Current use of microsurgery in lymphedema
Francesco BOCCARDO
Alberto MACCIÒ
Angelo ZILLI
Francesco SCHENONE
Surgery and Microsurgery
S. Martino’s Hospital, University of Genoa, Italy
SUMMARY
The authors, after a short introduction on historical role of surgery and microsurgery in lymphatic vessel pathology, report their own 25-year experience in lymphatic microsurgery and lymphatic-venous-lymphatic anastomoses.
In particular, the authors describe the derivative and reconstructive microsurgical techniques, pointing out the preoperative diagnostic, the indications and the obtained results concerning 798 patients affected by primary and secondary limb lymphedema.
Lymph – Lymphology – Lymphedema – Microsurgery – Lymphatic-Venous Anastomoses – Lymphaticvenous
– Lymphatic Anastomoses – Lymph Vessels – Lymphoscintigraphy.
INTRODUCTION
Lymphedema, refractory to nonoperative methods, may be managed by surgical treatment. Indications1 include insufficient lymphedema reduction by well-performed medical and physical therapy2-4 (less than 50%), recurrent episodes of lymphangitis,5 intractable pain, worsening limb function, and patients dissatisfied with the results obtained by nonoperative methods and willing to proceed with surgical options.
In 1908, Handley described his technique of “lymphangioplasty,” running silk threads subcutaneously to provide a conduit for lymphatic drainage.6 The procedure was abandoned, as postoperative infection and spontaneous extrusion of the implanted foreign material commonly occurred.
Excisional operations, such as Charles’ (Figure 1) total resection of subcutaneous tissue,7 Thompson’s subfascial drainage of a scarified skin flap,8 and Servelle’s total surface lymphangectomy,9 aimed at removing excess tissue to decrease the volume of the extremity. However, prolonged hospitalization, poor wound healing, long surgical scars, sensory nerve loss, residual edema of the foot and ankle, and poor cosmetic results can be important problems to use these highly debulking operations only in the most severe and advanced cases of elephantiasis, not responding to conservative measures.
In 1962, Cockett and Goodwin10 described the anastomosis of a dilated lumbar lymphatic to the spermatic vein to treat a case of chyluria.
Subsequent development of microsurgical techniques has enabled lymphatic-venous anastomosis to emerge as a potential treatment of lymphedema.
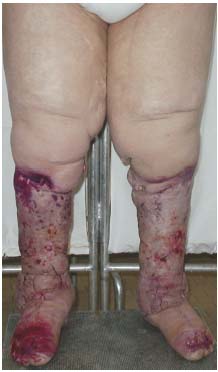
Figure 1. Long-term-results (30 years),
after radical Charles’s excisional operation.
EXPERIMENTAL AND CLINICAL STUDIES
In 1977, O’Brien and coworkers11 described microlymphatic surgery in the treatment of secondary obstructive lymphedema. They preferred three or more lymphaticovenous anastomoses at, or above, the elbow. They observed that the incidence of postoperative cellulitis was significantly less. The microlymphatic techniques were applicable to both upper and lower limbs, and perhaps could be extended to localized cases of obstructive lymphedema following trauma and congenital constriction bands. They underlined that considerable experience in microvascular surgery is required for doing this type of work. The results of microlymphatic surgery in obstructive secondary lymphedema were encouraging, even though the authors remarked that a long-term evaluation of the clinical outcome was required before judging the potential of those techniques.
In 1981, Degni12 introduced an original technique of lymphatic- venous anastomosis in cases of lymphedema of the limbs. The procedure was easy and could be indicated for both the upper and lower limbs, and also for thoracic duct or any blocked lymphatic vessels of the abdomen. He used this technique to treat lymphedemas due to surgical resection of benign tumours (lipomas of the thigh) or plastic surgery (pendulous abdomen, plastic surgery of the thigh), orthopedic operations on the knee, and after stripping of varicose veins. The purpose of the procedure was to divert the lymph to the vein in cases of blocked lymphatic vessels, particularly when lymphographic findings demonstrate good function and permeability of lymphatic vessels. A longitudinally divided needle was used to introduce the lymphatic trunk into the vein, pulling the lymphatic into the venous lumen and fixing the lymphatic to the upper venous wall with one suture.
Clodius,13 in 1982, observed that microsurgery for primary and secondary lymphedema, consisting of shunts between lymphatic vessels and veins, was a well-established surgical technique. The problems consisted of the irreversible changes in the peripheral lymphatic system and in the connective tissues, as well as the obliteration of the deep lymphatics, best suited for lymphaticovenous anastomoses. Therefore, lymphaticovenous shunts should be performed precociously before fibrotic tissural changes appear.
Huang and coworkers,14 in 1985, described their experience of 91 cases of lymphedema treated by microlymphaticovenous anastomosis, with very satisfactory immediate and long-term results in 79.1 percent. The data they obtained suggested that the quality of results is proportional to the number of anastomoses.
In 1987, Krylov and coworkers15 reported an experience of 510 cases of primary and secondary lymphedema in upper and lower extremities with two thirds of primary lymphedema cases among them. Most favorable results were obtained in secondary obstructive lymphedema cases (total 81.2%) due to a condition of hypertension in lymphatic vessels that contributes to better functioning of the lymphaticovenous anastomoses.
In 1987, Zhu and coworkers16 reported a clinical experience of 185 limbs with lymphedema treated by lymphaticovenous anastomosis with excellent results achieved in 72.9% of the cases.
Al Assal, Cordeiro and coworkers,17 in 1988, reported experimental studies in dogs using a new technique of microlymphovenous anastomosis to improve long-term patency rates and clinical results in lymphedema therapy. Technical points, such as an oval window on the wall of the vein and a few sutures piercing only two lymphatic layers, adventitia and media, outside the lumen for successful results were emphasized. Three methods for assessment of patency of anastomoses were used: (1) observation with operating microscope of dye transit across the anastomotic site; (2) lymphography, and (3) histopathologic examination. Based on the encouraging results obtained with this technique, the authors suggested that end-to-side anastomosis might be the technique of choice.
In 1988, Ho and coworkers18 described the use of microlymphatic bypass for the treatment of obstructive secondary lymphedema in the lower and upper limbs. They underlined the importance of preoperative assessment by lymphangiography and lymphoscintigraphy to assess suitability for the procedure. Postoperatively, patent lymph collectors were demonstrated by lymphoscintigraphy. Moreover, they were convinced of the fact that microlymphatic bypass should be carried out before the peripheral lymph collectors had been destroyed or permanently damaged by increasing back pressure and recurrent infection.
Olszewski19 published in 1988 his personal 20-year clinical experience in diagnosis and treatment of various types of lymphedema of the lower limbs with microsurgical lymph node-vein and lymph vessel-vein anasto- moses. He limited indications for surgical therapy of lymphedema to a carefully selected group of patients with the local, segmental obstruction of proximal lymphatics. Peripheral lymphatic should be patent and have their contractility at least partly preserved. He underlined that long-term penicillin therapy was indispensable prior to surgery in cases with a history of lymphangitis.
A critical review of microsurgical lymphovenous anastomois for the treatment of lymphedema was published by Gloviczki and coworkers20 in 1988. They performed lymphovenous anastomoses (LVA) to treat chronic lymphedema. Mean follow-up was 36.6 months. They reported that LVA offered ideal physiologic treatment, above all of secondary lymphedemas. Lymphoscintigraphy appeared to be a suitable method for both identifying patent lymph channels before surgery and determining function of LVA after operation.
Another experience in the treatment of lymphedemas by microsurgical lymphatic grafting was presented by Baumeister and Siuda21 in 1990. Lymphatic grafts were anastomosed to peripheral lymphatics distal to and central lymphatics proximal to the regional blockade. In the case of unilateral blockade at the groin or pelvis, the grafts connected the lymphatics of the thigh of the affected leg with lymphatics in the contralateral healthy groin.
In 1990, O’Brien and coworkers22 reported their clinical experience in the treatment of obstructive lymphedema by microlymphaticovenous anastomoses. They described a subjective improvement in 73 percent of patients and, objectively, volume changes showed a significant improvement in 42 percent of cases, with an average reduction of 44 percent of the excess volume. Authors also underlined the significant reduction in the incidence of cellulitis following surgery. Their long-term results (average followup of 4 years) indicated that microlymphaticovenous anastomoses had a valuable place in the treatment of obstructive lymphedema and should have been the treatment of choice in patients with obstructive lymphedema. Again, authors pointed out that improved results could be expected with earlier operations because patients referred earlier usually have less lymphatic disruption.
Despite advances in microsurgery, the most suitable operation for primary lymphedema remained unclear. A variety of tissue transplants and artificial substances had been used to facilitate drainage of peripheral lymph. The greater omentum was used experimentally in the treatment of canine obstructive lymphedema (O’Brien and co-workers,23 Abalmasov and coworkers,24). The findings indicated that experimental obstructive lymphedema in the dog could be reduced significantly by insertion of a vascularised omental graft. However, because there is no natural lymph nodal-venous (L-V) shunt within the greater omentum, the addition of a L-V shunt in dogs to omental transplantation seemed to increased effectiveness of the omentum for draining hind-limb lymph after its autotransplantation.
AUTHORS’ CLINICAL EXPERIENCE
AND LONG-TERM RESULTS
Lymphatic-venous (LV) derivative microsurgery
Clinical indications for lymphatic-venous (LV) derivative microsurgery include the following conditions:
• Appropriate lymph nodes and lymphatic collectors;
• No venous dysfunctions;
• Normal or increased lymphatic-venous pressure gradient.
It is recommended to treat patients with lymphedema as earlier as possible.25
Different techniques of LV anastomoses have been developed, end-to-end (Figure 2) or end-to-side, performed with different technical “tricks” and refined both through experimental studies and through extensive clinical practice.26
Over the last 25 years, 665 patients affected by peripheral primary or secondary lymphedema have been submitted to derivative microsurgical LV anastomoses.27 A total of 446 patients are available for long-term follow-up study, extending over more than 15 years in 11% of the cases. The age of the patients (whose female-to-male ratio was 1.5) ranged from 2 to 67 years (average 27 years). There were 231 patients with lymphedema of the upper limbs (37 primary and 194 secondary) and 434 patients with lymphedema of the lower limbs (230 primary and 204 secondary).
Primary lymphedemas (Figure 3) mainly included lymphnodal dysplasias (LAD II, according to Papendieck’s classification), consisting of hyperplastic lymph nodes with sinus histiocytosis, thick and fibrous capsule with microlymphangioadenomyomatosis.28 In these cases, lymph flow obstruction is revealed by alterations of the afferent lymphatics, which appear dilated and swollen with thickened walls; smooth muscle cells are reduced in number and appear fragmented by prevailing fibrous elements.
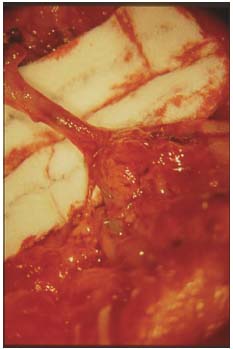
Figure 2. End-to-end multiple lymphatic-venous anastomoses
(25-30x). After passing the needle inside the vein, lymphatic
collectors are anchored by their adventitial and periadventitial
tissue. At the end of lymphatic–venous anastomoses, blue dye
passing inside the vein demonstrates the patency of
microanastomoses.
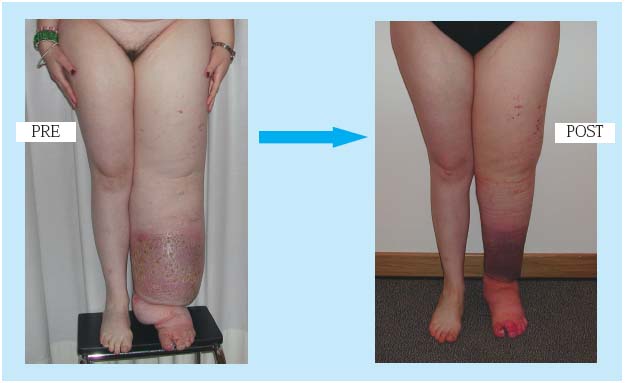
Figure 3. Primary left lower-limb lymphedema (V stage), before and 2 years after lymphatic-venous microanastomoses at the left groin.
In our experience, secondary lymphedemas (Figures 4, 5, and 6) were mostly due to lymphadenectomy and radiotherapy performed for oncological reasons (because of carcinoma of the breast, uterus, penis, bladder, prostatic gland, rectum, and seminoma of didymus), as well as to minor operations for varicose veins, crural and inguinal hernias, lipomas, tendinous cysts, or axillary and inguinal lymph node biopsies.
Most of the lymphedemas treated by microsurgery were at stages II (39%) and III (52%), while 3% of the patients were stage Ib and 6% were stages IV and V.
Derivative microsurgical LV anastomoses were performed both end-to-end and end-to-side. The end-to-end procedure was performed by a telescopic method with a single U-shaped stitch, anastomosing lymphatic collectors to a continent venous secondary branch. End-to-side LV anastomoses were performed by using the outlet of the vein as entry hole for lymphatic vessels, so that the risk of stenosis of the anastomoses is reduced to a minimum.
Particularly at pediatric ages, lymphatic-capsular-venous anastomosis was performed. The technique consists in anastomosing the lymph nodal capsular segment (which includes afferent lymphatics) directly to the vein, like a patch.
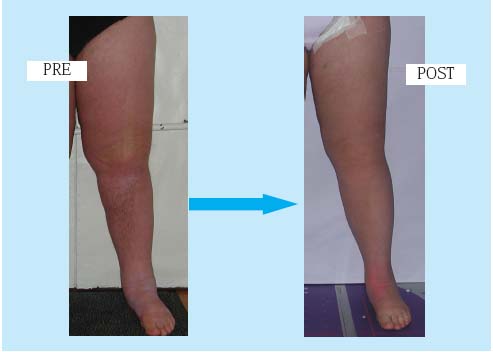
Figure 4. Secondary left lower-limb
lymphedema (IV stage), before and
immediately after lymphaticvenous
microanastomoses at the
left groin.
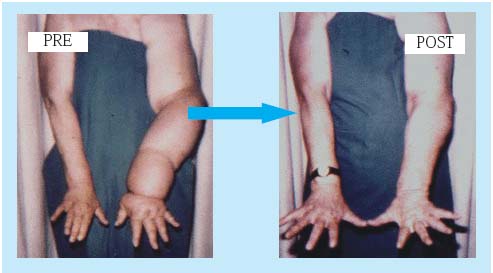
Figure 5. Left upper-limb lymphedema
(V stage) following breast
cancer treatment, before and after
microsurgical lymphatic-venous
anastomoses at the left arm.
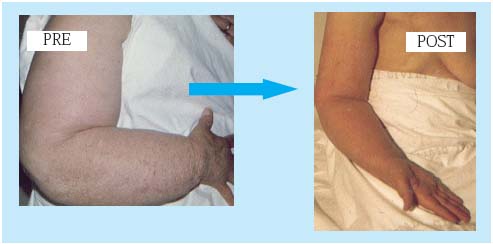
Figure 6. Right upper-limb lymphedema
(V stage) following breast
cancer treatment, before and after
microsurgical lymphatic-venous
anastomoses in the right arm
(long-term result).
Lymphoscintigraphy29 (Figure 7), performed with either 99mTc-labeled antimony sulfur colloid or 99mTcnanocolloid human serum albumin (90% of the particles > 80 nm in size), was employed in the diagnostic workup of patients with lymphedema and as a test for selecting patients for derivative microsurgical operations. Lymphoscintigraphy clearly discriminated whether or not edema was of lymphatic origin, and also supplied important data upon the etiologic and pathophysiologic aspects of lymphedemas.
Echo-Doppler was employed in all patients to identify any venous disorders possibly associated with lymphedema. In most patients, venous dysfunctions were corrected at the same time as microlymphaticovenous anastomoses (ie, valvuloplasty in case of vein insufficiency). In other cases, finding venous dysfunctions contraindicated derivative lymphovenous shunts, but it allowed to refer the patient to reconstructive microsurgical operations.
Conventional oil contrast lymphangiography was employed only in selected patients with lymphedema due to gravitational reflux, in order to better define the extension of the pathology and sites of lymphatic and chylous leakage.
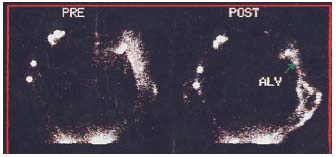
Figure 7. Lymphoscintigraphic demonstration of patency of
lymphatic-venous anastomoses. Marked reduction of dermal
backflow, appearance of preferential lymphatic pathways,
disappearance of the tracer at the site of microanastomoses
(Lymphoscintigraphy performed by Nuclear Medicine Unit,
University of Genoa).
Results have been evaluated in the short-medium-and long-term (over 15 years) after operation. Subjective improvement occurred in 578/665 patients (87%). Objectively, volume changes showed a significant improvement in 552 patients (83%), with an average 67% reduction of the excess volume. Out of the 446 patients included in the follow-up, 379 patients (85%) have been able to discontinue the use of conservative measures, with an average follow-up of over 7 years and an average 69% reduction in excess volume. There was an 87% reduction in the incidence of cellulitis following microsurgery. In those patients who improved their clinical condition, the restored lymphatic drainage resulted in increased softness of the limbs. Peripheral edema (hand and foot) diminished considerably in most patients.
Efficacy of microlymphatico-venous anastomoses was confirmed by the following lymphoscintigraphic patterns (Figure 7): 1) reduced dermal backflow; 2) rapid clearance in the bloodstream of the tracer at the site of microanastomoses, and 3) earlier tracer uptake by the liver indicative of more rapid entry into the bloodstream.
In particular, postoperative lymphoscintigraphy was performed in 119 patients (18%) with an average followup of over 7 years after surgery (maximum of 15 years in 7 patients). This procedure demonstrated patency of the microanastomoses in almost all patients (93%). In 9 patients, despite the absence of specific lymphoscintigraphic patterns positively proving the efficacy of microanastomoses, volume changes were clinically relevant anyway.
Reconstructive lymphatic microsurgery
Clinical indications for reconstructive lymphatic microsurgery (Figure 8) included patients with peripheral lymphedema (mostly of the lower limbs) under the following conditions:
• adequate lymphatic collectors;
• associated venous disorders that contraindicate derivative lymphovenous techniques;
• in whom the possible treatment of venous dysfunction can not ensure, anyway, a valid lymphatic-venous pressure gradient.
We used the technique of lymphatic-venous-lymphatic (LVL) plasty.30 It consisted of the interposition of autologous vein grafts between lymphatic vessels below and above the site of lymph blockage. This reconstructive method of interpositional LVL shunt transports a great volume of lymph because of the high number of lymph collectors which can be anastomosed to the venous graft. The technique (LVL), moreover, is easily feasible, esthetically satisfactory, and without risk of causing a secondary lymphedema in the uninvolved contralateral limb.
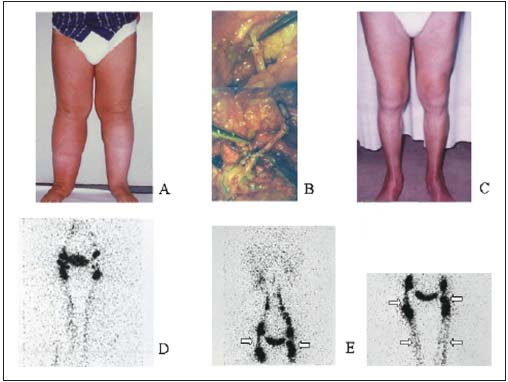
Figure 8. Primary lower-limb
lymphedema (A), treated by
LVLA (B), with 10-year followup
after microsurgery (C).
Lymphoscintigraphy – pre-op
(D) and post-op (E) – shows
preferential lymphatic pathways
and interposed vein grafts
(arrows).
Over the last 21 years, 133 patients with obstructive lower-limb lymphedema have been treated with interposition of autologous LVL shunts. There were 79 women and 54 male patients, with an average age of 43 ± 5 y. (age ranged from 27 to 68 yrs). Thirty-nine patients (27 women and 12 men) presented with bilateral lymphedema. From the etiopathogenetic point of view, 87 were primary lymphedemas and 46 secondary. Primary lymphedemas were due to lymphnodal fibrosclerosis (lymphadenodysplasia – LADII, according to Papendieck’s classification) with good, dilated, and hyperplastic lymphatic collectors.31 Secondary lymphedemas were caused by surgery and/or radiotherapy for oncological reasons (seminomas, penis cancer, lymphomas, bladder cancer, prostatic cancer, cancers of the female genitalia, melanoma). Ninetyseven patients had stage III lymphedema, 21 had stage II, and 15 had stages IV-V, based upon a clinical instrumental staging of lymphedemas of five stages. In both clinical settings (primary and secondary lymphedemas), there was the coexistence of venous disorders that could not be corrected during the same operation, thus precluding the possibility of using derivative lymphaticovenous shunt operations. All patients were studied by lymphoscintigraphy and echo-Doppler.
The microsurgical method used in these patients was interposition of autologous vein grafts between lymphatic collectors below and above the lymph obstruction (lymphatico-venous-lymphatic anastomoses — LVLA).32 The site of operation was at the groin. The lymphatics were colored blue by injection of blue dye (Patent Blue V) just below and above the site of operation. The lymphatic vessels afferent and efferent from the inguinal region were prepared for anastomoses, and the gap between them was bridged by interposing a venous graft, harvested from the same operative site (collateral branches of the great saphenous vein) or from the forearm. The anastomoses were performed by microsurgical technique, using 8/0 nonabsorbable suture material, microsurgical tools an the operative microscope with magnification variable from 25x to 35x. We employed the telescopic end-to-end lymphatico-venous technique, anastomosing several lymphatics altogether, at the same time, inside the proximal and distal cut-ends of the venous graft. Valved vein grafts were chosen to avoid possible lymphatic gravitational back flow, and the collateral branches of the vein were preserved for further lymphatico-venous anastomoses. Moreover, the number of lymphatics anastomosed to the distal vein cut-end was higher than that of the proximal end, in order to keep the vein graft filled with lymph and avoid vein graft fibrosis or ischemia. The patency and good closure of anastomoses was directly checked perioperatively at the microscope. A light functional bandage was applied to the leg for the first 2 to 3 postoperative days. The patients were discharged from the hospital after 5 to 7 days wearing proper elastic garments, which had the aim of maintaining an adequate lymph flow through the vein segment. As a consequence of the operation, lymphatic flow inside the vein graft quickly tended to reduce, owing to the fast reduction in lymphedema volume. For this reason, just for the significant decrease of limb volume immediately after microsurgical operation, the patient needed to wear stockings from 1 to 5 years after microsurgery (according to the stage of the disease before the treatment and to the entity of fibrose tissue) to maintain and improve the results with time.33
The only medical therapy these patients needed was antibiotics peroperatively, and long-acting penicillin if there were signs of recurrent episodes of erysipeloid lymphangitis.
Follow-up of the patients (Table I) included, besides preand postoperative photographs, water volumetry and lymphoscintigraphy. Ninety-five patients were available for the long-term follow-up study.
The excess volume percent reduction was higher in patients treated at the early stages (II-III) than at later stages (IV-V). The average reduction of the excess volume was over 75% in 63 patients (47%), between 50% and 75% in 45 patients (34%), between 25% and 50% in 20 cases (15%), and less than 25% in 5 patients (4%).
The average incidence of lymphangitic episodes decreased significantly, from 3 to 4 per year to 0 to 1 per year.
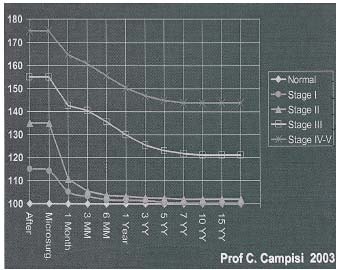
Table I. Table of long-term results (water volumetry).
The reduction in edema volume obtained by microsurgery was seen immediately after operation (already at the 1st, 2nd, and 3rd postoperative day), and a further decrease in lymphedema was observed also in the midand long-term after microsurgery, particularly between the first and the fifth year after operation. From the 5th year on, the clinical conditions of the limb remained stable with time, even at over 10 to 15 (in seven patients) years after operation.
Lymphoscintigraphy visualized lymph flow through the venous grafts, even over 10 and 15 years after operation, thus mirroring clinical improvement. The lymphoscintigraphic patterns consistent with efficacy of the microsurgical lymphatic reconstruction operations were as follows:
• reduced dermal backflow;
• appearance of preferential ways of lymph drainage;
• visualization of the intralymphatic interposition autologous venous grafts.
DISCUSSION AND CONCLUSIONS
Nowadays, primary and secondary peripheral lymphedemas are a quite well understood and curable problem. Present nonoperative measures are aimed at minimizing morbidity without removing the cause of the pathology. Microsurgical derivative and reconstructive operations can restore lymphatic drainage, both in the short and long term, and the best results are obtained when these surgical procedures are combined with physical rehabilitative methods.
Emphasis should be placed on prevention above all of secondary limb lymphedemas based on a proper understanding of the multifactorial etiology of the pathology. Presently, it is possible to identify preoperative factors that distinguish women at greatest risk of developing lymphedema, and alter the proposed management of the axilla without compromising the principles of cancer treatment. It is also possible to detect postoperative changes in the latent phase before the development of swelling, thus identifying those in whom lymphedema is most likely to occur. Early prophylactic initiation of nonoperative methods prove more effective than their institution when swelling has become established.
The authors’clinical experience in the prevention of secondary peripheral lymphedemas regards the use of a personal and original diagnostic and therapeutic protocol for lymphedema prevention which includes, apart from the clinical evaluation of the patient, also lymphangioscintigraphy. Lymphoscintigraphy may demonstrate the presence of pre-existing anatomical conditions of lymphatic circulation predisposing to specific lymphatic circulatory disorders, or prove the presence of impaired lymph drainage before the clinical evidence of the edema.
A proper therapeutic preventive protocol, including nonoperative measures and microsurgical operations, helps in avoiding the appearance of lymphedema or in treating it very early allowing to recover from the pathology completely and definitively.34
Finally, studies to investigate phenotype-genotype correlation are under way. In families where linkage or mutations are identified, testing of young clinically unaffected members permits early diagnosis and preventive management of congenital lymphedemas. Gene therapy aimed at stimulating new lymphatic growth in affected limbs is a possibility. Animal models exist to test gene therapy. New large families with primary lymphedema are required for further molecular genetic studies.

REFERENCES
2. Földi E, Földi M. Physiothérapie complexe décongestive. Paris: Editions Frison-Roche; 1993.
3. Leduc A. Le drainage lymphatique. Théorie et pratique. Paris: Masson; 1980.
4. Vodder E. La méthode Vodder – Le drainage lymphatique manuel. Institute. For Lymph Drainage; DK-2880, Bagsvaer; 1969.
5. Olszewski W. Recurrent bacterial dermatolymphangioadenitis (DLA) is responsible for progression of lymphoedema. Lymphology. 1996;29(Suppl):331.
6. Handley WS. Lymphangioplasty: a new method for the relief of the brawny arm of breast-cancer and for similar conditions of lymphatic oedema. Lancet. 1908;i:783-785.
7. Charles RH. A system of treatment. In: Latham A, English TC, eds. Churchill, London, 1912;3:504.
8. Thompson N. The surgical treatment of chronic lymphoedema of the extremities. Surg Clin North Am. 1967;47:2.
9. Servelle M. La lymphangiectomie superficielle totale. Traitement chirurgical de l’éléphantiasis. Rev Chir. 1947;294.
10. Cockett ATK, Goodwin WE. Chyluria: attempted surgical treatment by lymphatic venous anastomosis. J Urol. 1962;88:566-568.
11. O’Brien BM, Sykes P, Threlfall GN, Browning FS. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg. 1977;60:197-211.
12. Degni M. New microsurgical technique of lymphatico-venous anastomosis for the treatment of lymphedema. Lymphology. 1981;14:61-63.
13. Clodius L. Problems of microsurgery in lymphedema. Handchir Mikrochir Plast Chir 1982; 14: 79-82.
14. Huang GK, Hu RQ, Liu ZZ, Shen YL, Lan TD, Pan GP. Microlymphaticovenous anastomosis in the treatment of lower limb obstructive lymphedema: analysis of 91 cases. Plast Reconstr Surg. 1985;76: 671-685.
15. Krylov Vs, Milanov NO, Abalmasov KG, Sandrikov VA, Sadovnikov VI. Reconstructive microsurgery in treatment of lymphoedema in extremities. Int Angiol. 1985;4:171-175.
16. Zhu JK, Yu GZ, Liu JX, Pang SF, Lao ZG, Tang HY. Recent advances in microlymphatic surgery in China. Clin Orthop. 1987;215:32-39.
17. Al Assal F, Cordeiro AK, De Souza e Castro I. A new technique of microlymphovenous anastomoses. Experimental study. J Cardiovasc Surg. 1988;29:552-555.
18. Ho LC, Lai MF, Yeates M, Fernandez V. Microlymphatic bypass in obstructive lymphoedema. Br J Plast Sug. 1988;41:475-84.
19. Olszewski WL. The treatment of lymphedema of the extremities with microsurgical lympho-venous anastomoses. Int Angiol. 1988;7:312-321.
20. Gloviczki P, Fisher J, Hollier LH, Pairolero PC, Schirger A, Wahn HW. Microsurgical lymphovenous anastomosis for the treatment of lymphedema: a critical review. J Vasc Surg. 1988;7:647-652.
21. Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64-74.
22. O’Brien BM, Mellow CG, Khazanchi RK, Dvir E, Kumar V, Pederson WC. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg. 1990;85:562-572.
23. O’Brien BM, Hickey MJ, Hurley JV, Dvir E, Khazanchi RK, Pederson WC, Pribaz JJ. Microsurgical transfer of the freater omentum in the treatment of canine obstructive lymphoedema. Br J Plast Surg. 1990;43:440-446.
24. Abalmasov KG, Egorov YS, Abramov YA, Chatterjee SS, Uvarov DL, Neiman VA. Evaluation of the greater omentum in the treatment of experimental lymphedema. Lymphology. 1994;27:129-136.
25. Campisi C. Rational approach in the management of lymphedema. Lymphology. 1991;24:48-53.
26. Campisi C. Lymphoedema: modern diagnostic and therapeutic aspects. Int Angiol. 1999;18:14-24.
27. Campisi C, Boccardo F, Zilli A, Macciò A, Napoli F. Long-term results after lymphaticvenous anastomoses for the treatment of obstructive lymphedema. Microsurgery. 2001;21:135-139.
28. Badini A, Fulcheri E, Campisi C, Boccardo F. A New Approach in Histopathological diagnosis of Lymphedema: pathophysiological and therapeutic implications. Lymphology. 1996;29:190-198.
29. Mariani G, Campisi C, Taddei G, Boccardo F. The current role of lymphoscintigraphy in the diagnostic evaluation of patients with peripheral lymphedema. Lymphology. 1998;31:316-319.
30. Campisi C, Boccardo F, Tacchella M. Reconstructive microsurgery of lymph vessels: the personal method of lymphatic-venouslymphatic (LVL) interpositioned frafted shunt. Microsurgery. 1995;16:161-166.
31. Dellachà A, Fulcheri E, Boccardo F, Campisi C. Post-surgical lymphedema: Iatrogenic or pre-existing disease? Lymphology. 1998;31:562-565.
32. Campisi C, Boccardo F, Zilli A, Macciò A, Napoli F. The use of vein grafts in the treatment of peripheral lymphedemas: longterm results. Microsurgery. 2001;21:143-147.
33. Campisi C, Boccardo F. Frontiers in lymphatic microsurgery. Microsurgery. 1988;18:462-471.
34. Boccardo F, Campisi C et al. A pilot study on prevention of secondary lymphedema. Lymphology. 2000;33:222-225.
