Efficacy of a 6-month treatment with MPFF at a dose of 500 mg* in patients with venous edema (Efficacy of MPFF at a dose of 500 mg* in Edema Treatment. EDET)
Zuzana NAVRÁTILOVÁ1
and the EDET study group2
1 Ambulance dermatologické angiologie, Brno,
Czech Republic.
2 Andrš P. (Prost ějov), Diamantová D. (Olomouc), Felixová V. (Kralupy nad Vltavou), Flášarová V. (Brno), Hubáčková N. (St říbro), Jašková E. (Opava), Ješinová M (Teplice), Kazinota V. (Břeclav), Komárková K. (Praha), Komrsová H. (Praha), Koťátková D. (Pardubice), Krátký P. (Jablonec nad Nisou), Kreml M. (Kyjov), Kruková M. (Praha), Lánová M. (Ústí nad Orlicí), Michálková M. (Ostrava), Navrátilová Z. (Brno), Neumannová R. (Chomutov), Pacejka M. (Zlín), Pelikánová D. (Prostějov), Procházka P. (Kladno),
Scheetyová R. (Olomouc), Šmejkalová D. (České Bud ějovice), Špale Z. (Sedl čany), Švestková S. (Brno), Tomanová J. (Plzeň), Vogelová M. (Praha).
SUMMARY
Edema is often an early sign of significant fluid retention, which could eventually result in venous complications. It is commonly associated with venous symptoms. This study assesses the contribution of MPFF at a dose of 500 mg to improving symptoms and reducing venous edema in patients with chronic venous disease.
METHODS
Patients, aged β18 years, with venous edema without skin changes, assessed as at least 4 cm on a 10-cm visual analogue scale, complaining of at least 3 symptoms (among pain, heaviness, sensation of swelling, sensation of tension, and restless legs), were included.
They received MPFF at a dose of 500 mg 2 tabs/day for 6 months. Primary end points were the reduction of leg perimeter and leg volume assessed respectively by tape and the disk model method of calculation. Improvements in clinical symptoms using different scales were the secondary criteria.
RESULTS
From month 2, edema was significantly reduced by MPFF at a dose of 500 mg (P<0.001), in terms of both leg perimeter measurement and volume calculation. Symptoms (sensation of swelling, of tension, pain, heavy leg sensation, and restless legs) were significantly improved by MPFF at a dose of 500 mg from month 2 (P<0.001). No treatment-related side effects were reported and the acceptability was considered excellent by most patients.
INTRODUCTION
Edema of the lower limbs is not a disease per se, but a sign of underlying disorders which may accompany numerous diseases. The causes of edema are important to identify because they will have major consequences for treatment. Generally, the causes of lower-limb edema are suggested by the clinical examination of the patient. The clinical interview should aim to identify any history of the condition, establish whether edema is acute or chronic, and look for possible precipitating factors. To confirm the diagnosis of venous edema, the clinical examination should exclude edema related to other diseases and verify whether the edema is isolated or diffuse, painful or not painful, its consistency, the existence of urticaria-like lesions suggesting angioedema, the existence of a serous effusion (peritoneum, pleura), signs of thromboembolic disease, or clinical evidence of internal organ disease (heart, kidney, liver).1
Edema related to chronic venous disease (CVD) is sporadic, unilateral or bilateral, has no component of inflammation (leg skin is white, not red), is limited to the legs, but may also involve the foot and the ankle (not toes) and is increased by heating, hormonal load, prolonged standing or sitting, but decreases at rest or when walking. On examination, venous edema is initially manifested around the ankle and is variable in intensity, usually worsening during the day and then disappearing after prolonged elevation of the legs.
When edema becomes chronic, thickening of the skin and lymphedema may develop. It is not uncommon that venous and lymphatic edema are combined in the severe stages of CVD. The combination of edema and varicose veins, ankle telangiectasia, hyperpigmented dermatitis, white atrophy, cutaneous sclerosis, and eczema or venous ulcers on the lower limb is a good indication of the venous origin of edema.2
On the other hand, venous edema is often accompanied by symptoms like pain, heaviness, sensation of swelling or tension, and restless legs, the cause of which is believed to be linked to the pressure edema exerts on skin nerve endings.3
Micronized purified flavonoid fraction (MPFF) [MPFF at a dose of 500 mg] is a well-established oral flavonoid with venoprotective properties.4 It consists of 90% micronized diosmin and 10% flavonoids expressed as hesperidin, diosmetin, linarin, and isorhoifolin.5 The micronization of diosmin to particles with a diameter <2 µm has improved the oral absorption of MPFF at a dose of 500 mg.6
MPFF at a dose of 500 mg significantly decreases capillary hyperpermeability,7-9 resulting in a decrease in edema in two trials,7,8 and weight loss (1.5 kg) and a decreased sensation of swelling in one study,7 and by an improvement of the symptoms of capillary fragility (spontaneous ecchymosis, epistaxis, purpura, petechiae, gingivorrhagia, metrorrhagia and conjunctival hemorrhage) in another trial.9 These interesting product features made us choose MPFF at a dose of 500 mg for the present trial. In three further studies that have used different methods to quantify leg edema, beneficial effects of MPFF at a dose of 500 mg have also been demonstrated.10-12
CVD-related edema may be assessed by either ankle circumference or leg volume. Ankle circumference must be taken at the same height to avoid artefacts, which is made possible by the use of Leg-o-Meter.13 Water displacement is considered the most valuable method for assessing the volume of an edema, but it is timeconsuming and may pose implementation challenges in the clinical and clinical trial environments.14 Leg volume may be calculated indirectly using the disk model.15 A series of ankle and leg circumferences are used to calculate the volume of each cross-section in millimeters. The sum of the disk volumes provides an estimate of total leg volume.14
The intensity of symptoms and their impact on quality of life should be considered in edematous patients. The 10-cm visual analogue scale (VAS) has been extensively used to assess symptoms in all types of diseases, including CVD.16 A complement to the VAS and to the clinical, etiological, anatomical, pathophysiological (CEAP) classification of CVD is the Venous Clinical Severity Score (VCSS), which includes 10 hallmarks of venous disease that are likely to show the greatest response to therapy, each scored on a severity scale from 0 to 3. These include pain, edema, and inflammation. Scores obtained for each item are added up to comprise the overall VCSS, which ranges from 0 to 30.17 To fully assess an outcome, the effects on the physician, patient, and community should be reported. This notion is at the heart of quality of care that considers quality of life. A widely used and well-validated instrument is the CIVIQ- 20, which has over time been developed and validated in 13 languages, with questions on physical health, psychological and social well-being, and pain.18
The aim of the present study was to assess the contribution of MPFF at a dose of 500 mg to the reduction of CVDrelated edema and symptoms.
METHODS
Design
The study was conducted in 27 centers (dermatology and angiology departments) located in the Czech Republic, according to an open design.
Patients
Ambulatory male and female patients, aged ≥18 years, assigned C3 (edema without skin changes) in the CEAP classification, presenting with venous edema, assessed as at least 4 cm on a 10-cm VAS, complaining of at least 3 symptoms (from among pain, heaviness, sensation of swelling, sensation of tension, and restless legs), were included after having given their written informed consent and having met the following inclusion criteria: no history of concomitant disease, no abnormal laboratory values, and having stopped taking phlebotropic drugs for 2 weeks. The main patient exclusion criteria were congenital angiodysplasia, deep vein thrombosis, superficial phlebitis, arteriopathy, uncontrolled diabetes, renal failure, lymphedema or ankle ankylosis, vasculitis or blood disorders, allergy to a drug, unauthorized concomitant pharmacological therapy (mainly venotropics, nonsteroidal anti-inflammatory drugs, other local therapy, systemic steroids, diuretics, ergotamine, beta-blockers, calcium inhibitors) or nonpharmacological therapy (sclerotherapy, surgical treatment, normovolemic hemodilution, laser therapy, local UV and ultrasound therapy, oxygen therapy), alcohol or drug abuse, pregnancy or breastfeeding. Patients were also excluded if the cause of their edema was renal, hepatic, cardiac, iatrogenic, or other than venous.
Therapy
Following a 2-week run-in period during which patients had to stop any unauthorized treatment, patients received MPFF at a dose of 500 mg (2 tablets/day in the morning). The treatment duration was 6 months.
Assessments
The two primary end points of the study were reduction in calf circumference, measured with the Leg-o-Meter,13 and decrease in leg volume, as assessed using the disk model15 after 2 and 6 months of therapy. For the calculation of leg volume, leg perimeter was taken at several points at 3-cm intervals, starting 3 cm below the saphenofemoral junction and ending just above the malleolus. Main secondary criteria were the evaluation of venous symptoms with both the VAS16 and the VCSS,17 and the self-evaluation of the patients’ quality of life with the CIVIQ-2018 during the course of the study.
After the selection visit (day –14), patients were seen at baseline (day 0), then at months 2 and 6.
At each visit, (day 0, month 2, month 6) assessments were carried out as follows:
• clinical evaluation of the appearance of the edema
• assessment by the investigator of the therapeutic effect on calf circumference using the Leg-O-Meter
• assessment by the investigator of the therapeutic effect on leg volume using the disk model method
• evaluation of the intensity of pain, leg heaviness, sensation of swelling, of tension, restless legs using the 10-cm VAS
• self-assessment of the quality of life with CIVIQ-20
• screening for adverse effects and acceptability
Treatment compliance was assessed by tablet count.
Statistical analysis
Statistical analysis was performed using BMDP software. In all statistical tests, the level of significance was set at 5%.
The intention-to-treat (ITT) population, considered for acceptability analysis, was defined as all selected patients with at least one visit and corresponding assessments available. The per protocol (PP) population was defined as those selected patients who completed the study in accordance with the protocol.
The change in parameters during the course of the study was analyzed using one-way analysis of variance.
RESULTS
Twenty-seven centers participated in the study. Out of the 215 patients selected for participation, 213 were included, and 196 completed the study in accordance with the protocol. Main demographic and baseline clinical characteristics of the study population are displayed in Table I, while Table II presents the clinical findings on admission in relation to the venous status of included patients.
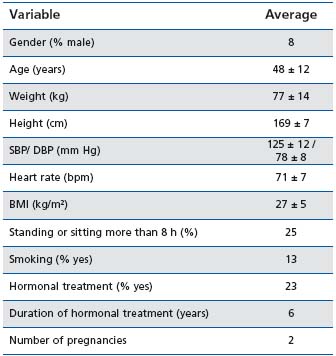
Table I: Demographics and clinical characteristics of the study
population at baseline
SBP/DBP: systolic/diastolic blood pressure; BMI: body mass index
(=weight/height²); bpm: beats per minute
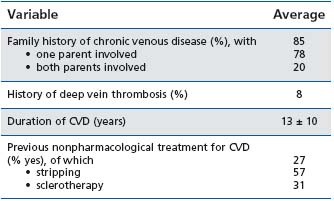
Table II: Characteristics of the venous status at baseline
SBP/DBP: systolic/diastolic blood pressure; CVD: chronic venous
disease
Patients were mostly women (92%) and a family history of CVD was found in almost 75%. A personal history of deep vein thrombosis was present in 8% of the patients and the duration of CVD was over 13 years in most of the cases.
Primary end points
Right from month 2, both the ankle and calf circumferences were significantly reduced as compared with those at baseline (24.4 cm vs. 25 cm; P<0.001 for ankle perimeter, and 39.9 cm vs. 40.6 cm; P<0.001 for calf perimeter). The reduction in perimeters continued until month 6, as shown in Figures 1 and 2.
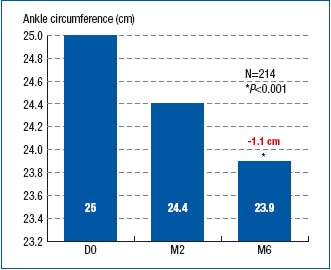
Figure 1. Ankle circumference at baseline (D0) and after 2- and
6-month treatment with MPFF at a dose of 500 mg (respectively at M2 and
M6)
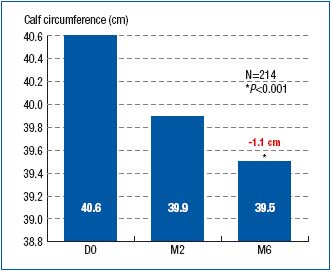
Figure 2. Calf circumference at baseline (D0) and after 2- and
6-month treatment with MPFF at a dose of 500 mg (respectively at M2 and
M6)
Leg volume had decreased by an average 78 cm3 after 2 months of MPFF at a dose of 500 mg treatment, and 121 cm3 after 6 months (Figure 3). This was statistically significant at both times (P< 0.001).
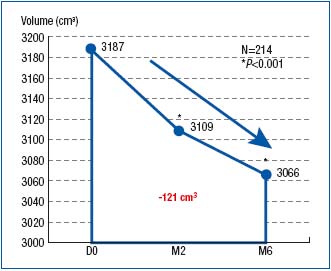
Figure 3. Assessment of leg volume by the disk model at baseline
(D0) and after 2- and 6-month treatment with MPFF at a dose of 500 mg
(respectively at M2 and M6)
Secondary criteria
The reduction in symptom intensity, as measured with VAS, was shown to be significant at both month 2 and month 6 of treatment (Figure 4). This was true for all symptoms. The VCSS assessment showed significant difference between baseline (score, 5.8), month 2 (score, 4.8), and month 6 (score, 4) scores (Figure 5). The CIVIQ score decreased substantially for all 20 questions, meaning that quality of life was improved with therapy (Figure 6).
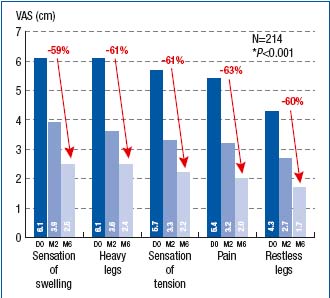
Figure 4. Assessment of symptom intensity using a visual
analogue scale (VAS) at baseline (D0) and after 2- and 6-month
treatment with MPFF at a dose of 500 mg (respectively at M2 and M6)
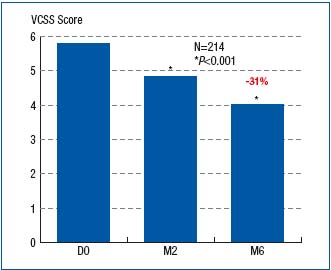
Figure 5. Assessment of Venous Clinical Severity Score (VCSS) at
baseline (D0) and after 2- and 6-month treatment with
MPFF at a dose of 500 mg (respectively at M2 and M6)
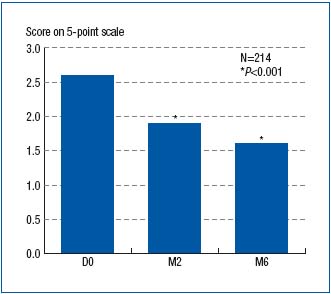
Figure 6. Assessment with a five-point Lickert scale of well-being
at baseline (D0) and after 2- and 6-month treatment with
MPFF at a dose of 500 mg (respectively at M2 and M6). Average score to the
following question: ‘During the past 4 weeks, to what extent did
you feel bothered/limited in your work or your other daily
activities because of your leg problems?’
The overall efficacy of the treatment was assessed by both the investigators and patients. For 93.5% of physicians, MPFF at a dose of 500 mg was considered to be good to excellent, while 91% of patients were satisfied or very satisfied, and for 82% of them it was decided to continue MPFF at a dose of 500 mg.
Acceptability
No change in body weight, heart rate, or blood pressure was reported during the study, and no side effects in relation to treatment were observed. The acceptability of the MPFF at a dose of 500 mg treatment was excellent, and no patient reported poor acceptability or deterioration.
Compliance
Assessment of treatment compliance during the course of the study showed 99 to 100% compliance.
COMMENTS
The present study confirms previously reported results on the beneficial effects of MPFF at a dose of 500 mg in the treatment of edema associated or not with venous symptoms.19 Our data show that pharmacological therapy with MPFF at a dose of 500 mg significantly reduces the perimeter and volume of lower limbs, while improving clinical symptoms and quality of life.
Edema is often an early sign of significant fluid retention, which could eventually result in complications. However, accuracy and consistency in assessing edema is a challenge. A variety of methods to measure edema quantitatively have been proposed (volumetry and ankle circumference). Among these, ankle and calf perimeter have shown excellent reliability.14 Previous research has shown that the disk model method of calculating leg volume is highly correlated with water displacement volumetry.20 The disk model method was previously chosen to assess the efficacy of venoactive therapy in reducing edema.21 In this last study of 253 consecutive outpatients in CEAP classes C3 to C4 who were treated for 4 weeks, the difference in leg volume was statistically significant (P = 0.0109) in favor of active treatment. This points to the usefulness of the disk model method in assessing edema in clinical research.
The efficacy of MPFF at a dose of 500 mg in reducing venous symptoms was also demonstrated from the second month of therapy. Subjective assessment with a VAS was complemented by the physician-related VCSS and the patient-reported quality of life questionnaire CIVIQ-20.
It is noticeable from this study that severity scores were not distributed over the whole 0 to 30 VCSS scale and that the mean score obtained at each study time was not very high (less than 6 at baseline). This was observed in a previous survey performed by French angiologists,22 in which a markedly lower clinical severity score was found in the [C1-C2-C3] group of patients compared with the [C4-C5-C6] group. This might be because the VCSS was designed by the authors to evaluate the most severe forms of CVD, above C4.
The CIVIQ-20 proved sensitive to clinical changes and well adapted to the assessment of efficacy of therapies even at early stages.23 The present study lends further weight to this observation
CONCLUSION
The results of the present study are conclusive evidence that MPFF at a dose of 500 mg is effective in the treatment of edema and the reduction of venous symptoms. Six-month treatment with MPFF at a dose of 500 mg significantly reduced leg edema, while improving some clinical symptoms of CVD.
The efficacy and the acceptability of this treatment were evaluated as good to excellent by a large majority of the investigators and patients as well, suggesting that better compliance may be expected from patients with MPFF at a dose of 500 mg, thus optimizing therapeutic efficacy.
This article is adapted from the previous publication ‘Z Navratilova. Efficacy of Detralex in edema treatment. Interni Med. 2009;11(2):87-90 [in Czech]’ with Dr Navratilova’s permission.

REFERENCES
1. Blétry O, Vignes S, Priollet P. OEdèmes. In: Godeau P, Herson S, Piette J-C, eds. Traité de Médecine. Paris, France: Flammarion Médecine- Sciences;2005:95-101.[in French]
2. Priollet P. Venous edema of the lower limbs. Phlebolymphology. 2006;13:183- 187.
3. Nicolaides AN. From symptoms to leg edema: efficacy of MPFF at a dose of 500 mg. Angiology 2003;54 (suppl 1):S33-S44.
4. Pascarella L, Lulic D, Penn AH, et al. Mechanisms in Experimental Venous Valve Failure and their Modification by MPFF at a dose of 500 mg. Eur J Vasc Endovasc Surg 2008;35:102-110.
5. Paysant J. Sansilvestri-Morel P, Bouskela E, Verbeuren TJ. Different flavonoids present in the micronized purified flavonoid fraction (MPFF at a dose of 500 mg) contribute to its antihyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81-85.
6. Garner RC, Garner JV, Gregory S et al. comparison of the absorption of micronized (MPFF at a dose of 500 mg) and nonmicronized 14C-diosmin tablets after oral administration to healthy volunteers by accelerator mass spectrometry and liquid scintillation. J Pharm Sci. 2002;91:32-40.
7. Behar A, Lagrue G, Cohen-Boulakia F, et al. Study of capillary filtration by double labelling I131-albumin and Tc99m red cells. Application to the pharmacodynamic activity of MPFF at a dose of 500 mg. Int Angiol. 1988;7 (Suppl. 2):35-38.
8. Cesarone MR, Laurora G, De Sanctis MT, et al. Capillary filtration and ankle edema in patients with venous hypertension: effects of Daflon. Angiology 1993;44:57-61.
9. Galley P, Thiollet M. A double-blind, placebo-controlled trial of a new venoactive flavonoid fraction (S 5682) in the treatment of symptomatic capillary fragility. Int Angiol. 1993;12:69-72.
10. Chassignolle J-F, Amiel M, Lanfranchi G, et al. Activité thérapeutique de MPFF at a dose of 500 mg dans l’insuffisance veineuse fonctionnelle [in French]. J Int Med. 1987;99 (Suppl):32-37.
11. Laurent R, Gilly R, Frileux C. Clinical evaluation of a venotropic drug in man. Example of MPFF at a dose of 500 mg. Int Angiol. 1988;7(Suppl):S34-S43.
12. Blume J, Langenbahn H, de Champvallins M. Quantification of oedema using the volometer technique: therapeutic application of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology 1992;(Suppl 2):37-40.
13. Bérard A, Kurz X, Zuccarelli F, Ducros JJ, Abenhaim L. Reliability study of the Leg-O-Meter, an improved tape measure device, in patients with chronic venous insufficiency of the leg. VEINES Group. (Venous Insufficiency Epidemiologic and Economic Study). Angiology. 1998;49:169-173.
14. Brodovicz KG, McNaughton K, Uemura N, et al. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin Med Res. 2009;7:21-31.
15. Latchford S, Casley-Smith JR. Estimating limb volumes and alterations in peripheral edema from circumferences measured at different intervals. Lymphology 1997;30:161–164.
16. Huskisson EC. Measurement of pain. Lancet 1974;2:1127-1131.
17. Rutherford RB, Padberg FT Jr, Comerota AJ, et al. American Venous Forum’s Ad Hoc Committee on Venous Outcomes Assessment. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307-1312.
18. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539-554.
19. Lyseng-Williamson A, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
20. Kaulesar Sukul DM, den Hoed PT, Johannes EJ, et al. Direct and indirect methods for the quantification of leg volume: comparison between water displacement volumetry, the disk model method and the frustum sign model method, using the correlation coefficient and the limits of agreement. J Biomed Eng. 1993;15:477-480.
21. Labs KH, Degisher S, Gamba G, Jaeger KA. Effectiveness and safety of calcium dobesilate in treating chronic venous insufficiency: randomized, doubleblind, placebo-controlled trial. Phlebology. 2004;19:123-130.
22. Perrin M, Dedieu F, Jessent V, Blanc M-P. Evaluation of the new severity scoring system in chronic venous disease of the lower limbs: an observational study conducted by French angiologists. Phlebolymphology. 2006;13:6-16.
23. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5(6):539- 554.
