Current concepts of venous malformation (VM)
MD, PhD, FACS
& Samsung Medical Center
Seoul, Korea.
SUMMARY
Introduction. Venous malformation (VM) is one of the most common forms of congenital vascular malformations (CVM). The VM is further classified into extratruncular (ET) forms and truncular (T) forms, depending on the embryonic stage when the developmental arrest occurrs. This new classification provides critical information on clinico-anatomo-pathophysiology.
Methods. Proper combination of the various noninvasive to minimally invasive diagnostic tests provided a precise diagnosis of the VM. Invasive studies were reserved mostly for differential diagnosis and/or for a treatment “road map.” Once the treatment was indicated, the crucial decision of the selection of proper treatment modalities and the time to begin was made through a multidisciplinary approach. Treatment was indicated in the case of hemorrhage, pain, functional disability, chronic venous hypertension, critical location which threatens vital functions or carries a high risk of complication, and severe cosmetic deformity. Various surgical and nonsurgical therapies were implemented: sclerotherapy, mostly for the surgically inaccessible or difficult lesions, and surgical (excisional) therapy of the surgically accessible lesion with or without preoperative embolo/sclerotherapy.
Results. Among a total of 294 VM, 99 surgically inaccessible ET forms received a total of 419 multisession ethanol sclerotherapy sessions, with an immediate success rate of 98.8% and excellent interim results (average 18.2 months). Most of the 25 surgically amenable ET forms received 36 sessions of preoperative embolo/sclerotherapy with N-butyl cyanoacrylate (16/25) and subsequent surgical excision, with excellent results with minimum morbidity (average 21.2 months).
Discussion. Absolute ethanol, accepted as the primary choice of the sclerotherapy for VM, can have various major and/or minor acute complications, even when used with extreme precaution. Chronic morbidity with or without sequelae still remains to be assessed.
Conclusion. A multidisciplinary approach based on a new classification will allow the best combination of treatment. Careful assessment and proper control of the potential risk involved with each treatment can deliver much-improved results.
INTRODUCTION
Venous malformations (VM) are one of the most common forms of congenital vascular malformations (CVM), after lymphatic malformations (LM). CVM are one of the vascular anomalies which can occur together with (infantile) hemangioma. The hemangioma is a true vascular tumor which develops after birth. It has a rapid growth course through its proliferative phase, but has self-limiting evolution (growth) followed by natural involution (regression).1 CVM are, in contrast, true vascular defects originating from defective embryogenesis of unknown etiology, and continue to grow proportionally to general body growth. The CVM are now classified into five groups, depending on their predominant component: arterial, venous, arteriovenous (AV) shunting, lymphatic, and combined (mostly hemolymphatic) defects, based on the modified Hamburg classification (Table I).2,3
The VM itself is further classified into two groups, like any other form of CVM; extratruncular forms and truncular forms, depending on the stage of embryonic life when the developmental arrest has occurred. The extratratruncular (ET) form is relatively common among VM. It is an embryonic tissue remnant following developmental arrest at the earlier stage of embryonic life. Any embryonic tissue remnants from the earlier stage (eg, reticular stage) of organogenesis can retain the characteristics of the mesodermal cell origin. Therefore, this ET form maintains its potential evolutive power like any other tissue originating from the mesenchymal cells.4 It can often grow explosively when the condition (eg, trauma, surgery, hormone therapy, pregnancy) adequately stimulates it. This, in turn, has serious consequences for clinicians in terms of “recurrence.” The truncular (T) form, in contrast, lacks this evolubility since it is a growth defect developed in the latter stages of embryonic life to grow as the normal (axial) venous system after it loses the mesenchymal cell characteristics.
Though classical (traditional) name-based nomenclature (eg, Klippel-Trenaunay syndrome, Parkes-Weber syndrome) is still popular among the clinicians, its fundamental liability is that it can not provide crucial information of the clinico-anatomo-pathophysiology of these complicated birth defects.5 The new modified Hamburg classification is finally able to provide this critical information and clear up confusion regarding the proper definition of the VM for the right diagnosis and management.6
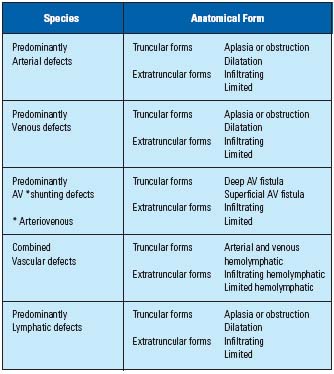
Table I. Hamburg Classification of Congenital Vascular
Malformation – 1988 with modification.2,3
METHODS – DIAGNOSIS
Proper combination of the various noninvasive to minimally invasive diagnostic tests were implemented in all the CVM patients registered at our Clinic, to provide accurate diagnosis of the VM.7
These newly introduced tests, mostly of a noninvasive to minimally invasive nature, based on advanced diagnostic technology, were able to provide the crucial hemodynamic and anatomophysiologic information for the diagnosis of the VM; duplex sonography (color Doppler image and spectral waveform analysis), whole-body blood pool scintigraphy (WBBPS) utilizing radioisotope-tagged erythrocytes, standard T1 and T2 MRI image study, transarterial lung perfusion scintigraphy (TLPS) utilizing radioisotope microalbumin, aeroplethysmography (APG) and/or lymphoscintigraphy.
Various invasive studies (eg, ascending, descending, and percutaneous phlebography and standard, selective, and superselective arteriography) were seldom needed for the diagnosis of the malformation per se. They were often reserved only for the differential diagnosis and/or for a treatment “road map.”7
Differential diagnosis with other CVMs were further included when indicated for the possible combined lymphatic malformation (LM) and/or AV shunting malformation (AVM). This is often critical for the proper management of the VM since there is potential risk of negative impact on VM management. Precise assessment of the deep vein system was mandatorily required when the T form of VM is involved the marginal (lateral embryonal) vein as a venous component of the hemolymphatic malformation (HLM).8
METHODS –
MULTIDISCIPLINARY APPROACH
Once the accurate diagnosis of the VM was established, further decisions were referred to the multidisciplinary board of the CVM Clinic. The summed-up results and opinions of each member were reviewed together by the team, involving 15 related specialists (Table II). The first crucial decision as to whether the lesion was indicated for treatment was made on the basis of a consensus among the multidisciplinary team members.

Table II. Multidisciplinary team*
* The Congenital Vascular Malformation Clinic, Vascular Center,
Samsung Medical Center & Sungkyunkwan University, Seoul,
Korea
Once the VM lesion was confirmed as needing treatment with various indications, the next decision for the selection of proper treatment modalities, as well as the time to begin, was made per-protocol.7 Unless the VM lesion is a life-, if not limb-threatening, condition, or seriously affecting functioning, the treatment is generally delayed until the child grows old enough to tolerate various treatment strategies (eg, age 6 to 9). Though with VM in general it is relatively safe to wait, buying enough time to observe its behavior for proper planning, VM in the lower extremity accompanying the vascular-bone syndrome was selected for earlier treatment, before abnormal long bone growth caused significant functional disability by the discrepancy between long bone length and pelvic tilt.9,10
The principle of the treatment strategy aimed at the primary lesion of the VM was first to control its hemodynamic impact. The treatment for secondary morbidity of the primary lesion (eg, Achilles tendon shortening) was deferred until the primary lesion was under adequate control.8
METHOD – INDICATIONS
AND TREATMENT MODALITIES
Various indications7 were implemented to select VM patients for treatment; hemorrhage, pain with or without functional disability, chronic venous hypertension with secondary morbidity, critical location (eg, proximity to the airway) threatening vital function, vulnerable location with increased risk of complication (eg, knee, ankle, and foot), severe cosmetic deformity decreasing quality of life, and vascular-bone syndrome.
Various surgical and nonsurgical therapies were implemented independently, or combined with other modalities of treatment depending on the indication. Sclerotherapy with various agents (eg, absolute ethanol) was given mostly to surgically inaccessible or difficult lesions. Surgical (excisional) therapy with or without preoperative supplemental embolo/sclerotherapy was selected for the surgically accessible lesion. A multidisciplinary approach to integrate the surgical therapy and embolo/sclerotherapy was strictly implemented in every possible case to reduce morbidity and/or complications, and also the recurrence rate.8
The treatment strategy was repeatedly reviewed by the multidisciplinary team to weigh the benefit over the risk of morbidity following the therapy before commitment to continuous treatment.
RESULTS
We performed retrospective analysis of a total of 294 patients with VM in order to assess the results of contemporary management of VM based on the new concepts.
Among a total of 797 patients with various CVMs, 294 patients (male-138, female-156, mean age 18.6 years, 3 months – 59 years) were confirmed as VM mostly located in the extremities (128/294: upper – 30 and lower – 98), and often as multiple lesions (73/294). They all were diagnosed based on the noninvasive tests only. One hundred and twenty four of a total of 294 VM were selected for treatment with various indications. Ninety-nine infiltrating types of the ET form indicated for the treatment but not for surgical therapy, received a total of 419 multisession ethanol sclerotherapy treatments. Twenty-five limited types of ET forms which were surgically amenable were excised surgically but mostly combined with preoperative embolo/sclerotherapy (16/25) with N-butyl cyanoacrylate (NBCA). Thirty-six sessions of NBCA embolotherapy were given independently or in conjunction with ethanol sclero- therapy as preoperative adjunct therapy for the subsequent surgical excision of 16 ET forms. Nine T forms of VM (eg, venectasia, venous aneurysm) were indicated for treatment hemodynamically, and underwent various surgical treatments as independent therapy successfully. Follow-up assessment of treatment results was made with the duplex scan, WBBPS, and/or MRI at regular intervals by the multidisciplinary team.
Ninety-nine ET forms, treated with ethanol sclerotherapy as independent therapy through a total of 419 sessions, showed an immediate success rate of 98.8% (414 sessions out of 419 sessions). The interim results were also excellent, with no evidence of recurrence of the treated lesions during the limited follow-up period of average 18.2 months after the completion of multisession therapy – average 3.2 sessions per patient – (Figures 1A and 1B). Sixteen ET forms which underwent various combinations of preoperative embolo/sclerotherapy (eg, ethanol, N-butyl cyanoacrylate) and subsequent surgical excision showed excellent results with minimum morbidity and no recurrence during the limited follow-up period (average 21.2 months) (Figure 2). Nine T forms underwent various surgical therapy (eg, venorrhaphy) as independent therapy, and showed excellent results.
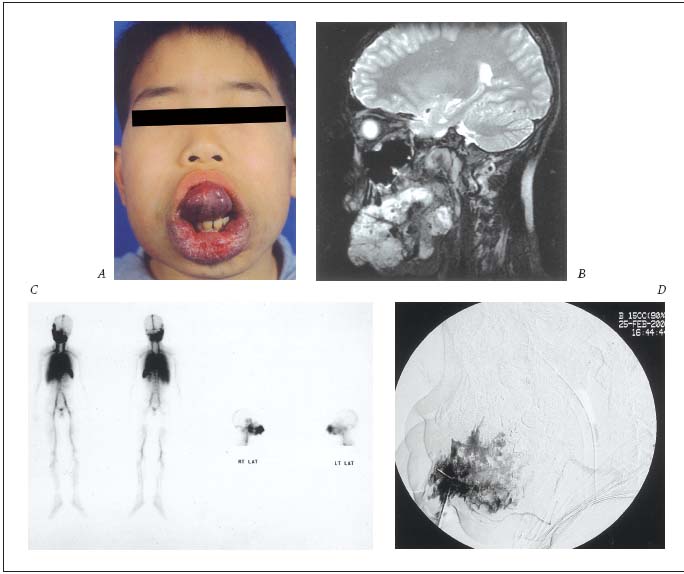
Figure 1-A. Management of the diffuse infiltrating ET form of the VM with ethanol sclerotherapy as independent therapy.
A. Clinical appearance of the extensive lesion affecting the tongue, cheek, lips, and mouth floor, etc. with severe functional disability.
B. MRI image of the lesions through whole mouth cavity involving multiple perioral structures – infiltrating type.
C. WBBPS* image of extensive abnormal blood pooling along the lesions.
D. Angiographic image of multisession ethanol sclerotherapy by percutaneous direct puncture technique with excellent response.
* WBBPS: whole body blood pool scan
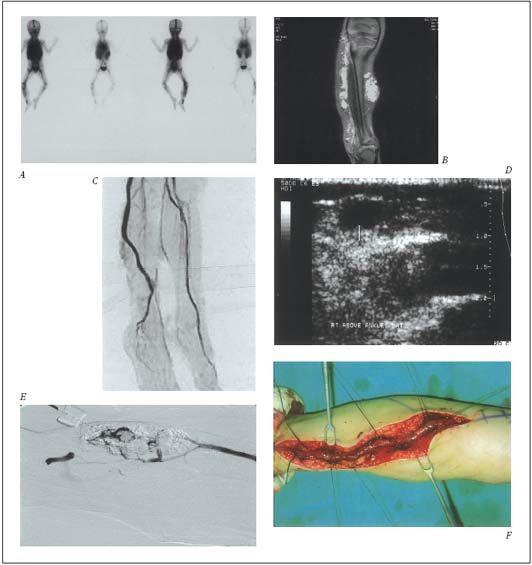
Figure 1-B. Management of the ET form of the VM with ethanol sclerotherapy and the T form with surgical therapy as
independent therapy.
A. WBBPS* image of extensive abnormal blood pool along right lower extremity by VM lesions.
B. MRI images of multiple infiltrating type of ET lesions, along upper thigh.
C. MR venography image of the marginal vein as one of T forms along right lower leg.
D. Ultrasonographic image of the superficially located marginal vein.
E. Angiographic image of the ethanol sclerotherapy to the infiltrating ET lesion with excellent response.
F. Operative image of the surgically isolated marginal (lateral embryonal) vein for the resection.
* WBBPS: whole body blood pool scan
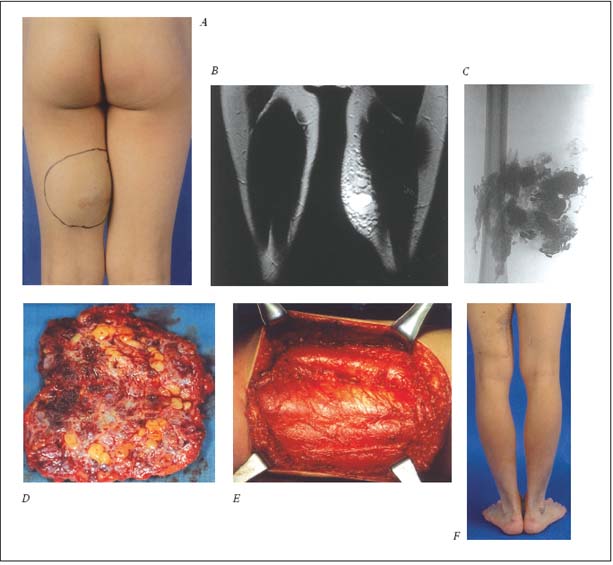
Figure 2. Management of the localized ET form of the VM with preoperative NBCA* glue embolotherapy and subsequent surgical
excision.
A. Clinical appearance of rapidly growing lesion at the posterior aspect of left thigh with recurrent bleeding following minor bodycontact
sport and progressive ache.
B. MRI image of the infiltrating type of the ET lesion as localized form.
C. Angiographic image of the lesion, filled with NBCA glue preoperatively for the subsequent excision.
D. Surgical specimen of the glue-filled ET lesion.
E. Surgical field following complete removal of the lesion.
F. Clinical appearance of the satisfactory result with no evidence of recurrence on the follow-up (2 years).
* NBCA: N-butyl cyanoacrylate
DISCUSSION
The critical value of close communication, not only among the multidisciplinary team members, but also with the patient and family before the acceptance of the risk involved in the treatment cannot be overemphasized. Though the “cure” can be achieved theoretically by the proper treatment, this is not always possible with minimum morbidity. It is wiser to choose “adequate control” of VM lesions with minimum adverse effects to improve the quality of life, if the morbidity for the curative treatment seems to be prohibitively high (eg, Malan operation I).
As we recently reported,7 we controlled most of the surgically inaccessible VM lesions with absolute ethanol quite successfully. Now, absolute ethanol has been accepted as the primary choice for sclerotherapy of VM at our Clinic. However, the price to pay for the satisfactory results with the promising long-term outcome with minimum recurrence was much higher than we wished. There were various major and/or minor acute complications, though mostly manageable skin complications, in spite of extreme precautions taken. The chemical toxicity of absolute ethanol is also an imminent threat during each session of the therapy, with potential risk of serious morbidity and/or mortality. All the procedures therefore should be done under general anesthesia, and close cardiovascular and pulmonary monitoring is essential for the immediate control of this acute morbidity. This potentially lethal pulmonary hypertension by the spillover ethanol reaching the pulmonary bed has to be aborted, if not minimized, by prompt control with adequate measurement. In addition to these acute complications and morbidity, the long-term results and chronic morbidity with or without sequelae following the sclerotherapy still remain to be assessed.11
CONCLUSION
New classifications can properly verify each component as well as characteristics of VM properly to become the basis of contemporary management of VM. A multidisciplinary approach with full integration of various modalities of surgical and/or nonsurgical treatment will allow the best combination of the treatment to be safely implemented for each different component of the VM. Through the careful assessment and control of the potential risk involved with each treatment, substantial improvement of the overall treatment results can be achieved. Complex forms of extensive VM which have been inaccessible to conventional treatment can now also obtain benefit from this contemporary strategy.
REFERENCES
2. Belov S. Classification of congenital vascular defects. Int Angiol. 1990;9:141-146.
3. Belov S. Anatomopathological classification of congenital vascular defects. Semin Vasc Surg. 1993;6:219-224..
4. Woolard HH. The development of the principle arterial stems in the forelimb of the pig. Contrib Embryol. 1922;14:139-54.
5. Malan E. Vascular Malformations (Angiodysplasias). Milan: Carlo Erba Foundation;1974:17.
6. Lee BB. Advanced management of congenital vascular malformation (CVM). Int Angiol. 2002;21:209-213.
7. Lee BB, Kim DI, Huh S, et al. New experiences with absolute ethanol sclerotherapy in the management of a complex form of congenital venous malformation. J Vasc Surg. 2001;33:764-772.
8. Lee BB, Bergan JJ. Advanced management of congenital vascular malformations: a multidisciplinary approach. J Cardiovasc Surg. 2002;10:523-533.
9. Mattassi R. Differential diagnosis in congenital vascular-bone syndromes. Semin Vasc Surg. 1993;6:233-244.
10. Belov S. Correction of lower limb length discrepancy in congenital vascular-bone disease by vascular surgery performed during childhood. Semin Vasc Surg. 1993;6:245-251.
11. Lee BB, Do YS, Byun HS, et al. Advanced management of venous malformation with ethanol sclerotherapy: mid-term results. J Vasc Surg. 2003. In press.