Cyanoacrylate ablation for chronic venous disease: a review and future applications

Kathleen Ozsvath, MD, FACS
Chief of Surgery, Samaritan
Hospital, Troy, New York; Vascular
Associates, St Peters Health
Partners, Albany, New York;
Professor of Surgery, Albany
Medical Center, Albany,
New York, USA
ABSTRACT
Many endovenous procedural modalities exist today, including endovenous thermal ablation (laser ablation, [EVLT] and radiofrequency ablation [RFA]), nonthermal nontumescent ablation, and ultrasound-guided sclerotherapy (UGS) and chemical ablation. Recently, cyanoacrylate has been used for the treatment of axial insufficiency. The most studied preparation of N-butyl cyanoacrylate is VenaSeal (Medtronic), which has been compared with the other ablative techniques. These studies have shown excellent results with patient satisfaction, outcomes, and low complication rates. Other cyanoacrylate preparations are being used, including VariClose (Biolas), and VenaBlock (Kalyon) outside the United States, but there are less than a dozen studies to date reporting on these formulations.
There is significant data with VenaSeal showing that post procedural pain was found to be less in patients undergoing ablation with cyanoacrylate than with thermal techniques. VenaSeal has been shown in many studies to be safe, with similar occlusion rates to thermal techniques. In a handful of studies published using VariClose and VenaBlock, closure rates have been shown to be similar to those with thermal techniques as well. Complications unique to cyanoacrylate include hypersensitivity reactions (early and delayed), glue extension, granuloma formation, and phlebitis. These complications are encountered with all 3 preparations of cyanoacrylate.
Nevertheless, cyanoacrylate is considered safe, and excellent results may be expected. Whereas VenaSeal has been extensively studied, and VenaBlock and VariClose have also been compared with thermal techniques, there are no studies to date comparing these 3 preparations with one another. There may be future uses for these compounds that may effectively treat additional venous beds.
Introduction
Venous disorders are the most common vascular disease in developed countries. Up to 40% of women and 17% of men have been reported to have chronic venous insufficiency, and upwards of 70% of women and 56% of men have been estimated to have varicose veins. Patients range in signs and symptoms from aesthetic issues to venous ulceration. Symptoms can include leg pain, aching, itching, bleeding varicosities, and restless legs. The cost of treating patients with venous disease is significant, and the treatment of venous leg ulcers (VLU) is a burden to the health care system. Issues affecting patients include misdiagnosis, incomplete care, limited access, and socioeconomic barriers to care.
Vein stripping and high ligation was the standard of care until approximately 25 years ago with the advent of less-invasive methods, including thermal ablation techniques. In the United States, thermal treatment is now regarded as standard of care. Treatment modalities include radiofrequency ablation (RFA) and endovenous laser ablation (EVLA). Ultrasound-guided foam sclerotherapy (UGFS) has also gained popularity among providers with slightly lower closure rates and higher risks of deep venous thrombosis (DVT) as compared with other modalities, including thermal ablation of the saphenous veins. Newer techniques evolved including pharmacomechanical treatment (also known as mechanochemical ablation [MOCA]) with ClariVein.
Cyanoacrylate closure (CAC) of refluxing veins was developed in Europe. Initially known as Sapheon, the proprietary glue is now commercially available from Medtronic under the name of VenaSeal. The product has been well studied in the United States and has shown great promise in the field of superficial venous treatment. VenaSeal has been used to treat saphenous axial reflux1 and may have future uses in other veins and tributaries and other venous beds. It is the most viscous with the longest polymerization time. Other cyanoacrylate formulations have been developed in Turkey, including VenaBlock and VariClose. VenaBlock is designed to be administered for catheter-directed as well as percutaneous injection. It has a very rapid polymerization time. A lighted catheter tip purportedly allows for better visualization. VariClose is the least viscous with a faster polymerization time than VenaSeal. Currently, VenaBlock and VariClose are not approved for use in the United States. There is growing evidence that these newer compounds may be efficacious as well in the treatment of refluxing veins.
In this article, we review the literature on VenaSeal as well VenaBlock and VariClose—2 compounds manufactured in Turkey and that are not available in the United States. It also includes a literature review on possible additional uses of cyanoacrylate in other applications within the venous system.
Results
VenaSeal is the most studied cyanoacrylate in the literature. The Turkish compounds have much fewer studies available to date. Similar closure rates and patient satisfaction have been found with the available cyanoacrylate products. No studies have been done to compare VenaSeal with VenaBlock or VenaClose.
Complications, including hypersensitivity reactions (HSR), exist for all 3 formulations. New applications on the horizon include the treatment of perforator veins. Other venous beds treated with cyanoacrylate include the treatment of varicocele, pelvic veins, and bleeding gastrointestinal varices.
Discussion
In 2019, Almeida et al treated 38 patients with VenaSeal. They found occlusion rates of 94.7% at 36-month follow-up. No adverse complications were noted.2 The multicenter, prospective eSCOPE study (European Sapheon Closure System Observational ProspectivE) showed occlusion rates of 92.9% at 12 months. There was significant improvement in quality of life (QOL) and in venous clinical severity scores (VCSS) at 12 months. Of 70 patients treated, 1 patient had phlebitis and 1 had thrombus extension beyond the saphenofemoral junction.
In a meta-analysis of 4 studies that included 378 patients undergoing CAC and 590 patients undergoing RFA, the authors found that CAC was comparable to RFA regarding closure rates, pain, VCSS, and Aberdeen Varicose Vein Questionnaire (AVVQ) scores in patients with incompetent saphenous veins.3 The CAC group had lower ecchymosis and paresthesias than the RFA group. In a systematic review by Farah et al, CAC was found to have better QOL and lower risk of recurrence and of complications than thermal ablation techniques.4 The multicenter, randomized control trial VeClose (VenaSeal Sapheon Closure System Pivotal Study) confirmed the results of the eSCOPE trial5,6; both show CAC to not be inferior to RFA. Kolluri et al reported a network meta-analysis comparing VenaSeal with other therapies.7 The authors compared CAC with RFA, EVLA, sclerotherapy, MOCA, and surgery. VenaSeal was found to be superior to surgery and to other comparators. VenaSeal was found to have the least probability of a number of adverse events and to be superior to all the other therapies in pain reduction scores and QOL measures.
The use of VenaSeal was found to be safe and effective in 37 Asian patients undergoing ablation for venous ulceration. All of the ulcers healed after the procedure.8 In another study, the authors retrospectively analyzed 170 small saphenous veins treated with VenaSeal in an Asian population with an occlusion rate of 96.3% and no noted complications.9 O’Banyion et al retrospectively compared CAC (via VenaSeal) (N=51) with RFA (N=68) in patients with venous ulcers. They found the ulcers in the CAC arm had faster healing and longer ulcer-free intervals than the RFA arm.10 These results suggest that ablation by CAC is as efficacious as RFA in the venous ulcer population.
Although VenaSeal and VariClose have similar properties, VariClose has a faster polymerization rate (Table I11). Additionally, there is a difference in delivery of these cyanoacrylate formulations—VariClose is a continuous delivery of the glue, whereas VenaSeal is segmental (Table I). In a single-center trial, 310 patients were treated with either CAC (via VariClose) or EVLT. They found the operative time to be shorter and the procedural pain to be less with CAC.12 Delivery of VenaBlock is via a pulse-system endovenous application of the glue. In a retrospective review, Yavuz et al investigated use of the VenaBlock catheter for embolization of incompetent greater saphenous veins in 538 patients. They found an improved QOL after treatment and no significant complications.13 Differences in viscosity among these 3 formulations is important to note as well (Table I). VenoSeal is more viscous than the other 2 formulations, with a longer polymerization rate, and the glue is administered segmentally, with manual compression between the segments treated. The Turkish formulations are less viscous, which runs the risk of glue potentially spilling into the deep system. However, these are faster in delivery, as the polymerization is much faster (Table I), and tout a faster treatment time, though this has not been directly studied. Unfortunately, VariClose and VeniBlock have been studied less than VenaSeal, and most of those studies compare the formulations with thermal ablation. Thus, for differentiation between these formulations, prospective randomized trials are lacking.
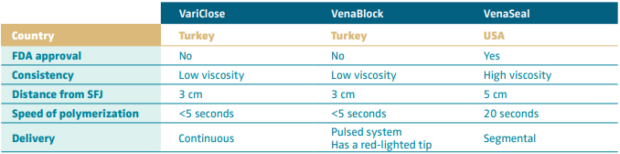
Table I. Cyanoacrylate formulations.
Based on reference 11: Bissaco et al. Minim Invasive Ther Allied Technol. 2019;28(1):6-14.
Complications
Hypersensitivity reaction
Type IV HSR is a delayed-type hypersensitivity that is a T-cell– mediated immune response occurring within 24-48 hours. Gibson et al reported a 6% frequency of hypersensitivity in patients treated with VenaSeal. They found 4.3% were mild, 1.3% were moderate, and 0.3% were severe. The patients with moderate HSR were treated with steroids; some had need for recurrent steroid treatment. The patient with the severe reaction had multiple recurrent reactions and ultimately had the vein removed surgically.14 Phlebitis-like allergic reaction (PLAR) was described by Park et al.15 They described PLAR as any skin condition that is sudden and presenting as erythema, itching, swelling, and pain over the treated veins. They recommend using antihistamines and steroids to manage this type IV HSR. As more papers are published regarding hypersensitivity to CAC, it is evident that those patients who are suspected to be at risk for hypersensitivity should avoid treatment with CAC. Allergic reactions will occur in a small subset of treated patients, no matter what formulation is used.
Granuloma
Sermsathanasawadi et al reported cyanoacrylate granuloma in 2.3% of great saphenous veins after CAC treatment.16 All patients in that study underwent incision, drainage, and removal of the glue.16 This led to a change in the instructions from Medtronic in regard to VenaSeal. Recapturing the tip of the catheter to minimize the extravasation of glue into the surrounding tissue is important. The thought is that the risk of granuloma formation is mitigated.
Other uses of cyanoacrylate
In lower-extremity venous disease, cyanoacrylate is a novel treatment for the treatment of pathologic perforator veins. In a study by Mordhorst et al, the authors treated 83 perforator veins in 62 patients with CAC injected directly into the perforator. They found no cases of DVT. More than half of the patients did receive additional sclerotherapy. The authors found 100% initial closure rate; however, in the second follow-up, they had an 86% closure rate.17 In a study by Prasad et al, 191 perforators were treated in inpatients with ulceration. All healed within 3 months of treatment.18 There may be concern that the application of direct pressure on the treated vein is sometimes difficult with these procedures, and technical success may be hindered. Given the thermal options for perforator treatment, CAC is certainly easier to use and less painful in a difficult anatomic area. Another concern is that glue could enter the deep system, leading to DVT. As practitioners improve their technique, this may be less of an issue.
There are reports of successful embolization of varicoceles using CAC as well. Urbano et al studied 41 patients with varicocele who were treated with cyanoacrylate as an embolic agent.19 The authors surmise that there is less pain compared with patients treated with sclerosants.19 There are no prospective randomized trials comparing cyanoacrylate with sclerosant with or without coil embolization, or open surgery, in the treatment of varicoceles.
Female pelvic venous disease is less understood and less treated, especially in the United States. Without published prospective, randomized studies, insurance companies deny treatment and consider it “experimental.” If better techniques can be developed that can show alleviation of pelvic pain, regression of vaginal/labial varicosities, and improvement in lower-extremity pain, it is hoped that care would be more available. In a study by Lorenzo et al, 29 out of 30 women were treated with bilateral ovarian vein embolization for pelvic venous disease.20 They reported no access-related complications, nontargeted embolization, or migration of the glue. At 1 year, pain upon standing, dyspareunia, and menstrual pain was greatly diminished. This study is a single-arm, nonblinded, nonrandomized trial. More research needs to be done to better evaluate the use of CAC in this patient population.
Cyanoacrylate has been used as an embolic agent in interventional radiology, interventional neuroradiology, and in the treatment of arteriovenous malformations, portal venous disease (gastric varices), and in aneurysms. Gastric varices have been treated with cyanoacrylate with some reported success. Treatment of the periprostatic venous plexus in patients with erectile dysfunction from venous leakage has been successful with CAC.21
Table II briefly summarizes treatment methods for venous pathologies mentioned in this section, for which cyanoacrylate may prove to be helpful. Future development of technique and further investigation will help guide treatment of venous disease for which present treatments do not deliver consistent outcomes.
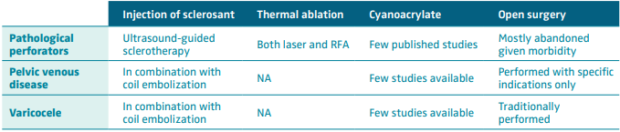
Table II. Treatment of venous pathology: cyanoacrylate may prove to be very helpful.
Abbreviations: NA, non applicable; RFA, radiofrequency ablation.
Conclusion
In choosing the best methods for treatment of axial reflux, physicians must also take into account the cost and reimbursement. In the United States, not all insurers will pay for CAC, stating it is experimental, despite growing research confirming its efficacy. A novel use for CAC in the future includes treatment of perforators, which may prove to be less cumbersome than other modalities of treatment. It would certainly be less painful as there is no need for tumescent anesthesia with injection of cyanoacrylate. There are small case studies and series reported in the literature to date. Treatment of pelvic venous disease in both men and women must be further studied. With the advent of standardized treatment options, venous specialists will be able to offer better treatments to their patients. Deciding upon the best technology is difficult as technology is evolving. Which glue formulations are better than others remains to be understood. Presently, there are no studies comparing VenaSeal with VariClose or VenaBlock; however, studies comparing VariClose and VenaBlock with thermal ablation have shown higher recanalization rates. Research is lacking presently, but as formulations and delivery systems improve and the techniques become standardized, patient care will continue to evolve.
References
1. Belramman A, Roshan Bootun R, Lane TRA, Davies AH. Endovenous management of varicose veins. Angiology. 2019;70(5):388-396.
2. Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1(2):174-180.
3. Chen MS, Shuangmeng Mou BS, Gengwu Dai BS, Jinliang Hu MS. Comparison between cyanoacrylate embolization and radiofrequency ablation for superficial venous incompetence: a systematic review and meta-analysis. Dermatol Surg. 2021;47(8):e214-e219.
4. Farah MH, Tarek Nayfeh MH, Urtecho M, et al. A systematic review supporting the Society for Vascular Surgery, the American Venous Forum, and the American Vein and Lymphatic Society guidelines on the management of varicose veins. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1155-1171.
5. Morrison N, Kolluri R, Vasques M, Madsen M, Jones A, Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36 month outcomes of the VeClose randomized controlled trial. Phlebology. 2019;34(6):380-390.
6. Proebstle TM, Alm J, Dimitri S, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3(1):2-7.
7. Kolluri R, Chung J, Kim S, et al. Network meta-analysis to compare VenaSeal with other superficial venous therapies for chronic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2020;8(3):472-481.
8. Chan SSJ, Yap CJQ Tan SG, et al. The utility of endovenous cyanoacrylate glue ablation for incompetent saphenous veins in the setting of venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2020;8(6):1041-1048.
9. Cho J, Joh JH. Cyanoacrylate closure of small saphenous vein insufficiency. DermatoL Surg. 2021;47:(3):381-384.
10. O’Banyion LA, Reynolds KB, Kochubey M, et al. A comparison of cyanoacrylate glue and radiofrequency ablation techniques in the treatment of superficial venous reflux in CEAP 6 patients. J Vasc Surg Venous Lymphat Disord. 2022;9(5):1215-1221.
11. Bissacco D, Stegher S, Callian FM, Viani MP. Saphenous vein ablation with new cyanoacrylate glue device: a systematic review on 1000 cases. Minim Invasive Ther Allied Technol. 2019;28(1):6-14.
12. Bozkurt AK, Yilmaz MF. A prospective comparison of a new cyanoacrylate glue and laser ablation for the treatment of venous insufficiency. Phlebology. 2016;31:106-113.
13. Yavuz T, Acar AN, Aydın H, et al. A retrospective study of a new n-butyl 2-cyanoacrylate glue ablation catheter incorporated with application guiding light for the treatment of venous insufficiency: twelve-month results. Vascular. 2018;26:547-555.
14. Gibson K, Minjarez R, Rinehardr E, Ferris B. Frequency and severity of hypersensitivity reactions in patients after VenaSeal cyanoacrylate treatment of superficial venous insufficiency. Phlebology. 2020;35(5):337-344.
15. Park I, Jeong MH, Park CJ, Park W, Park DW, Joh JH. Clinical features and management of “phlebitis-like abnormal reaction” after cyanoacrylate closure for the treatment of incompetent saphenous veins. Ann Vasc Surg. 2018:1-7.
16. Sermsathanasawadi N, Pruekprasert K, Chinsakchai K, Wongwanit C, Ruangsetakit C. Cyanoacrylate granuloma after cyanoacrylate closure of incompetent saphenous veins. Dermatol Surg. 2021;47(10):1372-1375.
17. Mordhorst A, Yang GK, Chen JC, Lee S, Gagnon J. Ultrasound guided cyanoacrylate injection for the treatment of incompetent perforator veins. Phlebology. 2021;36(9):752-760.
18. Prasad K, Joy B, Toms A, Sleeba T. Treatment of incompetent perforators in recurrent venous insufficiency with adhesive embolization and sclerotherapy. Phlebology. 2018;33(4):242-250.
19. Urbano J, Cabrera M, Alonso-Burgos A. Sclerosis and varicocele embolization with N-butyl cyanoacrylate: experience in 41 patients. Acta Radiol. 2014;55(2):179-185.
20. Lorenzo JIL, Madueno GG, Peral AA, Rodriquez EP, Santos RC, Burgos AA. Bilateral ovarian vein embolization from a unilateral basilic approach with n-2-butyl cyanoacrylate and crossover technique for pelvic congestion syndrome. Eur J Vasc Endovasc Surg. 2021;63(1):163-164.
21. Rebonato A, Auci A, Sanguinetti F, et al. Embolization of the periprostatic venous plexus for erectile dysfunction resulting from venous leakage. J Vasc Inter Radiol. 2014;25(6):866-872.
