Current status of venous stenting and a look at where we need to go
Stephen Black, MD
Professor of Venous Surgery and
Consultant Vascular Surgeon, Guy’s
and St Thomas’ Hospital and Kings
College, London, UK
ABSTRACT
Venous stenting has rapidly advanced over the last 10 years as the emergence of dedicated venous stents and advancement in thrombectomy devices has renewed interest in this field. This rapid advancement has seen the introduction of several new devices, which have now gained market approval. Inevitably, the advancement of technology has outpaced the evidence to support the use of such devices, and complications have arisen as the number of patients treated has rapidly expanded. The lack of evidence has been compounded by difficulty in completing and recruiting for randomized trials, which has meant guidelines have needed to rely on cohorts and expert consensus for recommendations. Despite the inevitable growth difficulties, the options now available for patients are significantly wider, and future advancements in technology will likely improve options and long-term results. This is needed for a group of patients who continue to suffer with the debilitating effects of chronic venous disease.
Introduction
Venous stenting for the treatment of chronic venous disease rose to prominence following a seminal publication by Neglen and Raju.1 These papers highlighted the potential for stenting to address the chronic venous hypertension caused by iliac outflow obstruction. In addition, it became clear as thrombectomy practice evolved that stenting of underlying lesions played an important role in the success of these treatments in preventing postthrombotic syndrome (PTS).2
This paper reviews the advancements we have seen in the last several years, addresses some of the problems that have arisen, and assesses potential future developments that may be needed.
Dedicated venous stents
The biggest development in the last 10 years has been the emergence of dedicated venous stents. The focus has been on stents designed specifically for the unique venous environment rather than using arterial stents off-label. The dedicated stents have also benefited from newer platforms that have improved ease and accuracy of deployment.3
This evolution has seen 4 stents receive market approval in the United States following Investigational Device Exemption (IDE) studies, and several new devices have emerged in the European and Outside United States (OUS) markets.4-7 The results of these studies are collated in Table I and show outcomes out to 3 years. In addition to the IDE studies, other studies have published outcomes with now several different stents on the market.8,9
All of the studies have shown broadly similar outcomes with no data to suggest that any one stent performs significantly better than another.10 However, the studies have confirmed that PTS patients with occlusion have worse outcomes than those with a stent placed following thrombolysis for acute thrombotic (AT) events. As expected, nonthrombotic iliac vein lesions (NIVLs) have significantly better long-term patency. Direct comparison is not straightforward though as classification and outcome measures were not universally the same. Despite these limitations, the conclusion remains that dedicated venous stents have resulted in a substantial increase in attention to the treatment of these patients. However, long-term results have not yet realized the patency gains promised by the initial technological advances.
Regardless of patency, several studies have shown that treatment of patients with PTS, NIVL, and AT lesions does significantly improve long-term quality of life (QOL) outcomes, with these improvements sustained over time.11-13 In addition, a study from Italy has suggested that venous stenting is likely to be cost-effective, especially when considering moderate and severe disease.14 This is particularly important when we consider the cost associated with the burden of leg-ulcer care in which venous stenting may play a significant role.15,16
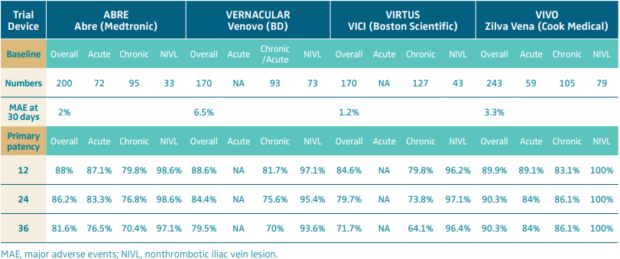
Table I. Primary outcomes (major adverse events at 30 days and primary patency) of the 4 completed Investigational Device Exemption (IDE) studies.
Guidelines and randomized trial data
Based on the available data, venous stenting was given a grade IIa recommendation in the European Society of Vascular Surgery guidelines for both acute and chronic disease.17,18 However, the recommendation was based primarily on expert opinion and limited studies, highlighting the paucity of robust evidence to support stenting.
Only 2 randomized controlled trials (RCTs) have been published. The first was a small series from Brazil that suggested a significant benefit.19 The second was the planned, larger STEVECO trial (Stent Versus Conservative Treatment in Patients With Deep Venous Obstruction), although it failed to meet its recruitment target. The difficulties faced in the later study highlight the difficulty in completing venous RCTs. This was clear in treatment of AT where both ATTRACT (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) and CAVA (CAtheter Versus Anticoagulation alone for acute primary [ilio]femoral DVT) trials took 10 years to complete recruitment.20,21 Larger RCT’s in PTS—C-TRACT (NCT030250247; Chronic venous Thrombosis: Relief with Adjunctive Catheter-directed Therapy) and BEST—PTS (NCT05622500; Best Endovenous Treatment, Including STenting, Versus Non-endovenous Treatment in Chronic Proximal Deep Venous Disease)—are experiencing similar issues with recruitment delays, and IGuideU (NCT04696354; Intravascular Ultrasound-Guided Intervention for Venous Leg Ulcers) was terminated early after recruitment problems meant the study could not meet its timeline.
These recruitment difficulties are the consequence of both patients and clinicians struggling with equipoise. In the case of the former, patients often demand treatment for conditions for which they have frequently struggled with inadequate medical management. When they are referred to centers that offer intervention, they refuse randomization. In the case of clinicians who are engaged in treatment, the decision to offer randomization is met with resistance.
Without this evidence, there will continue to be struggles to show clearly that these treatments should be offered. Importantly, this will not overcome resistance to refer patients for consideration for treatment from clinicians who are guided by current trial data. Trial evidence is a necessity to influence payors and organizations such as the National Institute for Clinical Excellence (NICE) in the United Kingdom who provide treatment guidance.
Therefore, there is a significant need to consider multiple alternative strategies to collect data that supports these treatments. This may be provided by registries established independent of the IDE studies, perhaps supported by societies. Well-established registries can provide the necessary multicenter prospective cohort data; however, a weakness lies in the absence of control groups. This weakness may be overcome, and it is necessary to do so to ensure there is an appropriate comparator arm. Nonetheless, even if this weakness is overcome, registries suffer from inherent bias that means the data produced is not viewed on a level with RCTs.
The stronger, and perhaps likely answer lies in so-called “big data.” In the case of large-scale data collection, opportunities arise in formal data analysis techniques such as causal inference, which allow a similarly robust bias mitigation afforded by RCTs.22 The advantage of these study designs is that patients do not need to be randomized and can be treated as the primary clinicians and patients choose.
Whatever choice is made, venous stenting requires more robust data, and it is incumbent upon clinicians who wish to see these treatments advance to commit to studies. This absence of data was critically highlighted when stent migration forced the withdrawal of the VICI stent (Boston Scientific).
Migration problem
The VICI stent withdrawal demonstrated the risk inherent in rapid technological advancement.23,24 Migration, though a known risk, had not been seen in the IDE studies and during the early phase of stent development had not been widely reported. However, a review of the literature suggests the problem may be underreported and concluded that it was principally associated with using short and undersized stents.25 Furthermore, it was highlighted that inappropriate patient selection was a factor.
The focus on appropriateness that has followed the migration debacle demonstrates that significant gaps in training and education need to be addressed. Clinicians and Industry need to work in parallel to build programs that support technical skill acquisition, as well as patient pathway and decision-making paradigms. The publication of an article in the New York Times that focused on peripheral arterial disease makes clear the dangers that lie ahead for this field if such issues are not addressed.26 Appropriateness involves ensuring treatment is only offered to patients who need it.
Future developments
The lack of data has been addressed above but will remain a central issue regardless of parallel technological advancements. A fundamental problem, in addition to those addressed previously, has been the choice of study outcome measure.27 ATTRACT has faced significant criticism for its use of the Villalta score, particularly in adopting a binary approach, whereas STEVECO likely failed by choosing a 14-point improvement in the VEINES-QoL questionnaire score (VEnous INsufficiency Epidemiological and economic Study – Quality of Life) as the primary outcome measure for the study. Both trials had significantly positive outcomes in favor of patient treatment, yet the primary measure did not show this. The future of studies rests on better defining outcome measures for venous disease.
Some efforts have been made through the International Consortium on Health Outcome Measures (ICHOM) process.28 That process highlighted several outcome measures that should be considered and, as a strength, incorporated patient-centered outcome measures. This review could only focus on existing tools, and it is clear from the lengthy list of necessary outcome measures that a better understanding of those features that really drive outcomes is needed. We are likely to see advances in this understanding and have seen some advances with the development by Houman Jaiaie of a system for classification of venous patients, which may help to standardize reporting and allow for more direct comparison between studies; however, at the moment, this classification has not yet been validated.
Technological advances in stent design are also inevitable and, as in other disease states, likely to outpace data collection. Current stents are largely all laser-cut nitinol designs with some variation between open and hybrid designs incorporating closed-cell elements. Future stent designs are likely to build on this by incorporating drug coating and alternative designs that attempt to influence factors like the Poisson effect or flow. The direction of travel is currently limited by a clear understanding of what factors drive patency loss and the biological mechanism of stent thrombosis and occlusion. It is imperative that a better understanding of the mechanism of stent failure is developed.
The IDE studies have all shown that the 3-year occlusion rate in PTS patients is approaching 30%. This underscores that improvements are needed but also indicates that there is a group of patients with blocked stents who will need treatment. We will see advances in creating tools suited to these patients with technology to remove the fibrotic tissue that builds up with stents. The current approach of simply ballooning stents is inadequate.
These advances are likely to focus on drug delivery; trials are already underway to assess the impact of dexamethasone administration into the vessel wall. Two DEXTERITY studies (NCT04858776 [Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation After Thrombus Removal to Yield Benefit in Subacute and Chronic Iliofemoral DVT (DEXTERITY-SCI)] and NCT04862468 [Perivenous Dexamethasone Therapy: Examining Reduction of Inflammation After Thrombus Removal to Yield Benefit in Acute Femoropopliteal DVT]) raise the possibility that so called “vessel preparation” may be a factor in improving outcomes in PTS patients, possibly negating the need for stenting in AT patients. Drug delivery to the wall in addition to coating on stents is an intriguing prospect, but it is not clear yet if a coating is to be added and what that coating should be.
Conclusion
Venous stenting has evolved rapidly in the last decade with several new devices reaching the market. This has seen treatment volumes increase but perhaps in advance of the data supporting these interventions. The next several years should see development of better reporting methods, as well as advancement in technology. The technological advancements are likely to focus more on adjunctive technologies that support the whole procedure rather than simply the stent itself.
CORRESPONDING AUTHOR
Stephen Black, MD
Guy’s & St Thomas’ Hospital, 1
Dpt of Vascular Surgery,
Westminster Bridge Road,
SE1 7EH London, UK
EMAIL: stephen.black@gstt.nhs.uk
References
1. Raju S, Owen S, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35(1):8-15.
2. Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10):e002772.
3. Lim CS, Black SA. Mechanical characteristics of venous stents to overcome challenges of venous outflow obstruction. Int Angiol. 2022;41(3):240-248.
4. Murphy E, Gibson K, Sapoval M, et al. Pivotal study evaluating the safety and effectiveness of the Abre venous self expanding stent system in patients with symptomatic iliofemoral venous outflow obstruction. Circ Cardiovasc Interv. 2022;15(2):e010960.
5. Razavi MK, Black S, Gagne P, et al. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12(12):e008268.
6. Comerota A, Hofmann L, McCann-Brown J. The VIVO clinical study evaluating the Zilver Vena venous stent in the treatment of symptomatic iliofemoral venous outflow obstruction: three-year subgroup outcomes. J Vasc Surg Venous Lymphat Disord. 2022;10(2):549. doi:10.1016/j. jvsv.2021.12.021.
7. Dake MD, O’Sullivan G, Shammas NW, et al. Three-year results from the Venovo venous stent study for the treatment of iliac and femoral vein obstruction. Cardiovasc Interv Radiol. 2021;44(12):1918-1929.
8. Lichtenberg M, Stahlhoff S, Özkapi A, Graaf R de. Braided nitinol stent for chronic iliofemoral venous disease – the real-world BLUEFLOW registry. VASA Z Fur Gefasskrankheiten. 2021;50(5):372-377.
9. Lichtenberg M, Graaf R de, Stahlhoff WF, Özkapi A, Simon M, Breuckmann F. Patency rates, safety and clinical results of the sinus-Obliquus venous stent in the treatment of chronic ilio-femoral venous outflow obstruction – data from the Arnsberg venous registry. VASA Z Fur Gefasskrankheiten. 2018;48(3):270-275.
10. Morris RI, Jackson N, Khan T, et al. Performance of open and closed cell laser cut nitinol stents for the treatment of chronic iliofemoral venous outflow obstruction in patients treated at a single centre. J Vasc Surg. 2022;75(5):1790. doi:10.1016/j.jvs.2022.03.028.
11. Pouncey AL, Morris RI, Hollins-Gibson JNC, Fernandes L, Quintana B, Black SA. Midterm disease specific quality of life outcomes following interventional treatment of iliofemoral deep vein thrombosis: results from a tertiary centre. Eur J Vasc Endovasc Surg. 2023;66(2):282-283.
12. Morris R, Pouncey A, Duval JL, et al. Quality of life outcomes for patients undergoing deep vein stenting for chronic deep venous disease: a tertiary center experience using the VEINES QoL/Sym. J Vasc Surg Venous Lymphat Disord. 2020;8(2):3
13. Abstract AFF9. doi:10.1016/j.jvsv.2019.12.013. 13. Marston W, Razavi M. Improvement in quality of life after iliac vein stenting in a prospective clinical study of a nitinol venous stent. J Vasc Surg Venous Lymphat Disord. 2019;7(2):300. doi:10.1016/j. jvsv.2019.01.035.
14. Rognoni C, Lugli M, Maleti O, Tarricone R. Venous stenting for patients with outflow obstruction and leg ulcers: cost effectiveness and budget impact analyses. J Comp Eff Res. 2020;9(10):705-720.
15. Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med. 2022;27(1):63-72.
16. Livingstone V, Johnson O, Peta S, et al. Leg Ulcer Pathway Acceleration (LUPA) study. Br J Surg. 2022;110(7):797-803.
17. Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61(1):9-82.
18. Maeseneer MGD, Kakkos SK, Aherne T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
19. Rossi FH, Kambara AM, Izukawa NM, et al. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6(2):183-191.
20. Vedantham S, Goldhaber SZ, Julian JAet al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252.
21. Notten P, de Smet AAEA, Tick LW, et al. CAVA (Ultrasound-Accelerated Catheter Directed Thrombolysis on Preventing Post-Thrombotic Syndrome) trial: long term follow-up results. J Am Heart Assoc. 2021;10(11):e018973.
22. Pearl J. An introduction to causal inference. Int J Biostat. 2010;6(2):Article 7. doi:10.2202/1557-4679.1203.
23. Ma Y, Dittman JM, Lavingia KS, Amendola MF. A comparison of patient and device issues reported for recalled venous stent systems. Ann Vasc Surg. 2022;87:95-99.
24. Ma Y, Dittman J, Lavingia KS, Amendola M. Analysis of publicly reported adverse events in the VICI and Venovo venous stent systems. Ann Vasc Surg. 2022;79:389. doi:10.1016/j. avsg.2021.12.034.
25. Sayed MH, Salem M, Desai KR, O’Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord. 2022;10(2):482-490
26. Thomas K, Silver-Greenerg J, Gebelhoff R. They lost their legs. Doctors and health care giants profited. New York Times. July 15, 2023.
27. O’Sullivan GJ, Graaf R de, Black SA. Just how attractive is the ATTRACT trial? Cardiovasc Interv Radiol. 2018;41(9):1313-1317.
28. Gwozdz AM, de Jong CMM, Fialho LS, et al. Development of an international standard set of outcome measures for patients with venous thromboembolism: an International Consortium for Health Outcomes Measurement consensus recommendation. Lancet Haematol. 2022;9(9):e698-e706.
