed on the surface of pericytes (or on microparticles released by the latter).
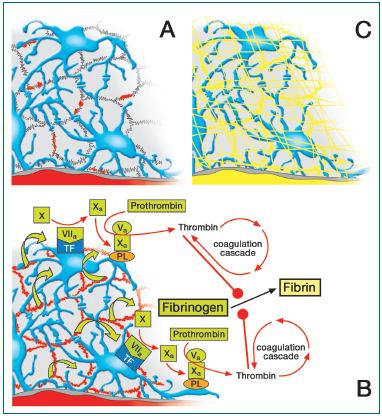
Figure 5: Valvular origin of venous thrombosis (view from the abluminal surface of the intimal coat of a valve).
A) Acute effect on endothelial permeability: endothelial clefts become leaky (small red areas). B) The plasmatic zymogens of the coagulatory system are extravasating into the interstitium (green arrows). The subendothelial pericytes unfold extremely high concentrations of tissue factor (TF) and prothrombinase (phospholipids PL, Va, Xa, Ca++), thus promptly initiating coagulation. C) The final result is envelopment of the respective vessel in a fibrin cuff that firmly anchors the fibrin thrombus that can also intrude into the lumen of the parent vessel.
In the nutritive microvessels of the vein wall, interconnected pericytes and their extracellular matrix form a sort of adventitial coat (Figures 3C-E), which is particularly well developed in the arterial limb of the respective microvascular systems.20 A net-like tissue of pericytes is also located in the subendothelium of the intima of larger arteries and veins (Figures 3A, 5 and left micrograph in Figure 6). All venous pericytes contain large amounts of tissue factor,15 which can be rapidly recruited to the cell surface under acute inflammatory conditions,21 eg in a fresh wound. No other cell types in the vessel wall or blood (besides activated monocytes) express this inducer molecule of nearly all clinically relevant thrombotic processes.22,23 Tissue factor, together with factor VIIa, activates factor X,15 as well as further membrane components that assemble the activated factors Xa and Va to form the prothrombinase complex (Figures 3 and 5), which in turn rapidly catalyzes the proteolytic conversion of prothrombin to thrombin.24 Immediately after injury, exposed pericytes in the intima, nutritive vessels, and microvascular networks of adjacent connective or parenchymal tissue all contribute to local thrombin formation and hence to the normally rapid initiation of hemostasis. Thrombin concentrations in blood as low as 1 nM (0.1 U/ml) produce fibrin clots that are turbid and composed of thick, loosely-woven fibrin strands.25 In parallel, immediate activation of platelets produces increasing numbers of aggregates, which recruit the entire coagulatory cascade to the rapidly enlarging surface area of this “white thrombus.”26 Thus, after a lag phase of 2-6 minutes, the generation of thrombin in the local environment of a thrombus can build up concentrations of between 100-500 nM.25 Release products from the platelets (eg platelet activating factor [PAF], see Figures 4, 7) in turn activate polymorphonuclear granulocytes (PMN),27 which immediately ensure an effective immune defense in the injured area.28 The final result of these processes is a highly organized “red thrombus”. All the activators that diffuse into the passing blood are rapidly inactivated due to their metabolic, thermal or chemical instability.13 Serpins in the plasma specifically contribute to the rapid removal of procoagulatory and profibrinolytic factors.29
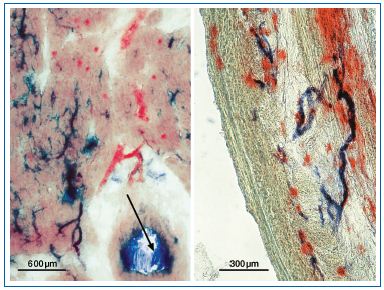
Figure 6: Similarity of coronary microvasculature in human left ventricular myocardium (left) and the vasa venarum of the saphenous vein wall (right). Specific demonstration of alkaline phosphatase on the pericyte tube of arterioles (blue), and dipeptidylaminopeptidase IV in the endothelial tube of postcapillary venules (red) by application of enzyme histochemical staining techniques. In addition, the arrow in the left figure identifies a sectioned branch of a larger coronary artery. The blue staining shows that alkaline phosphatase is also expressed also in the intima (here partly desquamated), indicating the presence of subendothelial pericytes (see Figure 3).
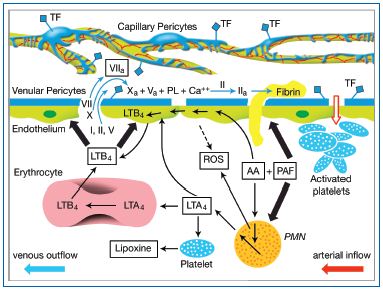
Figure 7: Scheme of the proposed cellular and biochemical mechanisms responsible for breakdown of the venular endothelial barrier, formation of fibrin cuffs, and the thread of venulo-thrombosis. Locally released inflammatory mediators activate the arteriolar endothelium, resulting in the adhesion/aggregation of platelets. Platelet activating factor (PAF) released from these platelets further activates the arteriolar endothelium as well as incoming neutrophils (PMN). The latter promptly synthesize leukotriene LTA4 from arachidonic acid (AA) (also released by the platelets). The LTA4 is metabolized by erythrocytes and endothelial cells to LTB4, which in turn activates, via specific receptors, the postcapillary venular endothelium. Via complex signal cascades this results, among other enzyme systems, in activation of the myosin light chain kinase (MLCK), contraction of the venular endothelial cells, and widening of the intercellular junctions and hence breakdown of the blood-myocardium barrier. Plasma now flowing into the subendothelial space encounters the activated pericytes.
The latter initiate activation of the coagulatory cascade and a rapid formation of fibrin cuffs, which can grow into the lumen of the parent vessel.
An important prerequisite for the physiological occlusion of wounds is that the antithrombogenic factors secreted continuously by the endothelial cells into the blood are evanescent, and thus disappear rapidly from the blood at the site of injury. This allows the autocatalytic prothrombogenic processes to proceed at that site, whilst their concentrations in the intact circulatory system are kept at a sufficient concentration by continuous endothelial re-synthesis. The contraction of integral contractile proteins released from aggregating platelets results in a retraction of the thrombus, whereby the latter becomes firm and extremely dense and thus prevents the contact of any further coagulation zymogens with the pericytes located outside the vessel. In this manner, the fulminant, self-reinforcing hemostatic process at the site of an injury is stifled as soon as its goal is achieved, whilst the remaining intact and perfused vascular bed remains free of thrombi.
INDICATION OF VENOUS THROMBOSIS AND VENOUS DISEASE
The previously described ingenious principle of hemostasis functions well at sites of tissue or organ injury and never spontaneously in a well perfused and healthy vascular bed. Under adverse perfusion conditions, as may occur in leg veins during upright stance, intravasal thrombotic processes can be initiated without mechanical damage, and the prothrombotic mediators accumulating in the venous blood are not inactivated with the necessary efficiency (see next section). In this context, the nutritive microvascular system within the vessel walls, particularly in the large veins (vasa venarum), appears to play a key role (although this is not yet widely recognized).
The vasa venarum3 in the vein wall correspond to the nutritive microvessels of the arterial wall, the vasa vasorum.30 In the saphenous vein, they are characterized by numerous postcapillary venules that stain highly specifically for dipeptidyl aminopeptidase IV, similarly to the smallest veins of the coronary microvasculature (Figure 6). In contrast, the arterioles of both microvascular systems stain selectively for alkaline phosphatase.31 This histological similarity is not surprising, as from an evolutionary point of view the heart is also a blood vessel, albeit with a specialized musculature in its wall. Indeed, some authors regard the coronary microvasculature as the “vasa vasorum of the myocardium,”32 and the numerous studies on the regulation of coronary blood flow and vessel density in the literature can therefore be regarded as meaningful models for the microvasculature of the arterial or venous walls.
The site at which the vena venarum of the leg veins drain into the lumen of the respective vein deserves special attention. In a recent article, Crotty describes the structure and function of the venous valve agger (a crescent-shaped fibroelastic sleeve, spanning the vein wall at the base of every vein valve): “The agger forms part of a complex that, in conjunction with its dedicated musculature, a reversible transmural pressure gradient and physiological turbulence in the valve sinuses, positively facilitates drainage from the local segment of the vasa venarum network when venous tone is normal. However, when venous tone is elevated the agger pumps and sucks blood from the lumen of the vein to perfuse the vasa venarum network.”33 Crotty concludes that disturbed agger function underlies the formation of varicose veins. Other authors also report direct entry of the vena venarum into the venous lumen.34 However, regardless of whether the blood from the vena venarum passes directly into the lumen of the large parent vein, or if it first enters a tributary, drainage of the wall venules into the lumen of the main vein proceeds within a few seconds and will allow reflux of venous blood into the vena venarum network, particularly during walking. As will be discussed in the next section, such reflux may trigger pathophysiological processes that disturb homeostasis in the vein wall and culminate in manifest venous disease under adverse, initially purely physical conditions.
Reflux is accompanied by hypoxia and local ischemia, which together rapidly activate blood platelets and PMNs.5,35-37 Both cell types cooperate metabolically. Thus, PAF released from platelets27 stimulates the PMN to increase the synthesis and release of leukotriene A4 (LTA4), which is the precursor for LTB4 synthesis and release by erythrocytes and endothelial cells (Figure 7). Using an in vitro model of the human coronary postcapillary venular wall, we have recently shown that this leukotriene elicits a rapid and specific contraction of the respective, highly specialized endothelium, which would most likely result in the breakdown of the venular barrier in situ.27 Very similar results have been obtained in studies on the mesenteric microvasculature in situ.38 All these findings are consistent with the generally accepted concept that postcapillary venules play a key role in the recruitment of leukocytes, in plasma exudation and hence in the genesis of inflammatory edema.6-8 At this point the pericytes come into play.20 These cells accompany and envelop the postcapillary venules (Figure 3). Their direct contact with plasma coagulation factors initiates the coagulation processes described above (Figure 4) and results in the frequently observed fibrin cuffs that surround the microvessels in the later stages of CVI,20,39,40 and which can also be recognized in the subendothelium of larger veins of CVI patients. Tissue coagulation processes are always accompanied by acute inflammatory reactions, involving in particular the complement system, monocytes and PMN. The risk is that these processes spread into the venular lumina, reinforce themselves autocatalytically, with the resultant thrombi extending into the lumen of the parent large leg veins. The stability of the highly structured thrombi and the evanescence of endothelial inhibitors, which are advantageous in the case of perforating injury, may now represent a serious disadvantage by creating permanent obstruction in more and more terminal vessels of the vein wall (or adjacent leg organs) and ultimately even in the larger vein. A similar mechanism is likely to take place in the subendothelial pericytes in the region of vein valves (Figure 5) making the latter fibrotic and incompetent. This could explain the postulated valvular origin of venous thrombosis.41
For purely geometrical reasons (Figure 1), the refluxing blood flowing back and forth within the larger leg veins is generally in contact with relatively little endothelium. As a result the concentration of the short-lived inhibitors from the endothelium can become critical (Figure 2). Thrombosis in the deep leg veins42 may thus result in an even greater delay in the return of venous blood to the heart and to the pulmonary circulation, where the enormous total surface area of the lung capillaries normally delivers adequate amounts of anticoagulatory and anti-inflammatory mediators to the mixed venous blood (Figure 2). Such thrombi in the deep leg veins can extend further downstream and, in the worst case, tear loose and cause a potentially fatal pulmonary embolus.43
The remodeling of the walls and functional loss of valves in the larger veins, leading to rarefication of the nutrient vessel networks, sclerosis and varicosis, becomes understandable in the context of the spreading microthrombotic and inflammatory processes described above, and the consequent breakdown of the venular barrier between the blood and vein wall connective tissue. Pericytes are generally characterized by profibrotic activities.20 A correspondingly altered wall structure is frequently seen in the leg veins of patients suffering from “postthrombotic syndrome”.44 The spectrum of symptoms is further complicated by the fact that the microcirculation in the skin, muscles, and bones also contains numerous venules, which will react similarly under conditions of reflux and obstruction and become pathophysiologically important disease foci. This spread of microcirculatory dysfunction and selfreinforcing processes thus represents a vicious circle, which is the basis for the typical progression of the later stages of CVI.
An acute predisposition for such events is created, in particular, by orthopedic surgery (especially hip and knee replacements), which is accompanied by a high risk of thrombosis, despite postoperative anticoagulation.45-47 Under these circumstances there is a massive, iatrogenically caused influx of pericyte fragments (microparticles) from the red bone marrow, via the numerous small veins that drain venous blood from the central vein in the main leg bones into the deep leg veins. With their high tissue factor content and prothrombinase activity, these particles constitute an extremely effective foci for the formation of fibrin thrombi, culminating in rampant thrombosis, and in the longer term, to secondary venous disease.

REFERENCES
1. Ojdana D, Safiejko K, Lipska A, et al. The inflammatory reaction during chronic venous disease of lower limbs. Folia Histochem Cytobiol. 2009;47:185- 189.
2. Bergan JJ, Pascarella L, Schmid- Schonbein GW. Pathogenesis of primary chronic venous disease: Insights from animal models of venous hypertension. J Vasc Surg. 2008;47:183- 192.
3. Kachlik D, Baca V, Stingl J, et al. Architectonic arrangement of the vasa vasorum of the human great saphenous vein. J Vasc Res. 2007;44:157-166.
4. Lametschwandtner A, Minnich B, Kachlik D, Setina M, Stingl J. Threedimensional arrangement of the vasa vasorum in explanted segments of the aged human great saphenous vein: scanning electron microscopy and three-dimensional morphometry of vascular corrosion casts. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1372- 1382.
5. Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nature Med. 2011;17:1391- 1401.
6. Grega GJ, Adamski SW. The role of venular endothelial cells in the regulation of macromolecular permeability. Microcirc Endothelium Lymphatics. 1988;4:143-167.
7. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678-689.
8. Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234-247.
9. Faioni EM, Mannucci PM. Venocclusive disease of the liver after bone marrow transplantation: the role of hemostasis. Leuk Lymphoma. 1997;25:233-245.
10. Hubscher SG. Central perivenulitis: a common and potentially important finding in late posttransplant liver biopsies. Liver Transpl. 2008;14:596- 600.
11. Rose AG, Cooper DK. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7:31-41.
12. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
13. Weiss DR, Juchem G, Eblenkamp M, et al. Search for optimized conditions for sealing and storage of bypass vessels: influence of preservation solution and filling pressure on the degree of endothelialization. Int J Clin Exp Med. 2010;3:10-27.
14. Nees S, Weiss DR, Senftl A, et al. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol. 2012;302:H69-84.
15. Weiss DR, Juchem G, Kemkes BM, Gansera B, Nees S. Extensive deendothelialization and thrombogenicity in routinely prepared vein grafts for coronary bypass operations: facts and remedy. Int J Clin Exp Med. 2009;2:95-113.
16. Bombeli T, Mueller M, Haeberli A. Anticoagulant properties of the vascular endothelium. Thromb Haemost. 1997;77:408-423.
17. Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem. 2004;11:2245-2260.
18. van Hinsbergh VW. The endothelium: vascular control of haemostasis. Eur J Obstet Gynecol Reprod Biol. 2001;95:198- 201.
19. Verhamme P, Hoylaerts MF. The pivotal role of the endothelium in haemostasis and thrombosis. Acta Clin Belg. 2006;61:213-219.
20. Nees S, Weiss DR, Juchem G. Focus on cardiac pericytes. Pflugers Arch. 2013;465:779-787.
21. Juchem G, Weiss DR, Gansera B, Kemkes BM, Mueller-Hoecker J, Nees S. Pericytes in the macrovascular intima: possible physiological and pathogenetic impact. Am J Physiol Heart Circ Physiol. 2010;298:H754-770.
22. Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327- 358.
23. Osterud B. Tissue factor expression in blood cells. Thromb Res. 2010;125(suppl 1):S31-34.
24. Nesheim ME, Eid S, Mann KG. Assembly of the prothrombinase complex in the absence of prothrombin. J Biol Chem. 1981;256:9874-9882.
25. Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131-142.
26. Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381-1389.
27. Juchem G, Weiss DR, Knott M, et al. Regulation of coronary venular barrier function by blood borne inflammatory mediators and pharmacological tools: insights from novel microvascular wall models. Am J Physiol Heart Circ Physiol. 2012;302:H567-581.
28. Petaja J. Inflammation and coagulation. An overview. Thromb Res. 2011;127(suppl 2):S34-37.
29. Pike RN, Buckle AM, le Bonniec BF, Church FC. Control of the coagulation system by serpins. Getting by with a little help from glycosaminoglycans. FEBS J. 2005;272:4842-4851.
30. Mulligan-Kehoe MJ. The vasa vasorum in diseased and nondiseased arteries. Am J Physiol Heart Circ Physiol. 2010;298:H295-305.
31. Nees S, Juchem G, Eberhorn N, et al. Wall structures of myocardial precapillary arterioles and postcapillary venules reexamined and reconstructed in vitro for studies on barrier functions. Am J Physiol Heart Circ Physiol. 2012;302:H51-68.
32. Han DG. The innateness of coronary artery: Vasa vasorum. Med Hypotheses. 2010;74:443-444.
33. Crotty TP. The venous valve agger and plasma noradrenaline-mediated venodilator feedback. Phlebology. 2007;22:116-130.
34. Dashwood MR, Anand R, Loesch A, Souza DS. Hypothesis: a potential role for the vasa vasorum in the maintenance of vein graft patency. Angiology. 2004;55:385-395.
35. Lim CS, Gohel MS, Shepherd AC, Paleolog E, Davies AH. Venous hypoxia: a poorly studied etiological factor of varicose veins. J Vasc Res. 2011;48:185-194.
36. Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24:363-369.
37. Sica A, Melillo G, Varesio L. Hypoxia: a double-edged sword of immunity. J Mol Med (Berl). 2011;89:657-665.
38. Casillan AJ, Gonzalez NC, Johnson JS, Steiner DR, Wood JG. Mesenteric microvascular inflammatory responses to systemic hypoxia are mediated by PAF and LTB4. J Appl Physiol. 2003;94:2313-2322.
39. Sandor T. Pathomechanism of chronic venous insufficiency and leg ulcer. Acta Physiol Hung. 2004;91:131-145.
40. Brown J. The role of the fibrin cuff in the development of venous leg ulcers. J Wound Care. 2005;14:324-327.
41. Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Ann Rev Physiol. 2011;73:527-545.
42. Hassouna HI. Thrombophilia and hypercoagulability. Med Princ Pract. 2009;18:429-440.
43. McManus RJ, Fitzmaurice DA, Murray E, Taylor C. Thromboembolism. Clin Evid (Online). 2011 Mar 8;2011. pii: 0208.
44. Kahn SR. The post-thrombotic syndrome: progress and pitfalls. Br J Haematol. 2006;134:357-365.
45. Januel JM, Chen G, Ruffieux C, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307:294-303.
46. Warwick D, Rosencher N. The ‘’critical thrombosis period’’ in major orthopedic surgery: when to start and when to stop prophylaxis. Clin Appl Thromb Hemost. 2010;16:394-405.
47. Calfon M, Seddighzadeh A, Piazza G, Goldhaber SZ. Deep vein thrombosis in orthopedic surgery. Clin Appl Thromb Hemost. 2009;15:512-516.
48. Busch C, Cancilla PA, DeBault LE, Goldsmith JC, Owen WG. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982;47:498-504.
49. Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209- 217.
