Histochemical insight into lymphangiogenesis
Oita University
Oita – Japan
SUMMARY
The lymphatic vascular system consists of a network of thin-walled vessels, and it plays an important role in transportation of extravasated fluid and macromolecules from tissues back to blood circulation, as well as in many pathological processes, including tumor metastasis and lymphedema. The structural organization and fine distribution of lymphatic vessels in the tissues are very important in the pathophysiology of a variety of microcirculatory disorders, infectious diseases, and cancer. The lymphatic system develops through a process known as lymphatic development (lymphangiogenesis). However, studies of the lymphangiogenesis of the lymphatic system have been hampered by a lack of lymphatic specific markers. Recently, the molecules that specifically control lymphangiogenesis and lymphatic regeneration have been identified.1 Thus, the discovery of new lymphatic endothelial cell (LEC)-specific markers has now provided new insights into the molecular mechanisms that control lymphatic development and function. We herein review the histochemical insight into the field of lymphangiogenesis, with special emphasis on the novel and reliable LEC markers, such as 5’-nucleotidase and VEGF-C/VEGFR-3, as well as on lymphatic regeneration after experimental injury.2,3
MARKERS OF LYMPHATIC ENDOTHELIAL CELLS
Enzyme histochemistry
5’-nucleotidase (5’-Nase), an important enzyme in the metabolism of nucleotides, has widely been employed as a marker of cell membranes. 5’-Nase histochemical staining has proved to be an effective method for differentiating initial lymphatics from blood capillaries, based on its much higher activity on lymphatic than on blood vascular endothelium (Figure 1a, 1b).4 In addition, blood endothelia, especially artery and arterial capillaries, have comparatively strong alkaline phosphatase (ALPase) activity. Thus, two types of vessels have been distinguished by using 5’-Nase-ALPase double staining (Figure 1c).5,6 Furthermore, dipeptidyl aminopeptidase IV (DAPase) activity is markedly higher in the endothelium of the venous part of the capillaries and venules (Figure 1d, 1e). Thus, a differential staining method using DAPase-ALPase double or DAPase-ALPase-5’-Nase triple staining for venous and arterial capillaries and/or lymphatics is effective for several tissues, not only in laboratory animals but also in humans (Figure 1f).7
SEM examination of 5’-Nase–stained tissues on wholemount preparations or tissue blocks after cryosection allows precise analysis of the abluminal aspects and threedimensional structure of the lymphatic network.8,9 Adequate treatment with NaOH effectively removes connective tissue matrices from the specimens and enables clear visualization of the three-dimensional structures of the immuno-histochemical stained lymphatics in both secondary emission and backscattered images.10
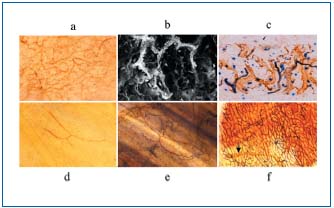
Figure 1. Light micrographs (a, c, d, e, f) and SEM-BEI view (b)
of whole-mount preparations and cryosections stained with
enzyme-histochemical staining. (a) 5’-Nase- positive lymphatics on
a whole-mount preparation of rat skin. (b) The 5’-Nase-positive
lymphatics are strongly highlighted. (c) 5’-Nase-positive lymphatics
(brown) and ALPase-positive blood vessels (blue) on a rat skin
cryosection stained with 5’-Nase-ALPase double staining. Figures
1d, e show a DAPase-positive vein and an ALPase-positive artery
on a whole-mount presentation of the rat cecal wall stained with
DAPase or DAPase-ALPase double staining. (f) Whole-mount preparation
of the rat stomach wall stained with DAPase-ALPase-5’-
Nase triple staining. a: x 45, b: x 200, c: x 250, d-f: x 100
Immunohistochemistry
5’-Nase mAb (JC815) and ecto-5’-Nase mAb (CD73): when 5’-Nase antigenicity, rather than its activity, is considered, 5’-Nase mAb specific for the lymphatic endothelium, instead of adenosine monophosphate, can serve immunohistochemically as a useful marker for cell selection and in vitro cultivation. Our 5’-Nase-mAb, (JC815) immunoreactivity was distinctly expressed on the lymphatic vessels of several tissues from mice and rats, in comparison with 5’-Nase staining controls.11 In the pancreas, JC815 strongly stained the interlobular lymphatic endothelium and was similar to the 5’-Nase staining pattern (Figure 2a, 2b). CD31 strongly stained arteries (Figure 2 C). Furthermore, in the rat tongue, immunohistochemical analysis of ecto-5’-Nase (CD73) is assumed to provide not only reassessment of the validity of 5’-Nase as a lymphatic endothelial marker, but also new information, including technical benefit (Figure 2d, 2e). The 5’-Nase activity, CD73 immunoreactivity and hybridization signals for its mRNA were colocalized in the lymphatic vessels, including central lacteals of the small intestine, suggesting that 5’-Nase is actually produced in lymphatic endothelial cells and allocated to their cell membrane as an enzyme to regulate lymph production and flow.12 These findings support our view that 5’-Nase is potential marker of lymphatics, and indicates the usefulness of the histochemical methods for 5’-Nase not only for demonstration of lymphatics, but also for examining the functional roles and dynamics of 5’-Nase in lymphatic endothelial cells in physiological and pathological conditions.
VEGFR- 3 Flt-4 and its ligand VEGF-C: a highly glycosylated class III cell surface tyrosine-kinase receptor, VEGFR- 3/Flt-4 was preferentially immunolocalized in the structures corresponding to the 5’-Nase-positive lymphatics in the lesion (Figure 2f), and the immunoreaction products were ultrastructurally distributed on the cell membrane of lymphatic vessels. In contrast, immunostaining for VEGF-C demonstrated significant reaction products in many stromal cells, which predominantly demonstrated signals for VEGF-C mRNA in in situ hybridization. Therefore, the combination of 5’-Nase mAb with other lymphatic endothelial cell markers, and enzyme histochemistry with immunohistochemistry, may provide new possibilities for lymphatic investigation.
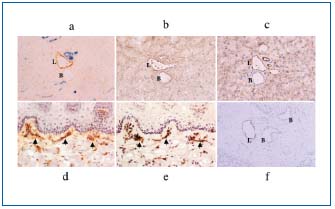
Figure 2. Enzyme- and immunohistochemical staining of the
lymphatics and blood vessels of several mouse and rat tissues. (a-c)
Serial sections of the mouse pancreas stained with 5’-Nase-ALPase
(a), JC815 (b), and CD31 (c) staining, respectively. (d, e) Serial
sections of the rat tongue stained with 5’-Nase (d) and CD73 (e)
staining, respectively. (f) VEGFR-3-positive lymphatics. x 160
LYMPHOSTASIS BY TD BLOCKAGE
The effects of experimentally induced lymphedema have been studied in the extremities and internal organs of various animals and the results have been discussed in the light of the clinical understandings of lymphedema.13,14 After thoracic duct (TD) blockage in rats, the mucosal and submucosal compartments of the small intestine in lymphostasis showed tortuous lymphatic networks and saccular dilations of the lymphatic vessels surrounded by fibrinoid materials, swollen collagen fibers, and focal accumulation of mononuclear cells. A tendency for reduced 5’-Nase activity in the TD endothelial cells became visible when the lymph flow was obstructed by TD blockage (Figure 3a).15 During TD blockage-induced lymphostasis, the 5’-Nase reaction product was almost undiscernible as a continuous demarcation of the endothelial layer within 2 weeks. Interestingly, the reduced 5’- Nase activity appeared earlier in the intramural intestinal lymphatics than in the TD (Figure 3b). Prolonged obstruction of intestinal lymph flow will progressively aggravate peripheral lymphostasis and lymphatic incompetence. The effect of TD blockage on the endothelial cells of the intestinal lymphatics and TD was temporary, and lasted for about 6 weeks after ligation. The gradual recovery of the structure and function of the endothelial cells might be due to the observation that effective circulation is established by the marked regenerative capacity of smaller lymphatics, rapid development of collateral pathways around the blockage, and a relatively high rate of lymphovenous anastomosis formation.16
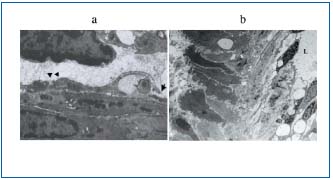
Figure 3. TEM views of small intestinal lymphatics in TD blockage
animals. (a) After 2 days of TD blockage, endothelial cells
frequently represent open intercellular (arrow) and interdigitating
(arrowhead) junctions. (b) After 4 days of TD blockage, 5’-Nase
activity of intramural intestinal lymphatics appears to reduce.
a: x 15 000, b: x 2300
LYMPHATIC DEVELOPMENT
(LYMPHANGIOGENESIS)
In early embryonic tissue, endothelial cells of newly formed lymphatic-like structures usually show extremely low 5’-Nase activity.17 Interrupted weak or absent staining of endothelial cells was seen on newly formed lymphaticlike structures in the early stage, although no 5’-Nase reaction product was observed in blood vascular endothelial cells. Lymphatic vessels in the rat stomach revealed increased 5’-Nase activity as the animals grew.18 Thus, 5’-Nase staining appears to be impractical for distinguishing developing lymphatics and blood vessels. On the other hand, in the early developing gastric wall, anti- VEGFR-3 was expressed in a cluster of circular lymphaticlike structures, which were gathered into several groups.18 VEGFR-3 makes it possible to identify developing and regenerating lymphatic vasculature by localizing the antigen. VEGFR-3 binding to endothelial cells showed variations in staining intensity in the lymphatic wall and among samples. Developing endothelial cells of lymphatic and blood vasculatures react with VEGFR-3 during the early embryonic stage (Figure 4a), although immature lymphatic vessels were usually stained with VEGFR-3 staining more intensely than typical lymphatic vessels. This suggests that lymphatic endothelial cells have similar molecular physiological features to blood vascular endothelia, and probably originate from the sprouting of small venous structures. The staining results for cell proliferation should be carefully analyzed. This opinion is not contrary to the observation that lymphangiogenesis originates from lymphatic vessels.19 The present findings are in close agreement with the view that VEGFR-3 is widely expressed to the lymphatic endothelium at later developmental stages and in postnatal life.20 Many circular and incomplete lymphatic-like structures expressing VEGFR-3 show an obvious accumulation, indicating that lymphangiogenesis occurs sequentially in definite regions in the early embryonic stage.
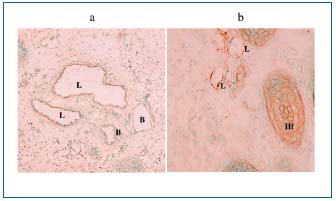
Figure 4. Photomicrographs of fetal monkey gastric cryosection
(a) and an adult mouse skin section (b) treated with VEGFR-3
staining. The VEGFR-3-expressing lymphatic-like structures (L)
are seen in the submucosa (a) and in the subcutaneous tissue (b).
Hf: hair follicle x 100, x 400
LYMPHATIC REGENERATION
Wound healing skin
Wound healing skins in mice were processed for 5’-Nase and VEGFR-3 histochemical staining to distinguish lymphatics from blood capillaries and analyze lymphangiogenesis.3 In the wound skin of the mice 3 to 5 days after injury, anti-VEGFR-3 immunopositive signals unevenly appeared in 5’-Nase-positive lymphatic vessels in the subcutaneous tissue. On days 7 to 15, numerous accumulated vasculatures were stained for 5’-Nase and PECAM-1/ CD31, extending irregularly along the wound edge. Ultrastructural changes in lymphatic vessels developed at different stages, from lymphatic-like structures to newlyformed lymphatic vessels with an extremely thin and indented wall. The generating signals of VEGFR-3 on lymphatic endothelial cells appeared as early as 3 days after injury in the subcutaneous tissue, much earlier than in the dermis. The expression pattern of VEGFR-3 in regenerating tissues was extremely uneven on the lymphatic wall, indicating that endothelial sprouting might begin from its up-expressing side. The most noteworthy finding was that numerous circular and irregular lymphatic-like structures with VEGFR-3 expression were distributed in the dermal and subcutaneous tissues along the wound edge (Figure 4b). According to the maturation of lymphatic vessels, the lymphatic wall became slender and irregular, and the endothelium protruded into the lumen and adjacent connective tissue. Intercellular junctions underwent morphological changes, from simple end-to-end to overlapping and interdigitating. The simple junction might facilitate separation, spreading, and migration of endothelial cells during lymphatic remodeling in compliance with tissue repair patterns. This finding appeared to be in good agreement with our previous observations in the early embryonic tissues of the monkey.18 However, 5’-Nase activity in the endothelial cells of newly-formed lymphatic vasculature is low during the wound healing process.
Combined histochemical staining for 5’-Nase and VEGFR-3 with multiple endothelial cell markers is useful for studying regenerating lymphatics and their relationship with blood vessels. VEGFR-3–expressing vasculatures occurred in the dermal-subcutaneous transitional area at the early stage of wound injury, whereas a 5’-Nasepositive lymphatic structure along the wound edge underwent morphological changes. These findings indicated that sprouting and growth of regenerating lymphatic vessels are active processes in the healing tissue response.
Regrowing an intestinal muscle coat after myectomy
The lymphatic regrowth from the surviving vessels in the severed stumps of the intestine occurred behind the regeneration of other tissue elements21 including blood vessels. The vascular arcades and terminal expansions, which were observed in the present lymphatic regeneration, are presumed to serve as growth points in lymphangiogenesis, as in angiogenesis.22 The unusual ultrastructural characteristics of the regrowing lymphatic endothelial cells, involving spindle-shaped and elongated cytoplasm, filopodium-like cytoplasmic projections and numerous intracellular thin filaments, probably indicate the high regenerative and migratory potential of the cells to establish new vascular channels.
The enzyme histochemistry for 5’-Nase demonstrated the manner of lymphatic regrowth, which was established by vascular sprouting from preexisting lymphatics and structural changes in the endothelial cells indicating their high migratory potential (Figures 5 a,b). The expression of 5’-Nase in the regenerating lymphatics was increased in proportion to their growth. These findings suggest that 5’-Nase may be correlated with the functional maturation of the regenerating lymphatics, because it is thought to facilitate membrane transport of lymph.12
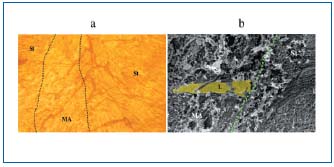
Figure 5. Light micrograph (a) and SEM view (b) of regrowing
lymphatics from a postmyectomized week-4 rat jejunum with
5’-Nase staining. MA: myectomized area, St: stump. The broken
line indicates the cut line of the muscle coat. a. Regrowing
lymphatics (arrows) with 5’-Nase activity show thickening and
varicose structures. b. Secondary emission image of regrowing
lymphatics arising from a vascular arcade in the lesion. 5’-Nasepositive
lymphatic endothelial cells are colored yellow. x 800
VEGF-C was expressed in a subpopulation of regenerating interstitial cells which are close to the regrowing 5’-Nasepositive lymphatics. Furthermore, the cells with VRGF-C transcripts showed a marked increase in cell number and intensity of mRNA signals during progression of tissue regeneration. These findings imply that VEGF-C is upregulated upon lymphatic regrowth in the repairing intestinal tissue following myectomy. The VEGFR-3 immunoreactivity was preferentially distributed in the 5’-Nase-positive regrowing lymphatics in the lesion and restricted on the cell membranes of lymphatic endothelial cells. Thus, the VEGFR-3 immunoreactivity demonstrated in this study is considered to be expressed in the regenerating lymphatics, although the presence of VEGFR-3 in the blood vasculature has been reported in cutaneous wound healing.23 The expression of 5’-Nase in the regenerating lymphatics was increased in proportion to their growth, VEGF-C, a highly specific lymphangiogenic factor, was highly expressed in a subpopulation of interstitial cells, being close to the regrowing lymphatics with the immunoreactivity of its receptor, VEGFR-3, in the regenerative area. The present findings suggest that transaction of the intestinal muscle coat affords a useful experimental model for the investigation of lymphatic regeneration in tissue repair, and that the interstitium may play a crucial role in lymphangiogenesis.
With regard to adhesion, differentiation, and migration of endothelial cells, some elements, including 5’-Nase and eNOS,15 the extracellular matrix of the basement membrane, and components of the surrounding connective tissues, are very important factors in the formation of developing vasculature. More recently, several other proposed markers for lymphatic endothelial cells, eg, LYVE-1, a homologue of the CD44 hyaluron receptor,24,25 podoplanin,26,27 and Prox 128,29 have emerged, but there are still questions about their reliability and specificity, and they need further study. However, coexpression of these new markers with so-called routine differentiating reagents, such as laminin, collagen type IV, and á-smooth muscle actin, is definitely helpful in analyzing the functional-structural properties of endothelial cells of both lymphatic and blood vessels.

REFERENCES
2. Shimoda H, Takahashi Y, Kato S. Regrowth of lymphatic vessels following transaction of the muscle coat in the rat small intestine. Cell Tissue Res. 2004;316:325-338.
3. Ji RC, Miura M, Qu P, Kato S. Expression of VEGFR-3 and 5’-Nase in regenerating lymphatic vessels of the cutaneous wound healing. Microvasc Res Tech. 2004;64:279-286.
4. Kato S. Enzyme-histochemical identification of lymphatic vessels by light and backscattered image scanning electron microscopy. Stain Technol. 1991;65:131-137.
5. Kato S. Organ specificity of the structural organization and fine distribution of lymphatic capillary networks: histochemical study. Histol Histopathol. 2000;15:185-197.
6. Kato S. Morphological characteristics of the lymphatics: enzyme-histochemical demonstration. Phlebolymphology. 2000;27:17-22.
7. Kato S, Itonaga I, Ji RC, Miura M. Enzyme triple staining for differentiation of lymphatics from venous and arterial capillaries. Lymphology. 1996;29:15-19.
8. Kato S, Miura M, Miyauchi R. Structural organization of the initial lymphatics in the monkey mesentery and intestinal wall as revealed by an enzyme-histochemical method. Arch Histol Cytol. 1993;56:149-160.
9. Kato S, Gotoh M. Application of backscattered electron imaging to enzyme histochemistry of lymphatic capillaries. J Electron Microsc. 1990;39:186-190.
10. Shimoda H, Kajiwara T, Takahashi Y, et al. Scanning electron microscopic observation of immunohistochemically specified stromal cells following the NaOH maceration method. J Electron Microsc. 2002;512:137-139.
11. Ji RC, Qu P, Kato S. Application of a new 5’-Nase monoclonal antibody specific for lymphatic endothelial cells. Lab Invest. 2003;83:1681-1683.
12. Shimoda H, Takahashi Y, Kajiwara T, Kato S. Demonstration of the rat lymphatic vessels using immunohistochemistry and in situ hybridization for 5’-nucleotidase. Biomed Res. 2003;24:51-57.
13. Casley-Smith JR, Clodius L, Foldi-Boresok E, Gruntzig J, Foldi M. The effects of chronic cervical lymphostasis on regions drained by lymphatics and by prelymphatics. J Pathol. 1978;124:13-17.
14. Olszewski WL. The treatment of lymphedema of the extremities with microsurgical lymph-venous anastomoses. Int Angiol. 1988;7:312-321.
15. Ji RC, Kato S. Histochemical analysis of lymphatic endothelial cells in lymphostasis. Microsc Res Technique. 2001;55:70-80.
16. Piller BB, Clodius L. Experimental lymphoedema: its applicability and contribution to our clinical understanding. In: Johnston MG, eds. Experimental Biology of the Lymphatic Circulation. Amsterdam: Elsevier; 1985:189-230.
17. Ji RC, Kato S. Enzyme-histochemical study on postnatal development of rat stomach lymphatic vessels. Microvasc Res. 1997;54:1-12.
18. Ji RC, Kato S. Lymphatic network and lymphangiogenesis in the gastric wall. J Histochem Cytochem. 2003;51:331-338.
19. Witmer AN, van Blijswijk BC, Dai J, et al. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490-497.
20. Wilting J, Neeff H, Christ B. Embryonic lymphangiogenesis. Cell Tissue Res. 1999;297:1-11.
21. Takahashi Y, Shimoda H, Kato S, Noguchi T, Uchida Y. Immunohistochemical study on myenteric nerves following transaction of the muscle coat in the rat small intestine, with special reference to glial cells line-derived neurotrophic factor(GDNF) and its receptor (Ret). Biomed Res. 2002;23:73-83.
22. Rhodin JAG, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21:1-34.
23. Paavonen K, Puolakkainen P, Jussila L et al. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol. 2000;156:1499-1504.
24. Banerji S,Ni J,Wang SX et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;1244:789-801.
25. Jackson DG, Orevo R. Clasper S, Banerji S. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317-321.
26. Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:358-394.
27. Weninger W, Partanen TA, Breiteneder- Geleff S, et al. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79:243-251.
28. Wigle JT, Harvey N, Detmar M. et al. An essential role for Prox 1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1501-1513.
29. Hong YK, Harvey N, Noh YH et al. Prox 1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351-357.
