Focus on venous embryogenesis of the human lower limbs
Paris, France
Abstract
Embryogenesis of the venous system is a complex phenomenon that is not fully understood. In spite of the studies done by Born, Hochstetter, and Lewis, we do not know the precise chain of events leading to vasculogenesis of the venous network in humans. Yet, this is an important topic in order to improve our anatomical knowledge, which is of the highest interest in clinical practice. A good knowledge of anatomy is mandatory to perform in-depth assessments of chronic venous disorders by ultrasound mapping. Understanding venous embryology is also crucial to investigate and treat congenital venous malformations. Further research projects, particularly using new techniques of 3D modeling combined with immunolabeling of the anatomical structures (computer-assisted anatomical dissection) will contribute precious data regarding the onset and maturation of the human embryo’s vessels, as well as providing data on the relationship between veins and nerves.
Introduction
Today, human embryology is one of the main topics in biomedical research.1 Several 3D atlases of the human embryo have been produced using the Carnegie embryo collection (National Museum of Health and Medicine, Washington, D.C., USA) and the Kyoto collection (Congenital Anomaly Research Center, Kyoto, Japan).2 The Visual Human Embryo with digital serial sections of human embryos from the Carnegie embryo collection illustrates the major stages of human embryonic development.3,4 The Kyoto human embryo collection5 is an example of computerized 3D modeling of human embryos by MRI, presented according to the Carnegie stages. However, today, to our knowledge, there have been no studies describing the different steps of development and the precise human anatomy of the venous system of the lower limbs, due to the lack of direct observation.
The aim of this paper is to focus on the updated knowledge about venous embryogenesis of the lower limbs and to discuss the interest of a new research technique for 3D modeling of human embryos– computer-assisted anatomical dissection (CAAD).6 A better understanding of embryology, and therefore venous anatomy, is of the highest interest for the clinical practice in phlebology and vascular medicine (Figure 1).
Figure 1. Usefulness of embryology and anatomy in clinical
practice, which is crucial for the investigation of CVDs and
management of CVMs.
Abbreviations: CVD, chronic venous disease; CVM, congenital
venous malformations.
Historical References
The Scandinavian method of Born7 was created in 1883 to build 3D reconstructions of embryos. It is based on the enlargement of serial anatomical slices, displayed on a tablet of colored wax. In 1893, Hochstetter also made huge progress on the embryogenesis of amniotes.8 However, the most innovative results were shown by Lewis in 1906 in the rabbit.9 In addition, the works of Bokova (1970)10 and Gilbert (1990)11 are also noteworthy.
Since then, several works on the Hox genes have brought about a revolution in our knowledge of limb organogenesis. Hox genes control the positional information, spatial orientation, morphogen gradients, and cellular differentiation.12 Molecular models and growth factors also condition the limb’s development.13 Finally, vasculogenesis is also tightly related to the skeleton formation.14
Development of the Human Embryo
and Lower Limbs
The human embryo development can be divided into three main stages: (i) embryogenesis occurs between postovulatory weeks 0 and 4; (ii) organogenesis between postovulatory weeks 4 and 8 (Figure 2); and (iii) the fetal period between week 9 and birth.
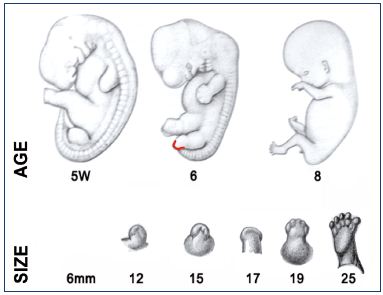
Figure 2 recapitulates the Carnegie stages of organogenesis, which is the main period of interest for the lower limb’s development, taking place between the 4th and the 8th postovulatory weeks (Carnegie stages 13 to 23). Limb development is a complex phenomenon directed by Hox genes, as we have seen previously. Here, we will only consider the simple morphological point of view. The lower limb’s development steps are shown in Figure 3. The first bud of the lower limb appears a couple of days after the upper limb, around 33 days (embryo size, 8 mm; Carnegie stage 15). At the beginning, it is reduced to a flat palette, which appears thicker at its extremity and along its caudal border at 6 weeks (embryo size, 15 mm). This is explained by the superficial migration of ectodermal cells, which increases the density of vessels in those locations, particularly in the marginal venous network. The toes appear at stage 20 (50 days; 18 mm) and the limb’s development ends at stage 23 (56 days; 30 mm).
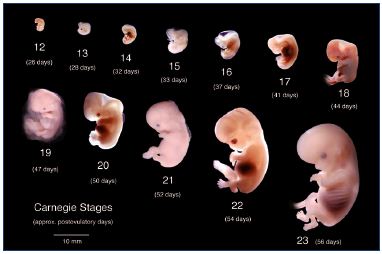
Figure 2. Organogenesis period from postovulatory weeks 4
to 8.
The lower limb buds first appear at Carnegie stage 15
(33 days; embryo size, 8 mm).
Copyrights Bradley R. Smith.
Classic “Basic” Knowledge about
Venous Embryogenesis
The early stages of development
The “primitive veins” were described by Lewis after observations made in the rabbit (Figure 4).9 Lower limb buds appear toward the end of day 10 during rabbit embryonic development. Starting on day 14, Lewis observed a concentration of the venous network at the caudal and distal aspect of the limb bud, which is called the marginal vein. This drains into a primitive fibular vein, then into an ischiatic and posterior cardinal vein (Carnegie stage 1). On day 17, he observed that the fibular vein became the main vein, while the lateral marginal vein disappears (Carnegie stage 2). At about 3 weeks of development (Carnegie stage 3), he noticed the existence of an anastomotic branch originating from the sciatic vein and connecting to a new vessel, the femoral vein. The latter forms the final deep venous system, while the sciatic vein disappears.
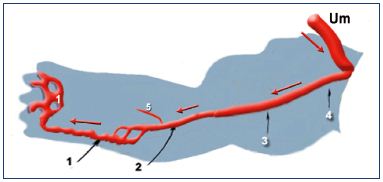
Figure 4. The primitive veins at week 5.
Note that the veins of the primitive limb are fed by the umbilical
vein (containing oxygenated blood): the blood flux goes from
the root to the extremity of the limb (black arrows). Therefore, the
veins appear first. The arteries then drain the blood back from
the limb (red arrows).
Abbreviations: 1, primitive marginal vein; 2, primitive fibular
vein; 3, primitive axial vein; 4, primitive ischiatic vein; 5, primitive
anterior tibial vein; Um, umbilical vein.
Theory of angio-guiding nerves
Later (after week 6), the arrangement of the venous network could be explained by the theory of the angio-guiding nerves proposed by Gillot15,16: the nerves appear at 6 weeks (embryo size, 18 mm; Carnegie stage 19; Figure 5). The axons and Schwann cells secrete a vascular endothelial growth factor (VEGF). It has a 2-fold role: (i) to attract the vascular plexuses to the vicinity of the nerves; and (ii) to induce their arterial, venous, or lymphatic specialization.17 The ephrin family (B2-B4) plays an important role in the differentiation of primitive endothelial cells.18
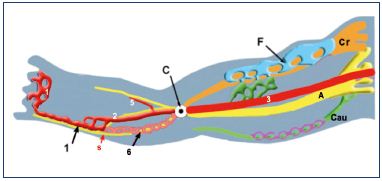
Figure 5. Growth of the three angio-guiding nerves at week 6.
Abbreviations: 1, primitive marginal vein; 2, primitive fibular
vein; 3, primitive axial vein; 5, primitive anterior tibial vein; 6,
intergemellar vein (vein of the sural nerve); A, axial (sciatic);
C, popliteal crossroad; Cau, caudal (small sciatic); Cr, cranial
(femoral); F, femoral plexus; purple, caudalplexus; s, sural nerve.
Fetal period
After postovulatory week 8, known as the fetal period, the venous plexuses along the three nerves condense and mature (Figure 6).
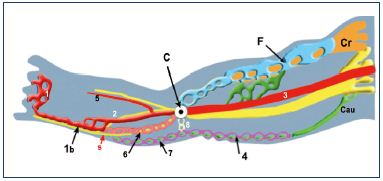
Figure 6. Venous system at weeks 7 to 8.
Abbreviations: 1, primitive marginal vein; 1b, SSV; 2, primitive
fibular vein; 3, primitive axial vein; 4, cranial extension of the
SSV (from caudal plexus); 5, primitive anterior tibial vein; 6,
intergemellar vein (vein of the sural nerve); 7, higher part of
the SSV (from caudal plexus); 8, arch of the SSV (anastomosis
between axial and caudal plexuses); A, axial nerve (sciatic);
C, popliteal crossroad (anastomosis between axial and cranial
plexuses); Cau, caudal nerve (small sciatic); Cr, cranial nerve
(femoral); F, femoral plexus; s, sural nerve; SSV, small saphenous
vein.
Data limitations
Data about the early stages are from small mammals and need to be confirmed by direct observations in human embryos. Angio-guiding nerves are just a hypothesis that is based on extensive anatomical observations in adults, but has not yet been confirmed in embryos.
Today’s Hypotheses and Proposals for the Steps of Venous Embryogenesis
Our knowledge is reduced to several hypotheses, which need to be confirmed by further direct observations and 3D reconstructions of human embryos (Figures 7 and 8):
• The lower limb bud first appears at 33 days (embryo size, 6 mm; Carnegie stage 15). The vascular layout is then reduced to a peripheral undifferentiated venous network, which is located subcutaneously and secondary to a central artery.
• Around week 5 (37 days; embryo size; 8 to 11 mm; Carnegie stage 16), the primitive veins emerge. A thickening appears at the distal and caudal aspect of the bud due to the migration of endothelial cells, and explains the location of the first veins of the limb, which are located superficially and are called the marginal venous sinus.
![Figure 7. Summary of the organogenesis period of an embryo’s development (postovulatory weeks (POW) 4 to 8; Carnegie stages 15 to 23). At week 4 (Carnegie stage 15), the reticular phase starts where the central artery (a) and superficial venous plexus (v) develop. At week 5 (Carnegie stage 16), the primitive veins (marginal [1,2]; fibular [3]; and axial [4]), umbilical vein (5), posterior cardinal vein (6), anterior tibial vein (7), and small saphenous vein (SSV [8]) appear. At week 6 (Carnegie stage 19), the three angio-guiding nerves (axial [sciatic; a]; cranial [femoral; b]; and caudal <div class="phlebo-small"></div>) appear. At week 7 to 8 (Carnegie stage 23), the following appear: marginal vein (1); fibular vein (3); axial vein (4); umbilical vein (5); anterior tibial vein (7); small saphenous vein (8); posterior tibial vein (9); femoropopliteal axis (10); deep femoral vein (11); epigastric vein (12); cranial extension of the SSV (13); great saphenous vein (14); arch of the SSV (A). Note the direction of blood circulation coming from the umbilical vein: from the root to the extremities of the veins (black arrows), and then circulated back by the arteries (red arrows).](https://www.phlebolymphology.org/wp-content/uploads/2015/08/12.jpg)
Figure 7. Summary of the organogenesis period of an embryo’s
development (postovulatory weeks (POW) 4 to 8; Carnegie
stages 15 to 23).
At week 4 (Carnegie stage 15), the reticular phase starts where
the central artery (a) and superficial venous plexus (v) develop.
At week 5 (Carnegie stage 16), the primitive veins (marginal
[1,2]; fibular [3]; and axial [4]), umbilical vein (5), posterior
cardinal vein (6), anterior tibial vein (7), and small saphenous
vein (SSV [8]) appear.
At week 6 (Carnegie stage 19), the three angio-guiding nerves
(axial [sciatic; a]; cranial [femoral; b]; and caudal [small sciatic;
c]) appear.
At week 7 to 8 (Carnegie stage 23), the following appear:
marginal vein (1); fibular vein (3); axial vein (4); umbilical vein
(5); anterior tibial vein (7); small saphenous vein (8); posterior
tibial vein (9); femoropopliteal axis (10); deep femoral vein (11);
epigastric vein (12); cranial extension of the SSV (13); great
saphenous vein (14); arch of the SSV (A).
Note the direction of blood circulation coming from the umbilical
vein: from the root to the extremities of the veins (black arrows),
and then circulated back by the arteries (red arrows).
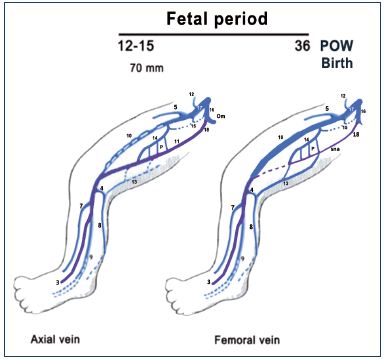
Figure 8. Summary of the fetal period of development (after
week 8).
The primitive veins are colored in purple. Notice that the fetus
(weeks 9 to 15) commonly has a big axial vein while the femoral
vein is smaller and plexus shaped. Later, the axial vein becomes
a small arcade along the sciatic nerve, and the femoral vein
becomes the main trunk of the thigh in the majority of cases at
birth, commonly with a collateral canal.
Abbreviations: 3, fibular veins; 4, arch of the SSV; 5, great
saphenous vein; 7, anterior tibial veins; 8, SSV; 9, posterior tibial;
10, femoral vein; 11, axial vein; 12, epigastric vein; 13, thigh
extension of SSV; 14, deep femoral vein; 15, obturator vein; 16,
hypogastric vein; 17, external iliac vein; 18, inferior gluteal vein;
cc, collateral canal; P, perforating branches of the femoral vein;
POW, postovulatory week; sna, axial arcade along the sciatic
nerve; SSV, small saphenous vein.
• The oxygenated blood comes from the placenta by the umbilical vein joining the posterior cardinal vein. It then reaches the root of the limb by the primitive ischiatic vein, feeds the primitive fibular vein, and finally, forms the lateral marginal vein located at the caudal aspect of the bud. The distal part of the marginal vein will form the “primitive small saphenous vein,” the very first vein present in the adult to appear in the embryo. At that time, the nerves do not exist. The blood goes back to the root of the primitive limb by the arteries. (Figure 7, left).
• Around week 6 (47 days; embryo size, 16 to 18 mm; Carnegie stage 19), the three angio-guiding nerves appear and quickly grow along the limb bud. This will initiate the development of the deep venous system and the great saphenous vein. In fact, due to the secretion of VEGF by the Schwann cells, the three angio-guiding nerves will attract the undifferentiated vascular plexuses and induce their maturation into veins, arteries, and lymphatics. These three angioguiding nerves are: (i) axially, the sciatic nerve; (ii) cranially, the femoral nerve; and (iii) caudally, the small sciatic nerve.
• Around week 7 to 8, the three venous plexuses of the lower limb grow and mature along the three angioguiding nerves. Along the sciatic (axial) nerve, the primitive axial vein becomes a huge vein and joins the deep femoral vein upwards. At this time, this is the main venous trunk of the thigh, but it will regress to become a small arcade in the majority of adults. Along the femoral (cranial) nerve from the cranial plexus, the great saphenous vein appears together with the femoral vein, which connects upward with the ischiatic vein to form the iliac vein. Downward, the anastomosis with the axial plexus will give birth to the popliteal crossroad. Along the caudal nerve (small sciatic), the caudal plexus forms the distal part of the small saphenous vein (SSV) below the knee and the cranial extension of the SSV at the thigh level. A possible anastomosis with the axial plexus will lead to SSV termination into the popliteal axis (saphenous popliteal junction or French “crosse”). Of note, around weeks 6 to 8, there is a medial rotation of the limb, where the cranial aspect of the limb becomes medial and the caudal aspect becomes lateral.
• At the end of the 12th week, organogenesis is finished and the venous anatomy is similar to an adult. However, some remodeling of the venous axis will occur, particularly at the femoral level. The axial vein, which is a large vein, will become a small arcade along the sciatic nerve, while the femoral vein commonly reduces to a thin network along the femoral nerve and will become the main venous axis of the thigh in 90% of the cases in adults.
• After 12 weeks, remodeling of the subcutaneous part of the superficial venous system (reticulum network) will lead to its definitive anatomy at birth. The venous valves appear and are closely related to the hemodynamic patterns of the anatomical arrangement, and thus, the blood circulation.
CAAD: A New Technique to Build 3D Models Depicting the Main Steps of Venous Embryogenesis
CAAD was first used by Yucel and Baskin to identify the penis nerves.19 Our laboratory then published several papers showing the benefit of CAAD for the studies of the male and female uretra20,21 and intrapelvic innervation.22 We recently dedicated the CAAD technique to the 3D reconstruction of the embryo’s limbs.6 Briefly, the principle of this technique is to make thin horizontal slices of the limbs. After digitalization and alignment of the slices, a different staining is used, in particular, proteins S-100 and D2-40 are stained to recognize the nerves and vessels, respectively. An example is shown in Figure 9 with the reconstruction of a big axial vein at the thigh.
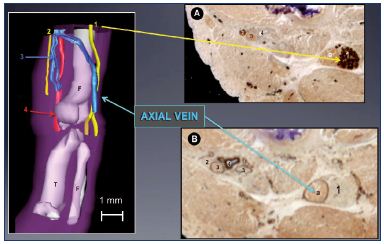
Figure 9. Immune markers for nerves and vessels in a 13-weekold
fetus.
Panel A. A 3D reconstructed limb showing a big axial vein
along the sciatic nerve, while the femoral vein is reduced to a
small plexus-shaped network located inside the femoral canal
with its companions the femoral artery and femoral nerve.
Slices of a 13-week-old fetus stained for nerve-specific immune
markers (slice A with protein S-100; Panel B) and vessel-specific
immune markers (slice B with D2-40; Panel C) using the CAAD
technique.
Abbreviations: 1, sciatic nerve; 2, femoral nerve; 3, femoral
vein; 4, femoral artery; a, axial vein; CADD, computer-assisted
anatomical dissection; f, fibula; F, femur; T, tibia.
The first results of these 3D reconstructed limbs were recently obtained from three embryos at postovulatory weeks 13 to 156 (discussed in the following section and illustrated in Figures 10 and 11), but it is a work in progress as detailed data about the earlier stages are still lacking.
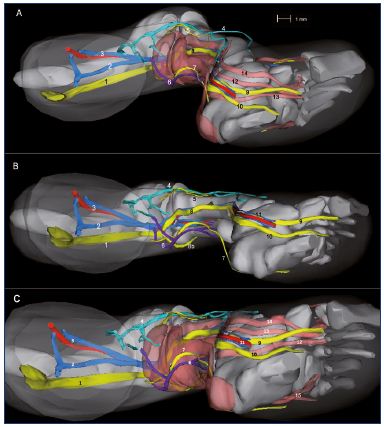
Figure 10. Three-dimensional reconstruction of a 14-week-old
fetus (right lower limb).
Medial view (Panel A), inferior view without muscles (Panel B),
and inferior view with muscles (Panel C).
Abbreviations Panel A: 1, sciatic nerve; 2, axial vein; 3, femoral
vein and artery; 4, great saphenous vein; 5, fibular nerve; 6,
small saphenous vein; 7, sural nerve; 8, tibial nerve; 9, medial
plantar nerve; 10, lateral plantar nerve.
Abbreviations Panels B and C: 1, posterior tibial artery and two
veins; 2, vastus medialis muscle; 3, semimembranous muscle;
4, rectus femoris muscle; 5, lateral gastrocnemius muscle; 6,
soleus muscle; 7, long fibular muscle; 8, tibialis anterior muscle;
9, extensor halluces longus.
Figure 10B has been reproduced from reference 6: Kurobe et al.
Surg Radiol Anat. 2015;37:231-238. © 2014, Springer Verlag
France.
Clinical Applications of Embryogenesis
Anatomical variations of the femoral vein
Three types of anatomical variations of the femoral vein have been observed in 12% of adults (Figure 12).23 If the large primitive “axial vein” of the embryo persists, then either an axiofemoral trunk (unitruncular layout, 3%) or axiofemoral vein (bitruncular layout, 9%) is found in adults. Most commonly, a modal layout (88%) will be found in the adult’s anatomy: the primitive axial vein becomes hypotrophic and reduced to a small arcade. The main vein of the thigh is a femoral vein inside the femoral canal.
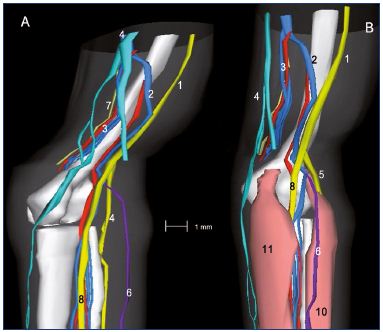
Figure 11: Three-dimensional reconstruction of a 15-week-old
fetal right lower limb without (Panel A) and with muscles (Panel B).
Abbreviations: 1, sciatic nerve; 2, axial vein; 3, femoral vein
and artery; 4, great saphenous vein; 5, saphenous nerve; 6,
small saphenous vein; 7, sural nerve; 8, tibial nerve; 8b, fibular
nerve; 9, medial plantar nerve; 10, lateral plantar nerve; 11,
posterior tibial artery and two veins; 12, tendon of the hallux
flexor longus; 13, flexor digitorum longus; 14, posterior tibial
tendon; 15, tendons of peronaeus longus and brevis.
Reproduced from reference 6: Kurobe et al. Surg Radiol Anat.
2015;37:231-238. ©2015, Springer Verlag France.
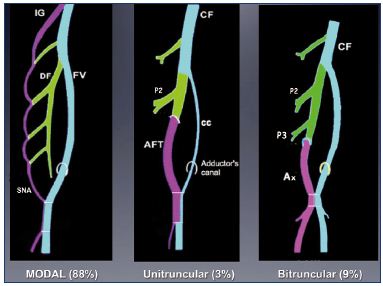
Figure 12. Anatomical variations of the femoral vein occur in
12% of adults.
If the primitive “axial vein” of the embryo persists, either an
axiofemoral trunk (unitruncular layout, 3%) or an axiofemoral
vein (bitruncular layout, 9%) can be found in adults. In the modal
layout (88%), the primitive axial vein becomes hypotrophic and,
reduced to a small arcade.
Abbreviations: AF, axiofemoral vein; AFT, axiofemoral trunk; cc,
collateral canal; CF, common femoral vein; DV, deep femoral vein;
FV, femoral vein; IG, inferior gluteal vein; P2, second perforator
vein; P3, third perforator vein; SNA, sciatic nerve arcade.
Anatomy of the lateral network of the leg and fibular perforators in the adult
A thin marginal venous network of the leg, commonly seen in adults, is a remnant of the primitive marginal vein. Just like in the embryo, it is connected to the fibular veins by several fibular perforators, as is nicely shown in Figure 13.
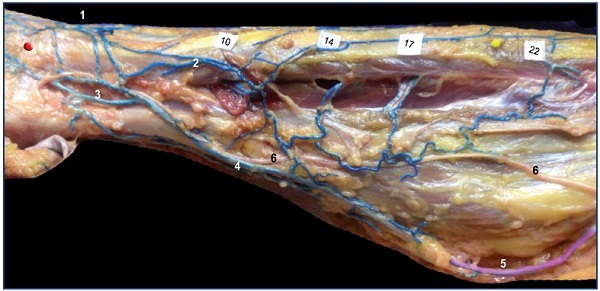
Figure 13. Remnant of marginal vein and fibular perforators in an adult.
Anatomical dissection after latex injection in the left leg (lateral view after resection of the fibula bone). Notice the alignment of the
fibular perforator veins connecting the thin marginal network to the fibular veins at specified locations (10, 14, 17, and 22 cm from
the apex of the lateral malleolus).
Abbreviations: 1, dorsal foot network connected to the anterior tibial perforators; 2, lateral marginal network connected to the fibular
veins; 3, calcaneus perforator vein; 4, Achilean vein joining the small saphenous vein.
Conclusion
Understanding embryogenesis is important mostly in terms of clinical applications: it improves our anatomical knowledge about the venous system and is particularly useful to perform a venous mapping for all CVD patients. Embryology is also essential for the diagnosis and management of congenital vascular malformations (CVM). As explained in the Hamburg classification of CVM,24 the severity of the malformations is related to the stage of the embryo’s development when they occur: (i) before week 4, they provide severe “extratruncular” malformations; (ii) between week 4 and 8, they are responsible for less severe “truncular” malformations; and (iii) after the organogenesis period (week 8), simple anatomical variations will occur.

1. Pepper MS. Angiogenèse et morphogenèse de l’arbre vasculaire: de la biologie cellulaire à la clinique [in French]. Médecine/Sciences. 2000;16:1378-1386.
2. Yamada S, Nakashima T, Hirose A, Yoneyama A, Takeda T, Takakuwa T. Developmental anatomy of the human embryo—3D-imaging and analytical techniques. In: Yamada S, Takakuwa T, eds. The Human Embryo. Rijeka, Croatia: InTech; 2012:111-126.
3. O’Rahilly R, Müller F. Developmental Stages In Human Embryos. Meriden, Connecticut: Meriden-Stinehour Press; 1987.
4. T he Virtual Human Embryo. A digital image database of serially sectioned human embryos from the Carnegie collection. New Orleans, Lousiana: Louisiana State University Health Sciences Center; 2011. http://virtualhumanembryo. lsuhsc.edu. Accessed January 27, 2015.
5. Yamada S, Uwabe C, Nakatsu T, et al. Graphic and movie illustrations of human prenatal development and their application to embryological education based on the human embryo specimens in the Kyoto collection. Dev Dyn. 2006;235:468-477.
6. Kurobe N, Hakkakian L, Chahim M, Delmas V, Vekemans M, Uhl JF. 3D-reconstruction of the lower limb’s venous system in human fetuses using the computer-assisted anatomical dissection (CAAD) technique. Surg Radiol Anat. 2015;37:231-238.
7. Born G. Die patten-modelier methode. Arch Mikrosk Anat. 1883;22:584-599.
8. Hochstetter F. Beiträge zur Entwicklunggeschichte des Venensystems der Amnioten. III. Säuger. In: Gegenbaur C ed. Morphologisches Jahrbuch: eine Zeitschrift für Anatomie und Entwickelungsgeschichte. Vol 20. Leipzig, Germany: Verlag Von Wilhelm Engelmann; 1893:543-648.
9. Lewis FT. Development of the veins in the limbs of rabbit embryos. Am J Anat. 1906;5:113-120.
10. Kokova J, Hoakova M. L’évolution des veines préet post-natales [in French]. Phlébologie. 1983;46:241-245.
11. Stephan G. Human Pictorial Embryology. Seattle, WA: University of Washington Press; 1998.
12. T owers M, Tickle C. Growing models of vertebrate limb development. Development. 2009;136:179-190.
13. T abin CJ. Retinoids, homeoboxes, and growth factors: toward molecular models for limb development. Cell. 1991;66:199- 217.
14. Eshkar-Oren I, Viukov SV, Salameh S, et al. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263-1272.
15. G illot C. Dispositifs poplités: hypothèses et certitudes. Phlébologie. 1998;51:65-74.
16. Uhl JF, Gillot C. Embryology and threedimensional anatomy of the superficial venous system of the lower limbs. Phlebology. 2007;22:194-206.
17. Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693-705.
18. Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741-753.
19. Yucel S, Baskin LS. Identification of communicating branches among the dorsal, perineal and cavernous nerves of the penis. J Urol. 2003;170,153-158.
20. Karam I, Droupy S, Abd A, et al. The precise location and nature of the nerves to the male human urethra: histological and immunohistochemical studies with three-dimensional reconstruction. Eur Urol. 2005;48:858-864.
21. Karam I, Droupy S, Abd-Alsamad I, Uhl JF, Benoit G, Delmas V. Innervation of the female human urethral sphincter: 3D reconstruction of immunohistochemical studies in the fetus. Eur Urol. 2005;47:627-633.
22. Alsaid B, Bessede T, Diallo D, et al. Computer-assisted anatomic dissection (CAAD): evolution, methodology and application in intra-pelvic innervation study. Surg Radio Anat. 2012;34:721-729.
23. Uhl JF, Gillot C, Chahim M. The anatomical variations of the femoral vein. J Vasc Surg. 2010,52:714-719.
24. Lee BB. New approaches to the treatment of congenital vascular malformations (CVMs)—a single centre experience. Eur J Vasc Endovasc Surg. 2005;30:184-197.
FURTHER READING: RECONSTRUCTION-TYPE BORN:
a. Peter K. Rekonstruktions methoden. Greifswald, Germany; 1922.
b. Born G. Uber die Nasenhchlen und den Thr’anennasengang der Amphibien. Morph Jb. 1876;2:577-645.
c. Born G. Die Plattenmodellirmethode. Archiv für mikroskopische Anatomie. 1886;22:584 (1883).
d. Born G. Noch einmal die plattenmodellirmethode. Z Wiss Mikrosk. 1888;5(4):433-455.
e. Kastschenko N. Die graphische isolierung. Anal Anz. 1887;2:426-435.
f. Kerr JG. The development of Lepidosiren paradoxa III. Development of the skin and its derivatives. Q J Microsc Sci. 1902;46:418-459.
g. Lewis WH. The use of guide planes and plaster of paris for reconstructions from serial sections: some points on reconstruction. Anat Rec. 1915;9:719-729.
h. de Beer GR. The Development of the Vertebrate Skull. Oxford, UK: Clarendon Press; 1937.