Effectiveness of micronized purified flavonoid fraction–based conservative treatment in chronic venous edema
Vadim Yu BOGACHEV, MD, PhD
investigators group.
Department of Faculty Surgery No. 2,
Pirogov Russian National Research
Medical University (RNRMU), Moscow,
Russia
Abstract
Aim: This study assessed the effectiveness of micronized purified flavonoid fraction (MPFF)-based conservative treatment in patients with chronic venous edema (CVE) as part of an observational program that evaluated the management of patients with CVE caused by the primary forms of chronic venous disease (CVD) in real clinical practice. Materials and methods. The VAP-C3 (Vein Act Prolonged-C3; NCT03722836) prospective, single-arm, observational study was conducted in Russia in adult outpatients with CVD of CEAP (clinical-etiological-anatomical-pathophysiological) class C3EpAsPr (CVE). Patients’ CVD symptoms, symptom severity, characteristics and location of edema, and ankle volume were recorded. Patients were treated by medical specialists according to conventional clinical practice and received compression and/or phlebotropic therapy with/ without surgical intervention and returned for follow-up visits. Primary efficacy end points were changes in severity of main CVD symptoms (by visual analog scale) leg heaviness, leg pain, and sensation of leg swelling, ankle volume (by disc-model method), and quality of life (QOL) parameters of the disease-specific questionnaire CIVIQ-14. This analysis is focused on the effectiveness of MPFF-based conservative treatment in patients with CVE. Results. VAP-C3 enrolled a total of 708 patients, including 176 (24.86%) males and 532 (75.14%) females; mean age was 48.6±12.6 years, with 25.56% of participants older than 65 years of age; 64.8% had a body mass index ≥ 25 kg/m2, and 61.30% had a family history of CVD. Mean study duration was 2.5±0.5 months. With MPFF-based conservative treatment, there were significant improvements in the main CVD symptoms such as leg heaviness, pain, and swelling and in CIVIQ-14–assessed QOL, and significant reduction in ankle volume. In comparative intergroup analysis, the reductions in ankle volume with MPFF-based conservative therapy and such therapy together with surgical intervention did not differ, whereas CIVIQ-14– assessed QOL was significantly improved when MPFF-based conservative therapy was used in combination with surgical intervention. Conclusion. MPFF-based conservative treatment, irrespective of addition of surgical intervention, was associated with a significant reduction in the ankle volume in patients with CVD of CEAP class C3EpAsPr. The antiedematous effect of conservative therapy with MPFF alone or in combinations including compression therapy suggests that it is reasonable to consider predominant use of MPFF in routine clinical practice in patients with CVD of CEAP class C3.
Introduction
Chronic venous edema (CVE) of the lower limbs, defined as a visible or palpable increase in the volume of interstitial fluid due to chronic venous disease (CVD), is the main objective criterion of the CEAP (clinical, etiological, anatomical, pathophysiological) clinical class C3 and also reflects the transition of the disease to a difficult-to-reverse stage of chronic venous insufficiency (CVI).1 The prevalence of CEAP class C3 CVD not only reflects the epidemiological situation of CVD, but also identifies the potential risks of trophic skin disorders and venous ulceration.2
The prevalence of CVE in the population is not clearly established and can vary significantly (from 7% to 20%) depending on the assessment method, age, and ethnic characteristics of the respondents, as well as circadian rhythms.3
Recent studies have shown that the development and progression of CVE is a complex pathophysiological process, caused not only by severe macro- and microcirculatory disturbances in the venous bed, but also by a significant deterioration in lymphatic drainage.4
CVE worsens the quality of life (QOL) of patients, causes technical issues during surgical interventions, and also increases the risk of adverse effects after surgery. In addition, it has been shown that even radical surgical intervention does not guarantee elimination or reduction in CVE.
For this reason, the complex conservative therapy, either as standalone method or in combination with surgery plays a crucial role in CVE treatment. According to the International Union of Phlebology (UIP) guidelines, a conservative approach in CVE is based on compression therapy and venoactive drugs (VADs). Other therapeutic techniques, such as intermittent pneumatic compression, neuromuscular electrical stimulation, and unloading exercises, play a secondary role.5
This publication presents an analysis focusing on the effectiveness of micronized purified flavonoid fraction (MPFF)-based conservative treatment in patients with CVE and is based on the results of the Russian national multicenter observational program Vein Act Prolonged-C3 (VAP-C3; an extension of the VEIN Act Program6), which was designed to evaluate treatment effectiveness on CVE in real clinical practice.
Materials and methods
The VAP-C3 observational program (CinicalTrials.gov identifier NCT03722836) was carried out in 2018–2019. It was a multicenter study performed in the framework of ordinary consultations and examinations of CVD patients with CEAP class C3. All the treatments were fully consistent with the established clinical practice, instructions for the use of drugs, and a specific clinical situation. In the program, the parameters that are usually assessed during the examination of patients with CVD, as well as additional linear dimensions and the volume of the parts of leg with the most severe edema at inclusion, were evaluated. This is the routine method in the sites included in the study. According to the study protocol, each doctor was to include at least 10 patients meeting all of the following inclusion criteria and none of the following exclusion criteria.
The main inclusion criterion was the presence of CVE of the ankle caused by CVD of class C3EpAsPr, according to the CEAP classification. Additional inclusion criteria were age over 18 years, patient’s written informed consent, absence of allergic reactions to a topical anesthetic and sclerosing agent, no intake of VADs within 4 weeks before inclusion in the program, and ability to come for a follow-up visit after the intervention.
Exclusion criteria were history of alcohol or drug abuse, secondary varicose veins, angiodysplasia, or neoplasm, lymphatic edema of the lower limbs, peripheral artery disease (ankle-brachial index <0.9), infectious disease within 6 weeks before inclusion, presence of one or more concomitant diseases that are able to affect treatment outcomes (ie, diabetes mellitus; hypertension; connective tissue diseases, including rheumatoid arthritis, intermittent claudication, diseases of bones or joints of the lower limbs; inflammatory bowel disease; renal failure; emphysema or chronic obstructive pulmonary disease; malignant neoplasm); history of deep-vein thrombosis within 1 year before inclusion; superficial thrombophlebitis within 3 months before inclusion; inability to walk (regardless of the cause); obesity (body mass index [BMI] ≥30 kg/m2); poor predicted adherence to the study protocol; participation in another clinical trial within the last 3 months before inclusion; for women: pregnancy, breast-feeding, or willingness to become pregnant within 2 months after the study.
A total of five visits for each patient were scheduled during the VAP-C3 program: inclusion visit (V0) and four follow-up visits (V1, V2, V3, and V4) at 14, 30, 60, and 90 days afterV0. The schedule used with data sources and measurements of the observation research program VAP-C3 in order to assess treatment efficacy is presented in Table I.
Based on a specific clinical situation, the doctor independently decided on the rationale of the prescription of compression therapy (and its type) and also recommended one VAD or another.
The criteria to evaluate the treatment outcome were changes in the severity of CVD symptoms as assessed by the VAS scores, changes in the QOL parameters of the CIVIQ-14 (14-item ChronIc Venous Insufficiency Quality of life questionnaire), as well as patient satisfaction with treatment outcome using the Darvall questionnaire (not addressed here). To measure and monitor ankle edema, the disc method was used.7,8 Moreover, all measurements were performed on the extremity with more pronounced edema.
Before inclusion in the observation program, all patients provided written informed consent, as well as gave permission to process personal data. Data processing and post hoc statistical analysis were carried out by an independent expert in medical statistics using two-sided parametric and nonparametric tests with a significance level of 0.05.
Results
Eighty-six Russian phlebologists from private clinics enrolled 708 patients (75% females; mean age 48.6±12.6 years; 25.6% patients aged over 65 years) with CVD of CEAP class C3, who fulfilled all the protocol requirements as stated in the methodology section. After the inclusion visit (V0), the patients were followed-up with four visits (V1, V2, V3, and V4) during a mean study period of 2.5±0.5 months. No adverse drug reactions were reported during the study.
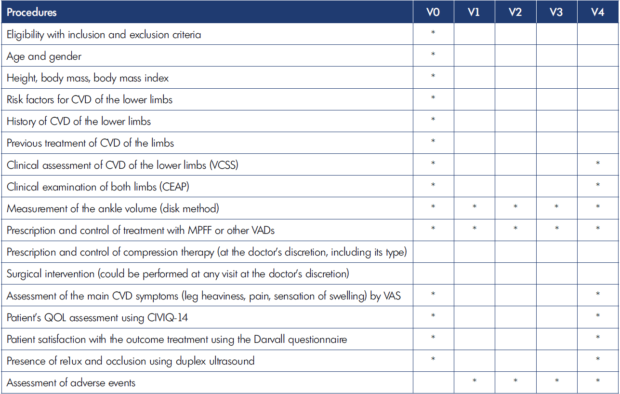
Table I. Schedule of the observation research program VAP-C3. CEAP, clinical, etiologic, anatomic, and pathophysiologic classification; CIVIQ-14, 14-item ChronIc Venous Insufficiency Quality of life questionnaire; QOL, quality of life; CVD, chronic venous disease; VAD, venoactive drug; VAS, visual analog scale; VCSS, Venous Clinical Severity Score.
The demographic and baseline clinical characteristics of these patients are presented in Table II.
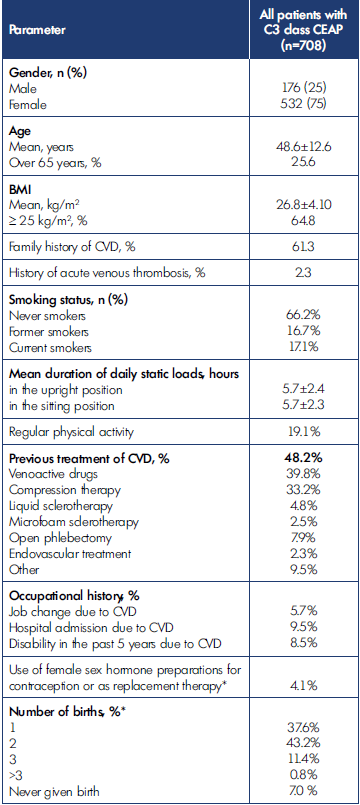
Table II. Demographic and baseline clinical characteristics of patients included in the study (n=708). BMI, body mass index; CEAP, clinical, etiological, anatomical, pathophysiological classification; CVD, chronic venous disease. *only for wome
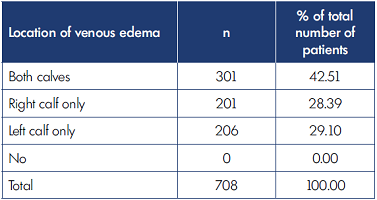
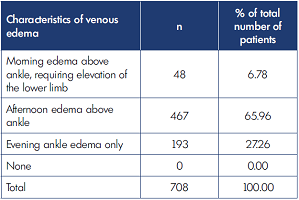
All patients in the study underwent ultrasound examination according to standard protocol. In the total sample, pathological reflux was identified in 78.7% of patients and was located in superficial veins (74.6%), perforating veins (3.7%), or deep veins (0.4%). The location and characteristics of the venous edema are presented in Table III and Table IV.
The allocation of patients depending on the treatment regimen is shown in Table V.
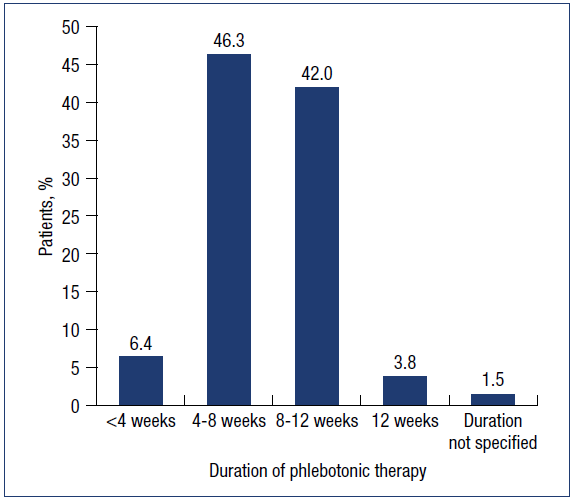
With regard to systemic phlebotonic therapy, 97.7% of patients were prescribed MPFF. The dosing regimen included intake of MPFF in the form of one 1000-mg tablet once daily in 77.9%, one 500-mg tablet twice daily in 4.8%, or a 1000-mg oral suspension once daily in 15% of patients. The duration of phlebotropic therapy recommended by doctors in the study are presented in Figure 1. The recommended duration for phlebotonics was for 8-12 weeks in 45.8%, 4-8 weeks in 46.3%, under 4 weeks in 6.4%, and unspecified in 1.5% (calculated percentages exclude those not prescribed MPFF).
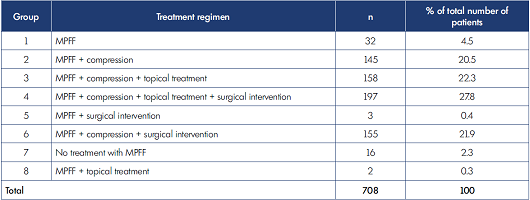
Table V. Allocation of patients by treatment regimen (n=708). MPFF, micronized purified flavonoid fraction; n, number of patients.
MPFF was used at V1, V2, V3, and V4 visits by 96.61%, 94.77%, 81.07%, and 54.94% of the total number of patients in the study, respectively.
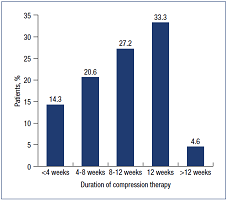
Graduated medical compression hosiery was prescribed at V0 visit to 92.5% of patients. The recommended durations for compression therapy as prescribed in the study are shown in Figure 2, with 8-12 weeks being most recommended, in 60.5% of patients, followed by 4-8 weeks in 20.6%, under 4 weeks in 14.3%, and over 12 weeks in 4.6% (calculated percentages exclude those not prescribed compression therapy).
Compression treatment was used at V1, V2, V3, and V4 visits by 91.95%, 84.60%, 75.56%, and 61.16% of total number of patients in the study, respectively. The compression class by RAL standard (European compression standard) was 1, 2, and 3 in 5.5%, 85.3%, and 0.3% of them, respectively.
Topical treatment was prescribed at V0 visit to 50.4% of the total number of patients in the study and was used at V1, V2, V3, and V4 visits by 36.44%, 35.03%, 28.11%, and 16.67% of patients.
Surgical intervention for CVI (including mostly endovenous laser treatment [EVLT], but also liquid sclerotherapy, radiofrequency ablation (RFA), miniphlebectomy or a combination of these techniques) was performed in 50.1% of the total number of patients in the study in combination with conservative treatments.
Due to an insufficient number of subjects in groups 5, 7 and 8, which are listed in Table V, these groups were excluded from further analysis. Characteristics of the 687 patients in the remaining groups thus selected for final analysis are provided in Table VI.
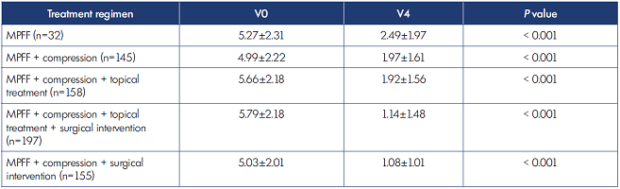
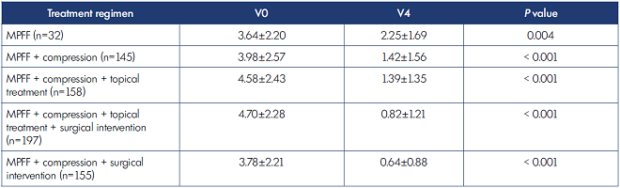
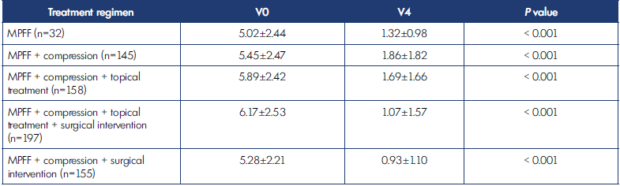
The study revealed significant positive changes at the final visit, compared with baseline, in the severity of the main CVD symptoms assessed by VAS, such as leg heaviness (Table VII), leg pain (Table VIII), and sensation of leg swelling (Table IX) in patients using MPFF alone, as well as in groups using MPFF in combinations including compression (P<0.001 in all groups).
Leg heaviness improved 52.8% in those receiving MPFF alone, 60.5% for MPFF+compression, 66.1% with MPFF+compression+topical treatment, 80.3% with MPFF+compression+topical treatment+endovenous surgery, and 78.5% with MPFF+compression+surgical intervention (Table VII).
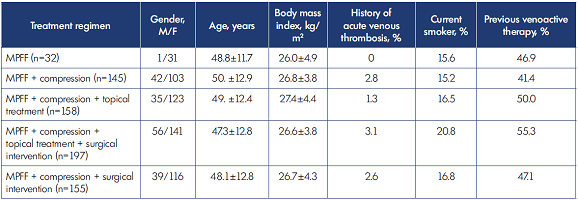
Table VI. Characteristics of patients allocated to groups included in the final analysis (n=687). MPFF, micronized purified flavonoid fraction.
Leg pain improved 38.2% in those receiving MPFF alone, 64.3% with MPFF+compression, 69.7% with MPFF+compression+topical treatment, 82.6% with MPFF+compression+topical treatment+surgical intervention, and 83.1% with MPFF+compression+surgical intervention (Table VIII).
Sensation of swelling improved 73.7% in those receiving MPFF alone, 65.9% with MPFF+compression, 71.3% with MPFF+compression+topical treatment, 82.7% with MPFF+compression+topical treatment+surgical intervention, and 82.4% with MPFF+compression+ surgical intervention (Table IX).
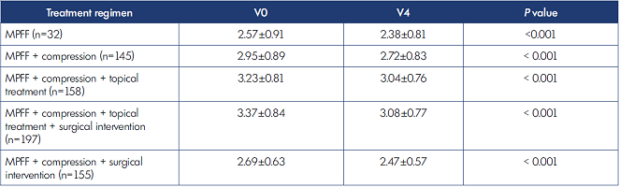
These positive changes were accompanied by significant improvement in the QOL of the patients, assessed by CIVIQ-14 global index score in all the respective treatment groups (all P<0.001; Table X).
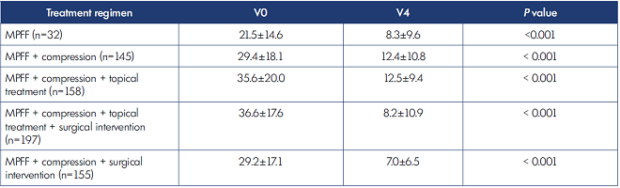
The ankle volume, as a main efficacy parameter during the study, significantly decreased in patients using MPFF alone, as well as in groups using MPFF in combinations including compression (all P<0.001; Table XI). Total reductions in ankle volume (ranging from 0.19 L to 0.29 L) with respect to different treatment strategies are shown in Table XII.
In the total treatment group (n=708), no differences in the CVE reduction were found between patients with or without surgical intervention (conservative treatment only) during the study period as presented in Table XIII.
Comparative intergroup analyses were also performed between patients who underwent surgical intervention and those who received only MPFF-based conservative treatments (excluding the 16 patients from the study who did not take MPFF; n=692). As in the total treatment group, no differences in observed CVE reduction were found between patients with or without surgical intervention (MPFF-based conservative treatment only) at the end of the treatment (Table XIV). However, there was significantly better improvement in the CIVIQ-14 global score in patients who were treated with both MPFF-based conservative treatment and surgical intervention (Table XV).
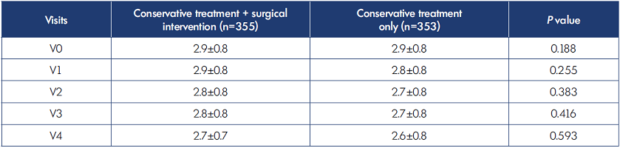
Table XIII. Comparison of changes in ankle volume (liters) in all patients with or without surgical intervention for chronic venous disease during the study (n=708).
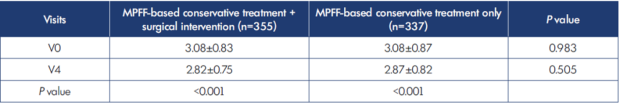
Table XIV. Changes in ankle volume (liters) in patients with MPFF-based conservative therapy with or without surgical intervention (n=692).
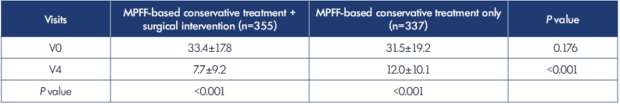
Table XV. Changes in the CIVIQ-14 global score in patients with MPFF-based conservative therapy with or without surgical intervention (n=692).
Discussion
The presence of CVE correlates with the worsening of other CVD symptoms and suggests decompensation of the drainage function of venous and lymphatic systems. Patients with CVD of CEAP class C3 not only report a significant reduction in all QOL parameters, but also fall into the high risk group for the development of trophic disorders of the soft tissues of the ankle.9,10
Regarding the conservative approach for CVD, a combination of compression therapy with VADs is often prescribed, which is sometimes complemented by other techniques, such as intermittent pneumatic compression, neuromuscular electrical stimulation, and various types of manual drainage massage. If a more aggressive approach is necessary, the preference is given to endovascular surgery. However, there is a large amount of evidence suggesting that even in such a case it is advisable to first reduce or eliminate CVE, ie, to transition the patient from class C3 to class C2 CEAP. Moreover, the UIP guidelines emphasize that surgical intervention or sclerotherapy does not guarantee a reduction in or elimination of baseline CVE, which suggests prolonged conservative therapy in the postoperative period may be necessary.11,12
The study aimed to assess the effectiveness of MPFF-based conservative treatment in patients with CVE in real clinical practice. The medical focus group comprised selected medical specialists who were regularly and professionally involved in the treatment of this population of patients. Thus, the doctors’ awareness in regard to this population of patients may also have played a role in decision making in this study.
All doctors started to treat patients with conservative therapy, including compression hosiery and VADs. The vast majority of patients were prescribed with class 2 medical hosiery (RAL standard), which is consistent with the International Compression Club guidelines. Rationale behind prescription of compression hosiery of RAL class 3 or to refuse prescription of compression therapy at all is unclear.13
Of interest is the recommended duration of compression therapy. Compression hosiery was prescribed for a period of more than 12 weeks, optimal for treating CVD, in only 4.2% of total patients, and not prescribed at all in 8.6% of patients. The reason for this finding is unclear. It can be assumed that the data shown in Figure 2 indirectly reflects patient adherence to compression therapy, which decreases with longer duration of prescribed therapy and which is aligned with the findings of VEIN Act Program.6
Regarding the phlebotropic therapy used in this study, the choice of MPFF in various forms and in a standard daily dose of 1000 mg was based on the Russian National Clinical Guidelines and findings from the meta-analysis of Allaert.14 This meta-analysis demonstrated the significant superiority of MPFF therapy for venous edema, assessed as the decrease in ankle circumference when compared with other VADs such as hydroxyethyl-rutosides, ruscus extract, and diosmin.14
In the study population that used MPFF-based conservative treatment, there were significant improvements in the main CVD symptoms such as leg heaviness, leg pain, and leg swelling, in CIVIQ-14–assessed QOL, as well as significant reduction in ankle volume. Whereas the observed improvement in ankle volume with MPFF-based conservative therapy was irrespective of surgical intervention, use of MPFF-based conservative therapy in combination with surgical intervention was associated with a significant improvement in CIVIQ-14–assessed QOL.
There is a need for a clearer management strategy in patients with CVD in respect to the duration of conservative therapy before referral to surgery, as well as in preparation for and following surgery. Study limitations include its observational nature and the absence of equivalent comparison groups. In addition, the location of patient enrolment being in specialized phlebology centers rather than general medical institutions might also affect the study results. Nevertheless, the design of the VAP-C3 program and the acquired results reflect real clinical practice to a greater extent than standard refined comparative studies. The data obtained in the VAP-C3 program and the current evidence from other studies have already allowed us to recommend the routine perioperative administration of MPFF in patients with CVD in real clinical practice.18
Conclusion
The results of the Russian national multicenter observational program VAP-C3 showed that treatment with MPFF-based conservative therapy significantly decreased the severity of CVE with reduction in the ankle volume and improved the main CVD symptoms, such as leg heaviness, pain, and sensation of swelling in patients with CVD of CEAP class 3. Such therapy also improved the quality of life of the patients, as assessed by the CIVIQ-14 global index score.
In addition, comparative intergroup analysis demonstrated a strong antiedematous effect of MPFF-based conservative therapy irrespective of surgical interventions, which may support the predominant use of MPFF in routine clinical practice in patients with CVD of CEAP class C3.
Bakhtin I.L. (Arkhangelsk); Khairutdinov S.V. (Balakovo); Alexandrova S.M. (Berdsk); Podyakov A.Yu. (Bryansk); Alekhin D.I. (Chelyabinsk); Kipaikin A.V. (Drezna); Lebedev A.K. (Drezna); Vasyagin A.N. (Dzerzhinsk); Shulikovskaya I.V. (Irkutsk); Pelevin A.V. (Ivanovo); Akhmetzyanov R.V. (Kazan); Bredikhin R.A. (Kazan); Larionov M.V. (Kazan); Bushnin S.S. (Khabarovsk); Storozhenko N.S. (Krasnodar); Lavrov R.N. (Krasnoyarsk); Ludkova L.F. (Krasnoyarsk); Khruslov M.V. (Kursk); Velikoretsky M.N. (Lipetsk); Mishin D.V. (Lukhovitsy); Allakhverdov N.V. (Moscow); Biryulin D.V. (Moscow); Volkov A.S. (Moscow); Voloshkin A.N. (Moscow); Vorobeva M.V. (Moscow); Golovanova O.V. (Moscow); Dolgov S.I. (Moscow); Doronin I.V. (Moscow); Kopylov B.E. (Moscow); Koreshkov A.E. (Moscow); Krylov A.Yu. (Moscow); Kuznetsov A.N. (Moscow); Lizanets Yu.M. (Moscow); Lishov D.E. (Moscow); Lobanov V.N. (Moscow); Manjikian O.P. (Moscow); Samokhin K.M. (Moscow); Semenov A.Yu. (Moscow); Solomakhin A.E. (Moscow); Sukhorukov E.A. (Moscow); Taranenko O.V. (Moscow); Fomichev D.O. (Moscow); Chizhikov N.N (Moscow); Sazhinov A.P. (Murmansk); Blinov D.V. (Nizhniy Novgorod); Lebedev S.S. (Novorossiysk); Mustafaev N.R. (Novosibirsk); Sevostyanova K.S. (Novosibirsk); Bazhenov V.N. (Omsk); Vasilevich V.V. (Omsk); Mironova T.I. (Omsk); Panyushkin D.V. (Perm); Shornikov V.Ø. (Petrozavodsk); Vagner D.O. (Rostov-on-Don); Khitaryan A.G. (Rostov-on- Don); Agapov A.B. (Ryazan); Krylov A.A. (Ryazan); Bykovsky A.V. (Saint-Petersburg); Gavva E.A. (Saint-Petersburg); Zhiruev M.S. (Saint-Petersburg); Shonov O.A. (Saint- Petersburg); Krasilnikov A.V. (Samara); Krygin S.G. (Samara); Khamidullin A.A. (Samara); Shakirov R.A. (Samara); Chabbarov R.G. (Saratov); Skorobogatov O.A. (Serpukhov); Pimenova N.Yu. (Tula); Ivanov E.V. (Tyumen); Nizamov F.Kh. (Tyumen); Ibragimov D.R. (Ufa); Minigalieva E.R. (Ufa); Plecheva D.V. (Ufa); Khafizov A.R. (Ufa); Kirdyashev A.V. (Ulyanovsk); Fefelov E.A. (Vladivostok); Larin S.I. (Volgograd); Simonov V.A. (Vologda); Lobtsov A.V. (Voronezh); Guzhkov O.N. (Yaroslavl); Potapov M.P. (Yaroslavl); Belentsov S.M. (Yekaterinburg); Smirnov O.A. (Yekaterinburg).
REFERENCES
1. Eklof B, Perrin M, Delis KT, et al. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(2):498-501. doi:10.1016/j.jvs.2008.09.014.
2. Perrin M, Eklof B, van Rij A, et al. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. Int Angiol. 2016;35(4)374-398.
3. Rabe E, Guex JJ, Puskas A, et al. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31(2)105-115.
4. Eklof B, Raju S, Kistner RL. Venous hemodynamic changes in lower limb venous disease: the UIP consensus document. Int Angiol. 2016;35(3)233- 235.
5. Cornu Thenard A, Scuderi A, Ramelet AA, et al. UIP 2011 C3 Consensus. Int Angiol. 2012;31(5):414-419.
6. Bogachev V, Arribas J, Baila S, et al. Management and evaluation of treatment adherence and effectiveness in chronic venous disorders: results of the international study VEIN Act Program. Drugs Ther Perspect. 2019;35(8)396- 404. Available at: https://doi. org/10.1007/s40267-019-00637-5.
7. Kaulesar Sukul DM, den Hoed PT, Johannes EJ, et al. Direct and indirect methods for the quantification of leg volume: comparison between water displacement volumetry, the disk model method and the frustum sign model method, using the correlation coefficient and the limits of agreement. J Biomed Eng. 1993;15(6):477-480. doi:10.1016/0141-5425(93)90062-4.
8. Rabe E, Carpentier P, Maggioli A. Understanding lower leg volume measurements used in clinical studies focused on venous leg edema. Int Angiol. 2018;37(6):437-443. doi:10.23736/S0392-9590.18.04057-9.
9. Jantet G. RELIEF study: first consolidated European data. Reflux assessment and quality of life improvement with micronized flavonoids. Angiology. 2000;51(1):31-37. doi:10.1177/0003319 70005100107.
10. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assessment and quality of life improvement with micronized flavonoids. Angiology. 2002;53(3):245-256. doi:10.1177/000331970205300301.
11. Wittens C, Davies AH, Bækgaard N, et al. Management of chronic venous disease. Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49(6):678-737. doi:10.1016/j. ejvs.2015.02.007.
12. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part I. Int Angiol. 2018;37(3):181-254. doi:10.23736/s0392-9590.18.03999-8.
13. Stout N, Partsch H, Szolnoky G, et al. Chronic edema of the lower limbs: international consensus recommendations for compression therapy clinical research trials. Int Angiol. 2012;31(4):316-329.
14. Allaert FA. Meta-analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31(4):310-315.
15. Kakkos SK, Nicolaides AN. Efficacy of micronized purified flavonoid fraction (MPFF) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: a systematic review and metaanalysis of randomized double-blind placebo-controlled trials. Int Angiol. 2018;37(2):143-154. doi:10.23736/ S0392-9590.18.03975-5.
16. Bush R, Comerota A, Meissner M, et al. Recommendations for the medical management of chronic venous disease: The role of micronized purified flavanoid fraction (MPFF). Phlebology. 2017;32(1 suppl):3-19.
17. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669. doi:10.3390/ijms19061669.
18. Mansilha A, Sousa J. Benefits of venoactive drug therapy in surgical or endovenous treatment for varicose veins: a systematic review. Int Angiology. 2019;38(4):291-298.