Duplex ultrasound investigation in pelvic congestion syndrome: technique and results
Milka GREINER2
78150 Le Chesnay, France
2 American Hospital of Paris,
Vascular Department, 63 Bd Victor Hugo,
92200 Neuilly sur Seine, France
Abstract
Chronic pelvic venous insufficiency is a common pathology, but it is overlooked and underdiagnosed as it sits on the edge of two medical specialties: gynecology and vascular medicine. It manifests most frequently as pelvic varicose veins and pelvic venous reflux toward the lower limbs. When symptomatic, it is expressed in the pelvic area as a sometimes-disabling pelvic congestion syndrome and/or in the lower limbs as it supplies the varicose veins. Consequently, the isolated treatment of lower-limb varices without treating pelvic leaks when there is pelvic congestion syndrome may cause early recurrences. The ultrasound exploration allows the positive diagnosis of pelvic venous involvement and the classification by pathophysiological types, which is a key step before any treatment. The echo Doppler is also essential for the diagnosis of superficial points of pelvic venous leaks supplying lower-limb varicose veins. In most symptomatic patients, ultrasound exploration provides sufficient arguments to evaluate the relevance of the pelvic-level venous treatment. It leads to additional imaging techniques, if necessary, or directly to a hyperselective descending pelvic venography. This last investigation provides an accurate mapping of varicose veins and pelvic venous reflux.
Introduction
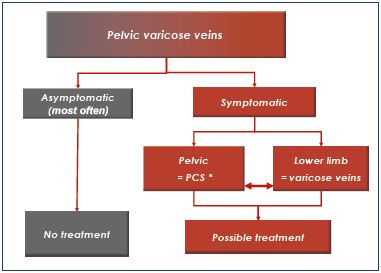
Chronic pelvic venous insufficiency includes all events related to the dysfunction of the pelvic venous system, whether congenital or acquired. Venous reflux and pelvic varicose veins represent the most common pathological expression. These pelvic varicose veins are very common in multiparous women, and they are most often asymptomatic. When symptomatic, they can be responsible for pelvic congestion syndrome and/or varicose veins in the lower limbs.1-4 reating pelvic veins should be considered when they are symptomatic at any level (Table I).
Semantic prerequisite
The female varicocele is only a particular form of pelvic varicose veins related to the dilatation of the pampiniform plexus.
Anatomical and hemodynamic prerequisites
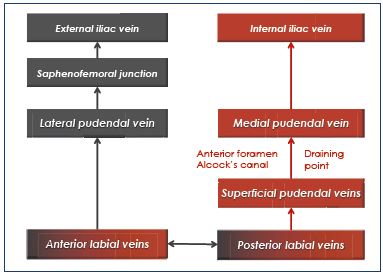
The pelvic veins drain into three main collector systems: the internal iliac, ovarian, and rectal veins.4-6 The common and internal iliac veins are generally valveless, but the visceral and parietal internal iliac tributaries are valved. This fact is clearly demonstrated by using hyperselective retrograde pelvic phlebography. These tributaries come from valveless plexuses that are extensively interconnected. Therefore, pelvic veins are not independent, but interconnected as a network and connected with other networks, particularly with the lower-limb veins. This connectivity explains why an abdominal or pelvic venous reflux can be the origin of a venous anomaly located in another area, ie, a left ovarian reflux can supply the right perineal varices.
Classifications
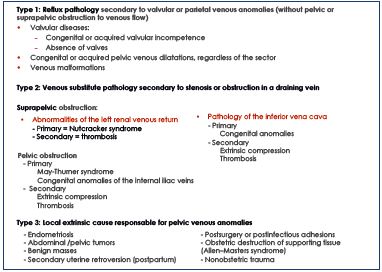
In 2005, Milka Greiner5 proposed a classification system based on pathophysiological findings. Three main types of vein damage diagnosed by ultrasound explorations and confirmed by cross-sectional imaging and selective phlebography were identified (Table II). These three types do not depend on the location of the pelvic venous pathology (ovarian or extraovarian coming from the internal iliac tributaries). Each type needs a specific therapeutic plan. Type 1 corresponds to valvular or parietal venous anomalies without pelvic or suprapelvic obstruction to venous outflow, which is responsible for the reflux. It is the most frequent physiopathology, and endovascular treatments are the preferred method of management. Type 2 relates to stenosis or obstruction in a draining vein responsible for symptomatic substitute collaterals. In type 2, the isolated treatment of reflux and varices without treatment of the obstruction can lead to worsening of abdominal, pelvic, and/or lower-extremity–related venous hypertension. It should be considered after a multidisciplinary assessment of the risk/benefit ratio. Type 3 pelvic vein anomalies and pelvic reflux are secondary to a local extrinsic cause. In this context, the treatment of varices could be only considered after the treatment of the cause.
From an anatomical point of view, two locations can be differentiated6: (i) genital varices are fed by the ovarian veins and/or the uterine veins; and (ii) extragenital pelvic varices are fed by the other internal iliac tributaries, particularly the extrapelvic parietal tributaries of the internal iliac, ie, the gluteal, obturator, and medial pudendal veins. These two locations can be associated.
Mode of pelvic reflux transmission toward the lower limbs
Two modes of leak transmission have been differentiated (Table III)6:
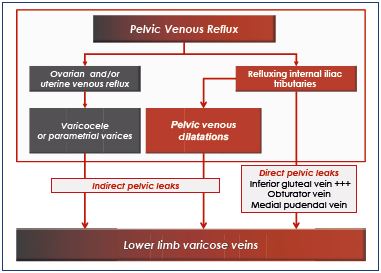
1. Direct pelvic leaks occur when the truncal pelvic reflux feeds the lower-limbs varices directly. The inferior gluteal vein and, to a lesser degree, the obturator and medial pudendal veins are involved.
2. Indirect pelvic leaks occur when the truncal pelvic reflux first feeds one or several clusters of pelvic varicose veins, which drain secondarily into the lower limbs. Genital varices are usually indirect leaks. Extragenital venous insufficiency can be expressed in the lower limbs by direct or indirect leaks.
Externalization mode of pelvic reflux toward the lower limbs
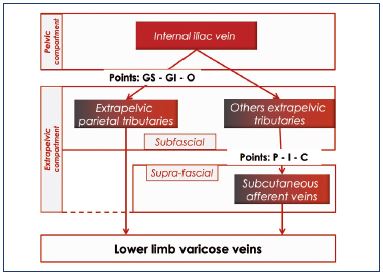
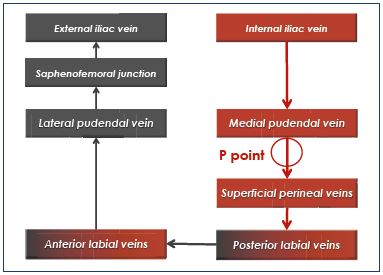
Whatever the direct or indirect mode, a venous reflux from the pelvis to the lower limbs requires communication between the two levels, these are called leak points. They can be nonsystematized, in which case, the pelvic venography will show a pelvic venous network that feeds the lower-limb varices. Conversely, the leak points can be systematized. In 2005, Claude Franceschi7 listed five systematized and symmetrical leak points: the buttock points (superior gluteal [GS] point, inferior gluteal [GI] point), perineal (P) point, obturator (O) point, and inguinal (I) point. A sixth point was described later: the clitoral (C) point. These leak points are different in terms of location and physiopathology because some are located in the anatomical drainage pathways of extrapelvic parietal afferent veins toward the left and right internal iliac veins and their pelvic collectors. Thus, the GS and GI points are located at the intrapelvic passage of the gluteal veins at the greater sciatic notch above (superior gluteal vein) or below (inferior gluteal vein) the piriformis muscle. The O point originates from the intrapelvic path of the obturator vein by the subpubic canal, at the obturator foramen. These three points are anatomically deep and difficult to study using ultrasound methods. The P, I, and C points7 correspond to the reflux of the extrapelvic infra-aponeurotic veins into the extrapelvic subcutaneous veins (Table IV).
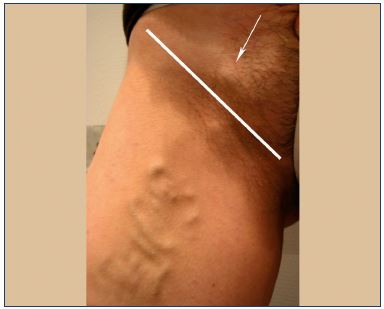
Perineal point
Superficial perineal veins drain the perineum, receive the anterior and posterior labial veins, and cross the superficial fascia of the perineum by an orifice that has been called the P point. These superficial veins ascend to the medial pudendal vein in Alcock’s canal. Refluxing veins follow the same pathway in the opposite direction. The P point is located at the union that is one-quarter posterior and three-quarters anterior to the vulvoperineal fold (near Alcock’s canal) (Figure 1). It is fed by the medial pudendal vein, and it could be the result of refluxing small tributaries of the ipsilateral medial pudendal vein and/or truncular pudendal reflux (Table V and VI).
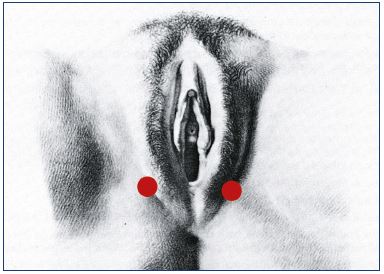
Figure 1. Schema showing the localization of the P point that is
three-quarters posterior to the vulvar fold.
Inguinal point
The I point corresponds to the superficial inguinal ring where the vein of the round ligament preferentially drains into the uterine vein. Given the valveless connection between the visceral plexus, the I point can externalize the venous flow from any pelvic area, but preferably from parametrial varices. It is located above the inguinal canal, outside the femoral vessels (Figure 2).
Clitoral point
The C point is related to incontinence of the medial pudendal vein, which generates increased venous pressure in the region of the peri-urethral plexus, followed by a reverse flow from the deep clitoral veins to the superficial clitoral veins. C points are located on each side of the clitoris. In practice, they are rarely identified.
Decisional algorithm
Our management of chronic pelvic venous insufficiency always follows the same decisional algorithm based on four steps (Table VII). Only the second step is related to this article’s topic, but it should be set within the global context. Initially, pelvic varices can be evoked in front of pelvic congestion syndrome or lower-limb varicose veins. The problem for the gynecologist is to recognize the venous origin of chronic pelvic symptoms. The vascular physician should recognize the pelvic origin of lower-limb varices. The second step, based on ultrasound exploration, will confirm, quantify, and classify the chronic pelvic venous insufficiency. This is a key step for advancing, in most cases, the decision-making stage by adding investigations, notably mini-invasive, cross-sectional imaging studies, eg, selecetive retrograde descending pelvic venography. Pelvic venography is the only exploration to provide up a precise mapping of pelvic varicose veins and pelvic venous reflux (P and I points included); therefore, it remains the gold-standard examination. It is a mandatory investigation when interventional treatment is considered.
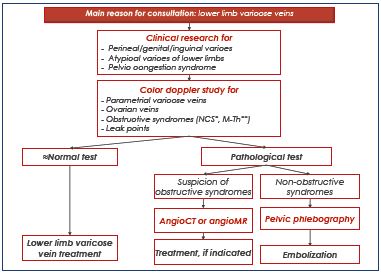
Table VII. Decisional algorithm of pelvic varicose vein
management
Abbreviations: M-Th, May-Thurner syndrome; NCS, Nutcracker
syndrome.
Principles of ultrasound exploration
Our ultrasound exploration always follows the same approach:
1. Search for varicose veins in the uterine area.
2. Examine the gonadal veins.
3. Search for anatomical and hemodynamic criteria of left renal vein compression.
4. Examine the iliac vessels.
5. Search for leak points.
6. Identify lower limb varices.
Search for varicose veins in the uterine area
The exam must be descriptive; anatomic and hemodynamic criteria must be noted. It begins with a suprapubic exploration using a macroconvex probe (frequency: 5 to 5 MHz). The normal venous plexus appears as a straight tubular structure with a normal diameter (<4 mm). In patients with pelvic varicosities, an ultrasound typically shows dilated and tortuous veins, with reversed and slow flow, that are located on both sides of the uterus, preferably on the left side. Genital varicose veins can form a cluster of little dilated veins; therefore, no specific diameter can be used to diagnose genital varicose veins (Figures 3, 4, and 5). It is important to search for dilated arcuate and/or intramyometrial veins communicating with bilateral pelvic varicose veins.
Flow imaging can confirm the presence of a reflux in lateral uterine venous dilatations and highlight some collateral pathways (Figures 6 and 7). This reflux can be spontaneous with breathing or caused by abdominal compression maneuvers (soft and prolonged) or the Valsalva maneuver.
1. The transperineal approach limits the artifacts related to the mobilization of the abdominal wall during the Valsalva maneuver: the patient is in a supine, gynecological position, and the probe is positioned at the middle perineum. This position has been used in a male patient to study the flow of the medial pudendal artery in the assessment of erectile dysfunction.
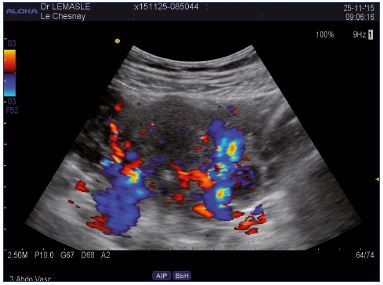
Figure 6. Abdominal approach.
Left lateral uterine varices draining of the lower pole pelvic
varicose in right medial pudendal vein.
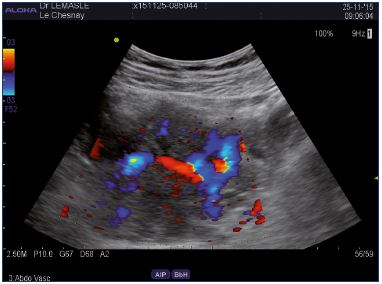
Figure 7. Abdominal approach.
Left lateral uterine varices draining by the right side uterine
network via arcuate veins.
2. We do not use the transvaginal ultrasound. It is useless when searching for iliac or renal vein compression and for superficial leak points (P, I, and C), but it provides the best image quality and resolution of lateral, arcuate, and myometrial uterine varices. The transvaginal ultrasound is helpful for assessing endometriosis cysts and tissular pelvic pathology (Type 3). High-resolution transvaginal ultrasound remains indispensable in the etiological evaluation of pelvic symptoms.
Examination of the gonadal veins
The venous territories of the parametrium, mesosalpinx, uterine fundus, and the pampiniform plexus are drained by the ovarian veins. They are formed by separate trunks, which become a single trunk at the level of the 4th lumbar vertebra. The right ovarian vein drains directly into the inferior vena cava and occasionally into the right renal vein. The left ovarian vein drains most often into the left renal vein. Variations are common in the number of trunks (double or triple) and in the mode of termination.8 These variations make the ultrasound exploration incomplete.
The best anatomical landmark is the psoas muscle (Figure 8). On the left, the upper part of the gonadal vein is located on the anterior side of the psoas muscle. In its inferior part, its way is internal, meaning that it runs along the anteromedial then medial side of the psoas muscle. In the pelvis, the left gonadal vein crosses in front of the common iliac vessels, so it is sometimes possible to see the gonadal vein, artery, and then the iliac vein on the same longitudinal section, from front to rear (Figure 9). The diameter of the vein has to be noted, but this measure is only a guidance element because a dilated gonadal vein can be draining, and conversely, a narrow gonadal vein may be refluxive.
Reflux is analyzed with the pulsed or color Doppler. Several types of reflux can be documented: spontaneous and intermittent retrograde flow; retrograde flow only on Valsalva maneuvers; and permanent retrograde flow in a standing position and/or in a supine position, and, in this last context, it can be more or less modulated with breathing.
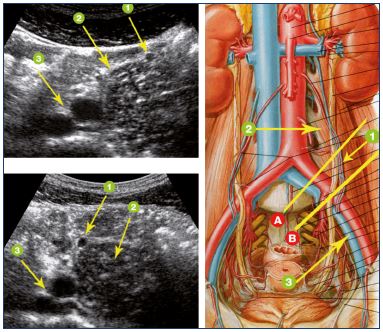
Figure 8. Ultrasound signs of left gonadal vein.
1: left gonadal vein; 2: psoas muscle; 3: iliac vessels; the
top image corresponds to section A and the lower image
corresponds to section B.
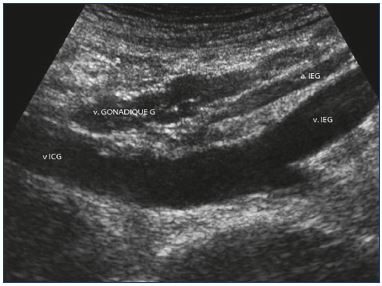
Figure 9. Abdominal approach.
The following veins are visualized, from front to rear: left gonadal
vein (v .GONADIQUE G), left iliac artery (a.IEG), left external
iliac vein (v IEG), and left common iliac vein (v ICG).
The hemodynamic status of the gonadal vein allows the type of varicose vein to be defined. In type 1 (reflux pathology without a pelvic or suprapelvic obstacle), the reflux can be spontaneous, major, even almost continuous in case of steal syndrome by the ovarian vein, but it is modulated by breathing. In type 2 (obstruction pathology), the gonadal vein will be the main way for renal drainage flow. In this context, gonadal reflux is spontaneous, permanent, and has little or no modulation by breathing. Type 3 may be suspected by the nonconformity between an important varicocele and little or no gonadal vein reflux.
Search for anatomical and hemodynamic criteria of the Nutcracker syndrome (NCS)
The NCS is characterized by the stenotic compression of the left renal vein (LRV) which is responsible for clinical symptoms. Several anatomical forms have been identified. LRV compression between the superior mesenteric artery and the abdominal aorta (or anterior LRV compression) is the most frequent. The posterior form, when the LRV is in a retro-aortic position and compressed between the aorta and a lumbar vertebral body, is less frequent. The circumaortic LRV with compression of its two branches is rare. Numerous attempts have been made to improve Doppler ultrasound diagnostic criteria. Some quantitative ultrasound criteria for anterior LRV compression has been described, based on small studies.9-11
LRV stenosis is detected of the basis of comparing peak velocity ratios and anterior-posterior diameter ratios of the aortomesenteric narrowed and hilar (distended) LRV portions. The sensitivity and specificity of a Doppler ultrasound ranges from 69% to 89%, respectively, for the hemodynamic criteria and from 80% to 94%, respectively, for the anatomic criteria. If we use a combined cutoff value of more than 5, the sensitivity is 90% and the specificity is 100% according to the authors.10 However, the measurement of LRV diameter is variable depending on systole, patient position, and an inappropriate angle of insonation. It is the same for the measurement of peak systolic velocity.
In practice, the most important criterion is indirect because it corresponds to the spontaneous, permanent, and nonbreathing-modulated flow of the left gonadal vein. The presence of such a reflux must always evoke a supplying flow from a significant stenosis of the LRV, which should lead to a search for direct criteria mentioned above and for other indirect criteria of stenosis, such as the visibility of the collateral pathways (Figure 10).11 The convergence of these anatomic and hemodynamic criteria suggests a symptomatic LRV compression that is responsible for a Nutcracker syndrome and must result in an angioCT or a contrast-enhanced MR angiography to help decide which procedure is the most appropriate.
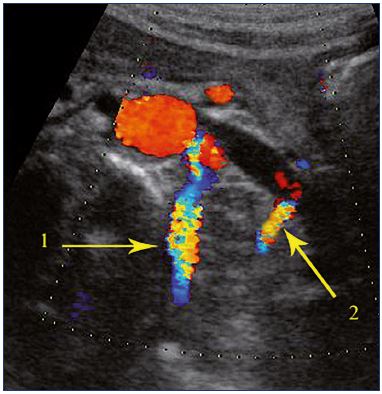
Figure 10. Abdominal approach.
Tight stenosis of the left renal vein in the aortomesenteric space.
Visualization speed velocities in the supplying venous ways. 1:
lumbar vein; 2: terminal part of the left gonadal vein.
Examination of the iliac vessels
At this step, the angiologist essentially searches for any postthrombotic residual sign or extrinsic venous compression. The most common venous compression is left common iliac vein compression. In its usual form, it is defined as the compression of the terminal part of the left common iliac vein between the right common iliac artery and the 5th lumbar vertebra associated with intraluminal fibrous spur-like bands. The challenge is to differentiate between a simple anatomical impression and a hemodynamic compression. When the later exists, it generates hypertension and stasis in the upstream venous network, thrombotic risk factors, and pelvic venous insufficiency. Symptomatic hemodynamic compressions generate specific ultrasound criteria: (i) slow speeds and decreased respiratory modulation of upstream venous flows, especially at the common femoral vein; (ii) a reversal flow in the ipsilateral internal iliac vein; and (iii) visualization of the draining network.
Search for leak points
The GS, GI, and O points are anatomically deep; therefore, they are rarely identified by direct ultrasound exploration. Conversely, varicose veins in the territory of their afferent veins are easy to identify and represent indirect signs of downstream truncal reflux:
1. The superior gluteal vein mainly drains gluteal muscles. Therefore, a truncal reflux of this vein feeds the buttock varices.
2. The gluteal inferior vein participates in the drainage of the buttocks, but also of the thigh and satellite veins of the sciatic nerve by its deep afferent vein. Gluteal varices associated with varices near the sciatic nerve are highly suggestive of an incontinent gluteal inferior vein.12
3. The obturator vein tributaries communicate with the medial circumflex veins that drain into the deep femoral vein or the femoral vein near the saphenofemoral junction. A preterminal reflux at the saphenofemoral junction, caused by Valsalva maneuver and fed by a medial afferent vein (but not by the lateral pudendal vein), should suggest an incontinence of the obturator vein. Of note, a reflux of the obturator vein can also feed the labial varicose veins by its genital afferent vein.
Conversely, the P, I, and C points are superficial and easily identified and localized by ultrasound investigation. Doppler ultrasonography is the most relevant investigation for identifying these leak points. The leak points must be characterized by their size and their hemodynamic status as mentioned above. If drainage is efficient, there will be no pelvic venous hypertension. This concept has two consequences:
1. Significant pelvic varicose veins, in terms of venous dilatation and reflux, can be asymptomatic at the pelvis if they are well drained. In other words, the absence of pelvic congestion syndrome is not a sufficient argument for not treating the pelvic varices.
2. Isolated ligation of a leak point,13 without treatment of their cause, does not seem an adequate response. This isolated ligation of a draining path can have two deleterious effects: (i) increases the intrapelvic venous pressure; and (ii) transforms asymptomatic well-drained pelvic varices into poorly drained pelvic varices, which become symptomatic varices. If the truncal reflux of the medial pudendal vein can no longer externalize after ligation of the leak point, it will feed Trendelenburg varices, vaginal and labial, and these veins are difficult or impossible to treat.
Conclusion
In the management algorithm of pelvic venous disorders, Doppler examination is the first-line imaging investigation to perform. It cannot identify all reflux, but is a very good tool for identifying pelvic varicose veins and highlights the essential pelvic leak points toward the lower limbs. It allows for the consideration of the main obstructive and supplying syndromes. In those cases, diagnosis will be confirmed by a second-line, cross-sectional imaging examination (angioMR, angioCT). The selective retrograde pelvic venography is the only exam that is able to achieve an accurate anatomic and hemodynamic mapping of the pelvic varicose veins, but it must remain a pretreatment exploration.
Acknowledgments
 We thank Doctor Jocelyne McGinnis, gynecologist at the American Hospital of Paris, for her linguistic assistance.
We thank Doctor Jocelyne McGinnis, gynecologist at the American Hospital of Paris, for her linguistic assistance.
REFERENCES
1. Hobbs JT. The pelvic congestion syndrome. Br J Hosp Med. 1990;43:200-206.
2. Villavicencio JL, Gillespie D, Durholt S, et al. Diagnosis and treatment of the pelvic venous disorders: pelvic congestion and pelvic dumping syndromes. In: Cann CC, ed. Surgical Management of Venous Disease. 1st ed. Williams and Wilkins: Baltimore, MD; 1997:462-483.
3. Perrin MR, Labropoulos N, Leon LR Jr. Presentation of the patient with recurrent varices after surgery (REVAS). J Vasc Surg. 2006;43(2):327-334.
4. García-Gimeno M, Rodríguez-Camarero S, Tagarro-Villalba S, et al. Duplex mapping of 2036 primary varicose veins. J Vasc Surg. 2009;49(3):681-689.
5. Greiner M. Syndrome de congestion pelvienne: diagnostic et traitement. Phlébologie. 2005;58:293-298.
6. Greiner M, Faye N, Thouveny F. Traitement endovasculaire par embolisation de l’insuffisance veineuse pelvienne chronique. In: Milka Greiner, ed. Thérapeutique Endovasculaire des Pathologies Veineuses. Springer-Verlag; 2012:236-264.
7. Franceschi C, Bahnini A. Treatment of lower extremity venous insufficiency due to pelvic leak points in women. Ann Chir Vasc. 2005;19:284-288.
8. Lechter A, Lopez G, Martinez C, Camacho J. Anatomy of the gonadal veins: a reappraisal. Surgery. 1991;109:735-739.
9. Arima M, Hosokawa S, Ogino T. Ultrasonographically demonstrated nutcracker phenomenon: alternative to angiography. Int Uro Nephrol. 1990;22:3-6.
10. Kim SH, Cho SW, Kim HD, et al. Nutcracker syndrome: diagnosis with Doppler US. Radiology. 1996;198:93- 97.
11. Takebayashi S, Ueki T, Ikeda N, et al. Diagnosis of the nutcracker syndrome with color Doppler sonography: correlation with flow patterns on retrograde left renal venography. Am J Roentgenol. 1999;172(1):39-43.
12. Lemasle P, Uhl JF, Lefebvre-Vilardebo M, Gillot C, Baud JM, Vin F. Veine du nerf sciatique et maladie variqueuse. Aspects écho-anatomiques et hémodynamiques. Phlébologie. 2001;54:219-228.
13. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
14. Franceschi C, Bahnini A. Treatment of lower extremity venous insufficiency due to pelvic leak points in women. Ann Vasc Surg. 2005;19:284-288.