Edema in venous insufficiency
Einar STRANDEN
Section of Vascular Investigations
Oslo Vascular Centre
Oslo University Hospital, Aker
ABSTRACT
Edema is a clinical state characterized by an accumulation of fluid in the interstitial or intracellular space. This accumulation develops when the net transcapillary filtration rate exceeds the lymphatic drainage rate over a period of time. In other words, increased filtration or reduced lymph flow or both. The present chapter focuses on the lower limb edema frequently associated with venous insufficiency. Because the key pathophysiological factor behind this edema is increased distal venous pressure in the upright position, much attention is given to the mechanisms leading to venous hypertension. Furthermore, because understanding edema mechanisms requires knowledge of the factors acting on transcapillary fluid balance, a basic review of these and how they may be measured experimentally is included.
TRANSCAPILLARY FLUID BALANCE
Fluid exchange between the intra- and extravascular space takes place across the capillary wall. This structure is considered to be semipermeable: impermeable to plasma proteins and freely permeable to water and lowmolecular- weight solutes. The interstitial fluid volume (IFV) is normally kept within narrow limits. Edema is likely due to an imbalance in the hydrostatic and colloid osmotic forces across the capillary wall, resulting in net transcapillary filtration exceeding lymphatic flow.1 Net transcapillary filtration (F) (see “Revision of the Starling principle” at the end) is classicaly described by the so-called Starling equation:
where CFC is the capillary filtration coefficient, which expresses the capillary permeability, ie, capillary “leakiness”. CFC is the product of capillary hydrodynamic conductivity (Kf) and the available capillary surface area (SA). If the gaps between the endothelial cells widen, then Kf increases, and SA increases if an increased number of capillaries are perfused. An increased CFC thus increases the rate of capillary filtration at a given net filtration pressure.
Pc and Pif are the hydrostatic pressures of the capillaries and interstitial fluid, respectively (Figure 1). COPpl and COPif are the colloid osmotic pressures of plasma and interstitial fluid. The colloid osmotic pressures are caused by proteins, mainly albumin. Sigma (σ) is the capillary reflection coefficient, expressing how efficiently the capillary wall is creating an osmotic pressure. For a capillary exchange system that is impermeable to proteins, σ = 1. If proteins pass freely, no osmotic pressure gradient is created and σ = 0. In subcutaneous tissue σ is probably between 0.9 and 1.0.2,3
Since the interstitial fluid volume is normally kept fairly constant, this implies that lymph flow (Jl) balances net transcapillary filtration (F).
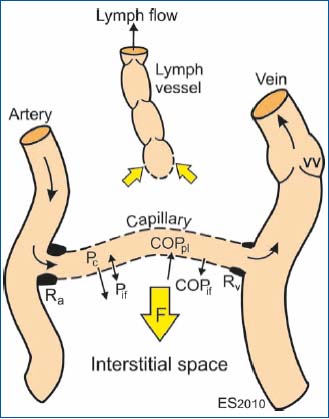
Figure 1. Schematic illustration of the factors involved in
transcapillary fluid transport. Ra and Rv are the pre- and
postcapillary vascular resistances, Pc and Pif are the hydrostatic
pressures in the capillary and the interstitial fluid, respectively,
COPpl and COPif are the colloid osmotic pressures of plasma and
interstitial fluid. VV denotes venous valve, and F is the net
filtration of fluid.
The tissue pressure (Pif) in normally hydrated leg subcutaneous tissue is weakly negative, but may increase to a few mm Hg in subcutaneous edema (Figure 1). In a study on patients with post-reconstructive edema, we found a low subcutaneous Pif, never above 5 mm Hg, even if subcutaneous tissue volume rose by more than 600% (Figure 2B). This indicates a very high compliance of that tissue, especially following the initial steep rise.4 When edema develops in muscle tissue of the legs, however, the pressure may rise from normally a few mm Hg to well above 50 mm Hg due to the non-elastic fasciae limiting tissue expansion. At these high pressures the risk of tissue necrosis is elevated (ie, compartment syndrome). During volume expansion the two compartments thus behave very differently, as indicated in Figure 2B. In this illustration the curve for subcutaneous tissue is based on our study of patients with post-reconstructive edema4 and the intramuscular pressure on our studies on patients with deep venous thrombosis.5
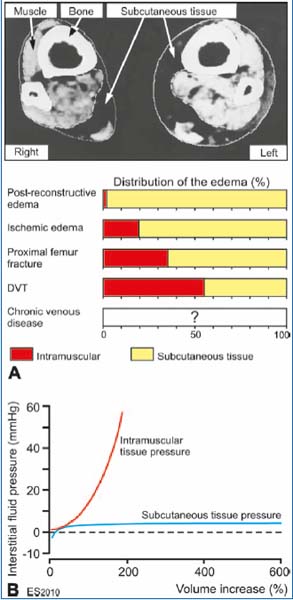
Figure 2. A. Distribution of edema within subcutaneous and
muscle tissue measured from CT scans of the legs in different types
of edema. The depicted cross-sectional CT scan was taken proximal
to the ankle in a patient with mainly subcutaneous edema on the
left side. B. Very different pressure/volume curves (compliance) are
seen in muscle and subcutaneous tissues. For more explanation, see
text.
Figure 2A indicates the distribution of edema between muscle and subcutaneous tissue from our previous studies on patients with post-reconstructive edema,6 critical ischemic edema,7 edema following proximal femur fracture (manuscript in preparation), and leg edema in deep venous thrombosis.8 The studies were based on CT scans proximal to the ankle () and calf. From these scans, for unilateral edema, subcutaneous and intramuscular swelling could be calculated by planimetry and later by computer area measurement, as the difference between the edematous and the contralateral extremity. There was an increasing fraction of muscular edema from the post-reconstructive edema (2%) to deep vein thrombosis legs (55%) (Figure 2A). The higher fraction of intramuscular edema in deep vein thrombosis renders these patients more likely to develop high intramuscular pressure, which may provoke compartment syndrome (ie, intramuscular pressure >30 mm Hg), especially in the early edema forming phase.5 In this group intramuscular Pif in some patients was above 50 mm Hg, but was reduced in the edema declining phase.
To the author’s knowledge, systematic studies of edema distribution in patients with venous insufficiency have not yet been carried out. Such studies are desirable, because the filtration load in the upright position may be very large. Vaughan9 did, however, report subcutaneous edema on CT scans in four patients with isolated superficial venous insufficiency. One might expect, though, that deep venous insufficiency represents a greater challenge in edema formation than a superficial disorder alone, because in the latter group the deep venous pump system may be partly intact.
INFLAMMATORY SWELLING
Recent research suggests the presence of inflammation in legs with venous insufficiency, probably caused by the long-lasting venous hypertension in the upright position. The inflammation may be responsible for remodeling of the venous wall and valve restructuring.10 Hemodynamic forces such as blood pressure elevation and mechanical stretching of the venous wall may activate both leukocytes and endothelial cells. Membrane adhesion molecules then facilitate adhesion of leukocytes and their transmigration through the vessel wall into the inflamed tissue. This leukocyte infiltration is followed by remodeling of the extracellular matrix, which again is responsible for the destruction of valves.10
Inflammation also opens the gaps between the endothelial cells. Gap formation is most likely caused by contraction of actin and myosin filaments within the endothelial cells. The gap opening may become very large, greatly enhancing the hydraulic conductance of fluid. It also raises the permeability to plasma proteins into the interstitial space, which reduces the gradient of COP that opposes filtration. In addition, increased gap openings reduce the capillary wall protein reflection coefficient (σ) to around 0.4, which further reduces the effective COP gradient (Figure 1).11
MEASUREMENT OF THE “STARLING COMPONENTS”
Measurement of interstitial fluid colloid osmotic pressure (COPif)
The collection of interstitial fluid for protein analysis represents a challenge. We have used three approaches: 1) Direct sampling by catheters, 2) Technique based on fluid equilibrium (“wick method”, Figure 3A), and 3) Blister method (Figure 3B).
The wick method (approach 2) is based on equilibrium between saline-soaked nylon threads (0.8 mm thick, 210 filaments) sewn subcutaneously at the antero-lateral part of the leg and left in place for one hour. During that time the wick equilibrates osmotically with the surrounding fluid, but does not reflect the true protein composition of interstitial fluid as the insertion trauma causes considerable efflux of proteins during the first 30 min. The wicks are then pulled out, protruding ends cut off, quickly transferred to centrifugation tubes filled with mineral oil and centrifuged. The small sample (2-10 μL) is then transferred to an osmometer developed by the author for small samples (“OncoLab”, Figure 3C). The osmometer was built with a dialyzing membrane with a cut-off of 30 000 Daltons, similar to that of the endothelial cells of leg capillaries.
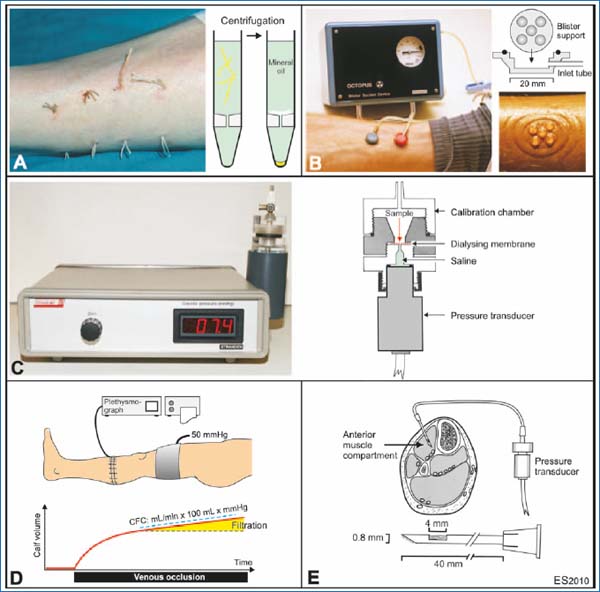
Figure 3. Methods for studying “Starling components”. Interstitial fluid for protein analysis may be collected by either the wick method A or the blister method B, blister suction device, Stranden). C. Colloid osmotic pressure in plasma and interstitial fluid is measured by a specially designed device (OncoLab, Stranden). D. Plethysmographic determination of the capillary filtration coefficient (CFC).
E. Subcutaneous and intramuscular pressure may be measured using the “wick-in-needle” technique.
Interstitial fluid may be collected by a blister technique (approach 3) as described by Kiistala and Mustakallio.12 Subatmospheric pressure was obtained by a manually working pump (blister suction device, developed by the author) with two suction cups made of PVC. The 20 mm wide suction cups each has five concave 5 mm holes into which the blisters are formed. Blisters normally appear after 60-90 minutes of suction with a subatmospheric pressure of 200 mm Hg. After puncture with a needle the blister fluid (approx. 20 μL) is collected in unheparinized glass capillaries and later transferred to the osmometer mentioned above.
Blood sampling and plasma colloid osmotic pressures (COPpl)
Blood from antecubital veins can be collected for analysis. Following centrifugation, colloid osmotic pressure of plasma is measured by the osmometer technique described above.
Measurement of capillary filtration coefficient (CFC)
CFC may be measured by plethysmography (Figure 3D). A venous occlusion cuff is applied proximal to the measuring site. Cuff pressure of 50 mm Hg (or stepwise increase in pressures) is maintained during the measurement. This permits unrestricted arterial flow into the limb while venous outflow is compromised, resulting in an increased leg volume. The volume curve reaches a steady state after approximately 3 minutes. The initial, relatively steep part of the calf volume recording coincides with the filling of veins. After the volume curve flattens, a secondary very small, but distinct increase in volume is measured, which signifies a limb volume increase due to transudation of fluid through the capillary wall. This increase in volume over time denotes CFC, and is expressed as mL/min · 100 mL of tissue · mm Hg increase in filtration pressure.
We have measured CFC for different types of lower limb edemas as listed below. The values refer to contralateral side, or healthy controls in bilateral edema:

There seem to be no studies so far focusing on CFC for patients with venous insufficiency. Capillaroscopy studies using a tracer technique have, however, revealed increased permeability in toe nailfold capillaries in patients with venous insufficiency.
Interstitial fluid hydrostatic pressure (Pif)
The interstitial fluid pressure may be measured by the “wick-in-needle” technique. The method is based on fluid equilibrium between a pressure transducer and the interstitium (Figure 3E). Hypodermic needles (0.8 mm OD, 40 mm length) are provided with a 4 mm long sidehole approximately 7 mm from the tip. The needles are filled with cotton thread and sterilized by autoclave. The thread provides a continuous water connection between tissue and needle lumen. The pressure is adjusted to zero before insertion and checked after each measurement. By this technique Pif may be measured in both subcutaneous and intramuscular tissue.
Capillary pressure (Pc)
Blood flow through the capillaries is primarily regulated by variation in the arteriolar/precapillary resistance (Figure 1, Ra). This variation in vasoconstriction also influences capillary pressure, Pc. Vasoconstriction reduces pressure; the pressure increases during dilatation. Hence, Ra affects transcapillary filtration— higher pressure means higher filtration.
On the venous side of the capillary there is another site for adjusting vascular resistance Rv (Figure 1). This is smaller than Ra, but contributes to the regulation of filtration. When this resistance is high, the capillary pressure tends to rise, much the same way as the pressure build-up within a garden hose whose outlet is squeezed. Mean capillary pressure thus depends on the balance between the two, the pre- to postcapillary resistance ratio (Ra/Rv). Ra is typically four times larger than Rv and capillary pressure is therefore more sensitive to changes in venous pressure than arterial pressure.18 This is why venous obstruction affects filtration rate so markedly (eg, deep vein thrombosis).
Increase in Ra/Rv is an important edema-limiting mechanism when standing up. Arteriolar constriction then normally limits the increase in capillary pressure to about 2/3 of the increase in arterial pressure because of the very potent veno-arteriolar response (VAR). There have been reports that the VAR is reduced in patients with chronic venous insufficiency,19 which may in itself contribute to the formation of edema, in these patients.
The capillary pressure is not readily available in a clinical setting. However, because of the low post-capillary resistance, the level of venous pressure may to some extent mimic capillary pressure. High venous pressure is transmitted retrogradely to the capillaries; low venous pressure permits Pc to remain relatively low. Because of the key role of Pc in influencing transcapillary filtration, and its dependency on venous pressure, a large part of this chapter is about the venodynamics of the lower limb in healthy limbs and in venous insufficiency.
VENODYNAMICS OF THE LOWER LIMB
The venous system in the lower limb is composed of a subcutaneous superficial system, a deep system within the muscular fasciae, and connecting perforating veins. Dysfunction, mainly of the valves, may occur in each system and in combination. The great variability in venous anatomy and function makes pathophysiological understanding rather complex. The following description of four clinical conditions is therefore simplified. The pressure and flow curves of the examples summarize numerous published studies, eg, studies of venous pressure by CC Arnoldi,20-27 the studies of pressure and flow by RI Bjordal,28-34 and noninvasive investigations.
THE VENOUS PUMPS IN THE LOWER LIMB
In the upright position a significant amount of blood is translocated to the lower extremity veins. During quiet standing, the muscles in the lower extremity contract and relax rhythmically, causing a swaying motion of the body. During muscular contraction blood is squeezed in a proximal direction, and the veins are refilled during the relaxation phase. This cyclic muscular action and the venous valves form a powerful pumping system aiding the venous return to the heart.35 The return of blood from the extremity does not totally depend upon properly functioning pumps; cardiac activity alone is sufficient to maintain return flow (vis-a-tergo blood flow). The pump system is, however, of vital importance in preserving the integrity of the microcirculation, by reducing distal capillary pressure when standing.36
Pumping occurs in all veins containing valves and is subject to oscillating surrounding pressure. Even without functioning venous valves, leg motion, by virtue of venous compression, promotes venous return.36 The venous pumping system may be divided into three portions with different working mechanisms (Figure 4):
1. The muscle pumps27
2. The distal calf (“piston”) pump35-37
3. The foot pump35, 38-40
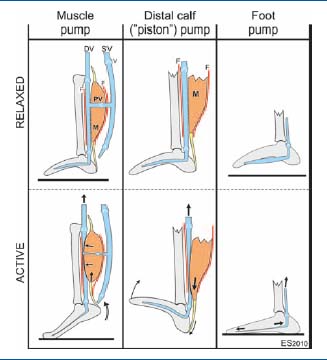
Figure 4. Schematic illustration of the venous pump systems of
the foot and calf in the relaxed and active states. The muscle pump
unit consists of muscles (M) ensheathed by a common fascia (F)
and veins within the same compartment. Contraction of the calf
muscles (muscle systole), as in plantar flexion of the ankle joint
during walking (below), expels blood into the proximal collecting
vein. During relaxation (muscle diastole, above) the blood is
drained from the superficial veins (SV) into the deep veins (DV) in
addition to the arterial inflow, making the pump ready for the
subsequent ejection. V: venous valve. The distal calf (“piston”)
pump is indicated in the middle. On dorsiflexion of the ankle
(passive or active), the bulk of the calf muscle (M) descends within
the fascial sheath (F), and expels blood in the distal veins like a
piston. The foot vein pump is illustrated on the right. The plantar
veins are connected like a bow-string from the base of the fourth
metatarsal in front to the medial malleolus. On weight-bearing the
tarso-metatarsal joints are extended and the tarsal arch is
flattened. Thus the veins are stretched, causing them to eject their
blood content.
During normal walking the three vein-pumping systems are synchronized to form a complete network of serial and parallel pumps aiding the return of blood towards the heart. The mechanism may be summarized as:
1. Before weight-bearing the ankle is dorsiflexed, emptying both the anterior muscle compartment (muscle pump) and the distal calf (“piston” pump).
2. At weight-bearing the foot veins are emptied (foot pump).
3. The plantar flexion of the foot to ensure forward locomotion activates the proximal calf pump of the posterior muscles (muscle pump).
Normal venodynamics
In the upright position, the hydrostatic vascular pressure is greatly increased in the lower part of the body. The increase is similar in arteries and veins, and should per se have little effect on the overall blood flow through the lower limb. However, the very potent veno-arteriolar response initiated by distension of veins, at transmural pressures above 25 mm Hg, causes arteriolar vasoconstriction which reduces blood flow in the dependent limb by approximately 50%.
In passive dependent legs, the pressures in all veins at the same height are equal, and are approximately equal to (slightly higher than) the hydrostatic fluid pressure in a column of blood from the point of measurement to the level of the heart. In this state, phlebography indicates that blood returns to the heart through both deep and superficial veins (Figure 5).27
Muscle contraction (systole) at weight-bearing is accompanied by a rise in pressure in all veins of the limb (Figure 5). Within the muscular compartment the increase is largest, typically 60-70 mm Hg, three times higher than the rise in superficial veins. During systole the muscle contraction may cause venous outflow obstruction, further enhancing deep systolic vein pressure. In extreme cases the pressure is raised by more than 200 mm Hg in a fraction of a second.27 The systolic venous pressure increase in the collecting conduits is smaller (appr. 20 mm Hg, popliteal vein), and the resulting pressure gradient rushes blood from calf to thigh. Competent valves prevent distal flow or outward flow through the perforators. In addition, the higher deep venous pressure does not allow inward flow through the perforating veins during systole.
During muscle relaxation (diastole) the pressure falls below that at rest, especially in the deep veins (Figure 5), ensuing an inward flow through the perforating veins. In healthy subjects patent vein valves prevent flow in the distal direction in both deep and superficial veins.
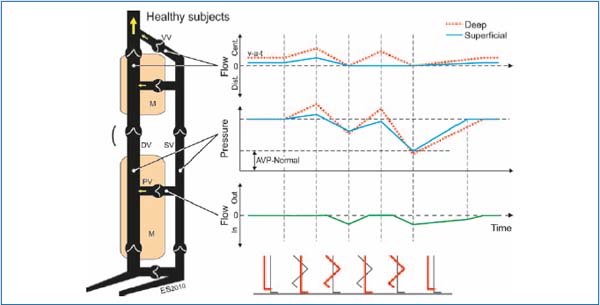
Figure 5. Schematic representation of normal anatomy and dynamics of lower extremity veins. The simplified venous system consists of superficial veins (SV), deep veins (DV) within muscular compartments (M) of the calf and thigh, and perforating veins (PV). Venous valves (VV) ensure unidirectional flow of blood in central (cent.) direction in deep and superficial veins, and inward direction in the perforating veins. The diagram on the right depicts idealized pressure and flow characteristics of different areas of the veins during steady state at passive dependency (the leftmost walking-phase symbol) and two subsequent walking cycles—during weight-bearing (muscle systole) and elevation of the leg (muscle diastole). The red line indicates the extremity described. In a passive relaxed state the blood is forced almost solely through the deep system by the pumping action of the heart, often referred to as the vis-a-tergo (v-a-t) blood flow. AVP-Normal is the normal ambulatory venous pressure in superficial veins.
In repeated muscle contractions, as in normal walking, the systolic pressures in the deep and superficial veins will gradually fall and fluctuate at levels considerably below the pressures during a single contraction (Figure 5). The superficial venous pressure in the ankle region during walking is typically 30 mm Hg and is referred to as the ambulatory venous pressure (AVP).
Superficial and perforator dysfunction
Relatively few patients referred to hospital have dysfunction of superficial veins combined with normal valvular function in the perforating veins. In this group the calf vein pumps are normal and ambulatory venous pressure in the deep veins at the ankle is low, which explains the absence of edema and trophic changes in the skin.27
Most patients with venous dysfunction have incompetent valves in both superficial and perforator veins. This causes a significant reflux in the superficial vein trunk and a smaller reduction in superficial venous pressure than normal, often referred to as “ambulatory venous hypertension”. Although the venous pumps may be normal, the pressure in the deep veins is rapidly restored to the blood column pressure upon standing, because of backfill from the superficial veins (Figure 6). The clinical picture is varicose veins and sometimes leg edema, often in combination with trophic changes. The more extensive and the more distal the venous reflux, the greater the probability of ulcer formation.
In steady state at passive dependency blood flows primarily through the deep veins, and the pressure in the veins corresponds, as in healthy subjects, to the hydrostatic pressure from the blood column to the heart. Consequently, the pressure at rest is not affected by valvular dysfunction. Weight-bearing with muscular contraction causes a steep rise in deep venous pressure, as in healthy subjects. The increase in superficial venous pressure is considerably higher than normal, due to the extensive retrograde flow from the deep venous system through incompetent dilated perforating veins in muscle systole.
During relaxation the pressure in the muscular deep veins falls abruptly, and to a larger extent than in popliteal and superficial veins. This causes an inward flow through the perforating veins, whereas reflux from the popliteal vein is prevented by valves. The absence of competent valves in the superficial system allows retrograde flow, most often through the saphenofemoral junction. Bjordal28 quantified this reflux in the superficial main trunk during normal walking as an average of 300 mL/min, and thus verified the hypothesis of “a private circulation” as suggested by Trendelenburg. According to his finding a large fraction of the blood from the deep venous trunk is “spilled” through incompetent superficial veins and re-enters the deep veins at a lower part of the limb.
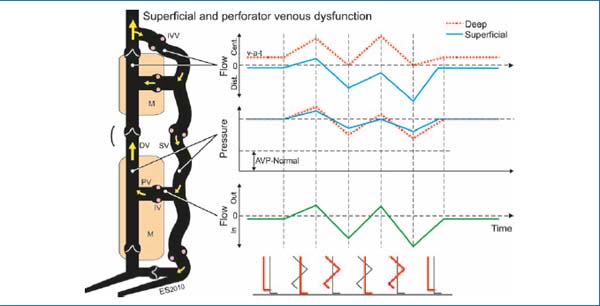
Figure 6. Schematic representation of patients with superficial and perforator venous dysfunction. The most striking difference from healthy subjects is the distal (dist.) blood flow in the superficial veins due to incompetent venous valves (IVV). During ambulation there is oscillating flow in incompetent perforators, outward during muscle contraction and inward at muscle relaxation. Furthermore, the state is characterized by less reduction in venous pressure in superficial veins during walking (“ambulatory venous hypertension”). Annotations are as in Figure 5.
The high retrograde flow in superficial veins during walking refills the deep veins during muscle diastole, greatly enhancing the venous pump capacity by increasing the expelled volume. The net increase in expelled volume is, however, due to the superficial retrograde circuit and does not represent effectively increased drainage from the extremity. The result of this rapid back-flow is that the systolic pressures in the deep and superficial ankle veins remain high during walking.
Proximal occlusion of the superficial veins normalizes ambulatory venous pressure in these veins and the pressure recovery time after standstill. This effect is the dynamic basis for the detection of superficial venous dysfunction by venous pressure measurements. The pressure test does not, however, assess the patency of the perforating veins.
Combined superficial, perforator, and deep dysfunction
The deep, perforating, and superficial veins of the leg may all be more or less involved in skin ulcer formation. Deep venous incompetence is usually secondary to previous deep venous thrombosis, although venous dilatation and subsequent valvular insufficiency may also be the result of increased pressure and flow load from isolated superficial insufficiency. The latter condition may be reversed following treatment of the superficial veins. The venodynamics are characterized by ambulatory venous hypertension in both superficial and deep veins. In this state the capillary pressure in the upright position is high, the only relief being elevation of the legs.
During walking, the pressures in superficial and deep veins oscillate around the level during passive standing, ie, with minimal net reduction in ambulatory venous pressure (Figure 7). Flow in perforating veins is bidirectional, with an outward net flow, as opposed to the situation with superficial and perforator incompetence only (Figure 6). The flow in superficial veins may be bidirectional, without net flow, or a net flow directed centrally or distally.
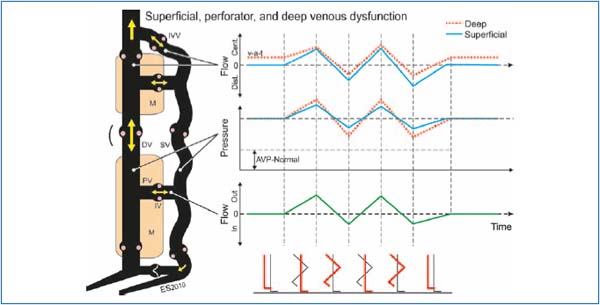
Figure 7. Venodynamics in patients with superficial, perforator, and deep venous dysfunction. During walking, the pressures in superficial and deep veins oscillate around the pressure in the passive upright position, ie, with minimal net reduction in ambulatory venous pressure. Flow in perforating veins is bi-directional, with outward net flow, the opposite of what is found in patients with superficial and perforator incompetence only (Figure 6). Annotations are as in Figures 5 and 6.
Outflow obstruction
Venous outflow obstruction may be the result of occluded or partially recanalized veins subsequent to deep vein thrombosis. In proximal (outflow) venous thrombosis, increased outflow resistance and venous pressure during muscle contraction may lead to venous claudication (Figure 8).
Ambulatory venous hypertension often leads to distension of the perforators and valve dysfunction. The pressure and flow is then directed towards the superficial veins, which may become the principal venous conduits. A resulting overload of the superficial veins may lead to dysfunction, including varicose veins. Figure 8 shows mean pressure curves during and after ambulation in the four states listed. The ambulatory venous pressure typically increases from healthy subjects to patients with superficial and perforator dysfunction to those with additional deep venous dysfunction and to those with deep venous obstruction. These venous pressure profiles, along with the recovery times (time from end of walking until the vein pressure reaches the level of passive dependency), with and without superficial venous occlusion (occlusion test), form the diagnostic basis of venous pressure measurement.
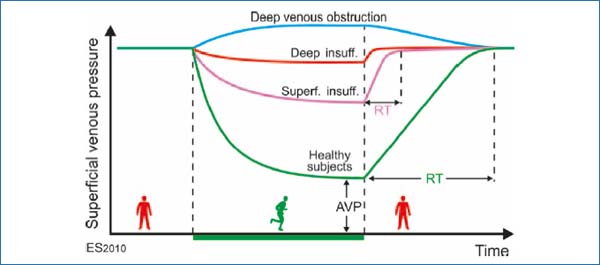
Figure 8. Schematic illustration of the superficial venous pressure at rest, and during ambulation. The ambulatory venous pressure (AVP) represents the lowest mean pressure during walking at the site of measurement, and the recovery time (RT) is the time interval between the termination of walking until the vein pressure reaches the pressure level at passive dependency. In healthy subjects, AVP at the distal calf is about 30 mm Hg and RT is 20-30 s.
REVISION OF THE STARLING PRINCIPLE
This traditional form of Starling’s principle has recently been challenged. In a recent review article,41 Levick and Michel argue that sustained fluid absorption into the capillaries from the interstitium does not normally take place (except in a few specialized regions like the kidneys and intestines). Rather there is merely a unidirectional fluid shift from the capillaries to the lymphatics via the interstitium. The structural basis for this idea is the endothelial glycocalyx small pore system covering the relatively wider intercellular clefts that form the semipermeable membrane of the capillary wall. Hence the area for colloid osmotic pressure build-up outside the capillaries is not in the interstitium; it lies within the intercellular clefts. This has important functional consequences. During filtration, the interstitial proteins that may previously have entered the intercellular clefts are washed into the interstitium, effectively reducing the COP just underneath the glycocalyx to a very low level and rendering it insignificant in the fluid balance, and the filtration force is reduced. In the opposite situation, during the initial phase of absorption, interstitial proteins are trapped in the cleft, like in a sieve, thus building up a large COP which reduces and may stop the fluid transport. These new ideas challenge the relevance of the COPif presented in studies so far.
ACKNOWLEDGEMENT
This paper is based on the book chapter:42 StrandenE. Edema in venous insufficiency. In: Wittens C, ed. Best Practice in Venous Procedures. Turin, Italy: Edizioni Minerva Medica; 2010:131-140. The permission given by the editor is highly appreciated.

REFERENCES
1. Starling EH. On the absorption of fluids from the connective tissue spaces. J Physiol. 1896;19:312-326.
2. Aukland K, Nicolaysen G. Interstitial volume: Local regulatory mechanisms. Physiol Rev. 1981;61:556-643.
3. Taylor AE, Granger DN, Brace RA. Analysis of lymphatic protein flux data. 1. Estimation of the reflection coefficient and permeability surface area product for total protein. Microvasc Res. 1977;13:297-313.
4. Stranden E, Myhre HO. Transcapillary forces in patients with lower limb ischemia. Scand J Clin Lab Invest. 1983;43:233-239.
5. Seem E, Stranden E. Transcapillary forces in muscle compartments of lower limbs with deep venous thrombosis. Scand J Clin Lab Invest. 1990;50:325-330.
6. Stranden E, Enge I. Computed tomography in the investigation of leg edema following arterial reconstruction. Europ J Radiol. 1982;2:113-116.
7. Khiabani HZ, Anvar M, Rostad B, Stranden E, Kroese AJ. The distribution of edema in the lower limb of patients with chronic critical limb ischemia (CLI). A study with computed tomography (CT). VASA. 1999;28:265- 270.
8. Seem E, Stranden E, Stiris MG. Computed tomography in investigation of leg edema in limbs with deep venous thrombosis. Europ J Radiol. 1985;26:727- 730.
9. Vaughan BF. CT of Swollen Legs. Clinical Radiology. 1990;41:24-30.
10. Pascarella L, Penn A, Schmid- Schönbein GW. Venous hypertension and the inflammatory cascade: major manifestations and trigger mechanisms. Angiology. 2005;56:S3-S10.
11. Levick JR. An introduction to Cardiovascular Physiology. Oxford, UK: Butterworth-Heinemann Ltd.;1991:142-170.
12. Kiistala U, Mustakallio KK. Dermoepidermal separation with suction. Electron microscopic and histochemical study of initial events of blistering on human skin. J Invest Dermatol. 1967;48:466-477.
13. Stranden E. Transcapillary fluid filtration in patients with leg edema following arterial reconstructions for atherosclerosis. VASA. 1983;12:219- 224.
14. Seem E, Stranden E. Transcapillary filtration in lower limbs with deep venous thrombosis; the role of the capillary filtration coefficient. Scand J Clin Lab Invest. 1990;50:331-336.
15. Stranden E. Is increased capillary leakage per se a possible mechanism in idiopathic leg edema? (In Norwegian). Vitenskapelige Forhandlinger. 1992; Abstract 179.
16. Anvar MD, Khiabani HZ, Kroese AJ, Stranden E. Alterations in capillary permeability in the lower limb of patients with chronical limb ischaemia and Edema. VASA. 2000;29:106-111.
17. Kazmi SSH, Stranden E. Pathophysiological aspects of lower limb Edema in patients with proximal femoral fractures. Scand J Clin Lab Invest. 2009;69:741-747.
18. Pappenheimer JR, Soto-Rivera A. Effective osmotic pressure of the plasma proteins and other quantities associated with the capillary circulation in the hindlimbs of cats and dogs. Am J Physiol. 1948;152:471-491.
19. Belcaro GV, Nicolaides AN. Acute effects of intermittent sequential compression in venous hypertension. J Cardiovasc Surg (Torino). 1993;34:493-497.
20. Arnoldi CC. Venous pressure in patients with valvular incompetence of the veins of the lower limb. Acta Chir Scand. 1966;132:628-645.
21. Arnoldi CC. The venous return from the lower leg. A synthesis. Acta Orthop Scand. 1964;(Suppl. 64):1-75.
22. Arnoldi CC. Venous pressure in the leg of healthy human subjects at rest and during muscular exercise in the nearly erect position. Acta Chir Scand. 1965;130:570-583.
23. Arnoldi CC. The influence of posture upon the pressure in the veins of the normal human leg at rest and during rhythmic muscular exercise. Acta Chir Scand. 1966;131:423-431.
24. Arnoldi CC, Greitz T, Linderholm H. Variations in cross-sectional area and pressure in the veins of the normal human leg during rhythmic muscular exercise. Acta Chir Scand. 1966;132:507.
25. Arnoldi CC, Linderholm H. Venous blood pressures in the lower leg at rest and during exercise in patients with idiopathic dysfunction of the venous pump of the calf. Acta Chir Scand. 1969;135:601-609.
26. Arnoldi CC, Linderholm H. Intracalcanean pressure in various forms of dysfunction of the venous pump of the calf. Acta Chir Scand. 1971;137:21-27.
27. Arnoldi CC. Physiology and pathophysiology of the venous pump of the calf. In: Eklöf B, Gjöres JE, Thulesius O, Bergqvist D, eds. Controversies in the management of venous disorders. London: Butterworths; 1989:6-23.
28. Bjordal RI. Simultaneous pressure and flow recordings in varicose veins of the lower extremity. Acta Chir Scand. 1970;136:309-317.
29. Bjordal RI. Pressure patterns in the saphenous system in patients with venous leg ulcers. Acta Chir Scand. 1971;137:495-501.
30. Bjordal RI. Circulation patterns in incompetent perforating veins on the calf and in the saphenous system in primary varicose veins. Acta Chir Scand. 1972;138:251-261.
31. Bjordal RI. Blood circulation in varicose veins of the lower extremities. Thesis, Universitetsforlaget, Oslo 1973.
32. Bjordal RI. Haemodynamic studies of varicose veins and the postthrombotic syndrome. In: JT Hobbs, ed. The Treatment of Venous Disorders. Lancaster, UK: MTB Press Ltd.;1977:37-55.
33. Bjordal RI. Die Zirkulation in insufficienten V.V. perforantes der Wade bei venösen Störungen. Klinische und Therapeutische Konsequenzen der beobachten hæmodynamischen Strömungsmuster. In: R May, H Partsch, J Staubesand eds. Venae Perforantes. München, Germany: Urban and Schwarzenberg; 1981:71-93.
34. Bjordal RI. Pressure and flow measurements in venous insufficiency of the legs. In: Eklöf B, Gjöres JE, Thulesius O, Bergqvist D, eds. Controversies in the management of venous disorders. London: Butterworths; 1989:24-35.
35. Gardner AMN, Fox RH. The return of blood to the heart: venous pumps in health and disease. London, UK: John Libbey & Co. Ltd.; 1989.
36. Thulesius O. Vein wall characteristics and valvular function in chronic venous insufficiency. Phlebology. 1993;8:94-98.
37. Gershuni DH, Yaru NC, Hargens AR et al. Ankle and knee position as a factor modifying intracompartmental pressure in the human leg. J Bone Joint Surg. 1984;66A:1415-1420.
38. Le Dentuy A. Circulation veineuse du pied et de la jambe. Paris, France: Adrien Delahaye, 1867.
39. Gardner AMN, Fox RH. The venous pump of the human foot. Bristol Med- Chir J. 1983;98:109-112.
40. Gardner AMN, Fox RH. The return of blood to the heart against the force of gravity. In: Negus D, Jantet G eds. Phlebology ‘85. London, UK: John Libbey; 1986:56-67.
41. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198- 210.
42. Stranden E. Edema in venous insufficiency. In: Wittens C ed. Best Practice in Venous Procedures. Turin, Italy: Edizioni Minerva Medica; 2010:131-140.
