Histopathology of great saphenous vein valves in primary venous insufficiency
Vascular Surgery), Italy
Udine, Italy
Florence, Italy
University of Florence, Italy
Pharmaceutics, Italy
University of Florence, Italy
ABSTRACT
Objective: To verify some of the previous findings of venous valves described in the literature, their pathophysiological significance, and the clinical implications. Materials and methods: The elementary components of 65 proximal valves of the great saphenous vein and their interrelationships were subjected to histopathological examination. Valves were taken from patients subjected to great saphenous vein surgical removal for varicose veins of the lower limbs. Measurements and morphological evaluations were performed by optical microscopy.
Results: The valvular sinus, leaflet insertion segment, and proximal portion of the cusp undergo parallel variations of thickness. The thickening of the proximal portion of cusp is related to the smooth muscle cells increasing in the leaflet insertion segment, and the elastic layer dissociation. The thickening of the distal portion of cusp depends on the collagen component, and it may shorten, crumple, and lead to the formation of a thickened border. The vein wall in a commissural aneurysm is usually thinner than in the valvular sinus.
Alterations in the intima, in the elastic membrane, and in the media were found in the 98% of the valvular anulus. Ectasia and asymmetry of the venous wall are mainly related to the muscular hypoplasia of the media.
Conclusions: The development of primary venous insufficiency seems to be due to the following tissue alterations: dilatation of the valvular anulus and hypotrophy of the cusp. The hemodynamic mechanical injury increases the tissue damage of both anulus and cusps. This pathophysiological interpretation of venous insufficiency leads to the need a detailed diagnostic procedure before reparative surgery of valves.
INTRODUCTION
Starting from the beginning of the century and up until about 10 years ago, several authors have developed the theory of “primary vein wall dilatation and late cusp degeneration” in order to explain the development of primary venous insufficiency. On these bases, various techniques for surgical valve repair in deep and superficial veins were developed and performed with satisfactory results. However, some discrepancies with the basic pathophysiological concept were observed, and the theory of “primary valve degeneration” gave no satisfactory explanation for either the onset or the development of the disease.
A recent retrospective study performed on 72 cases affected with saphenofemoral junction insufficiency subjected to external valvuloplasty demonstrated that the best results can be obtained in cases with early disease, that in 18% of the early cases the valvular cusps were already damaged, and that in 50% of the late cases the cusps still had normal structure and function.
Different parts and surfaces of cusps were taken into consideration in the previous studies: the leaflet insertion segment and the parietal and luminal parts of the leaflet, as well as the proximal and distal portions and border. The main tissue alterations observed were the following: increase in smooth muscle and connective tissue cells in the leaflet insertion segment; intimal plaques below the leaflet insertion segment (endophlebohypertrophy); leaflet irregular thinning or thickening with elastic membrane fragmentation and dissociation and fibroblast proliferation; segmentary irregular thickness of leaflet parietal surface (crypts); and cyst-like structures containing erythrocytes beneath the leaflet endothelium in some cases, and thickening of the free borders. Postthrombotic-like alterations in subterminal valves of long saphenous varicose veins led several authors to develop the theory of postthrombotic valvular destruction. Inflammatory cells were found in cases with inflammatory general diseases.
Some normal cusps were observed in dilated or aneurysmatic veins and, in some others, pathological cusps were found in undilated valvular anulus. The same finding emerged on a first verification performed in our departments by clinical, echographic, angioscopic and histopathological studies on 42 saphenofemoral valves. However, the conclusion of the study was that in the majority of the cases primary anulus dilatation and some early hypotrophic cusp damage can develop simultaneously.
OBJECTIVES
To detect the relationships between the elementary alterations of the venous wall and the cusps, to define the concept of cusp hypotrophy, and its clinical significance, and to clarify the pathophysiology of primary venous insufficiency in order to perform a more detailed diagnostic assessment of venous valves before reconstructive surgery.
MATERIALS AND METHODS
The valvular sinus, commissure, leaflet insertion segment, and leaflet thickness were evaluated and measured (microns) (Figure 1).
The clinical and anatomical characteristics of the patients and histological techniques employed are summarized in Table I. Seventeen longitudinal and 48 cross-sections (two were oblique) of proximal saphenous valves were studied. The protocol of the microscopic evaluation is summarized in Table II. The anatomical and histological details subjected to microscopic evaluation are schematically described in Figure 1.
Correlations between thickness, morphology, and elementary tissue alterations of the valvular apparatus were investigated, evaluated, and underwent statistical analysis.
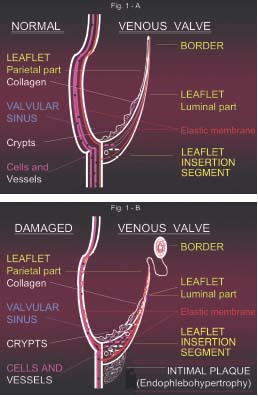
Figure 1. A – Schematic example of a great saphenous vein
proximal valve in a longitudinal section. The valvular apparatus
appears to be free from elementary tissue alterations, as it should
be in normal conditions. The different valvular parts and the
anatomical and histological components studied are shown.
B – In this example of a damaged valve we summarize the main
alterations observed: thinning of the venous wall and distal portion
of the leaflet, where the border is often crumpled and thickened;
thickened leaflet insertion segment with an increased number of
cells and vessels; elastic membrane fragmentation and dissociation;
flattened crypts; and endophlebohypertrophy below the valve
leaflet insertion segment. The more frequent and significant
combinations of these elementary tissue alterations are described
and analyzed in the text.

Table I. Histopathological examination of 65 proximal great
saphenous vein valves. Clinical and anatomical characteristics and
histological techniques.
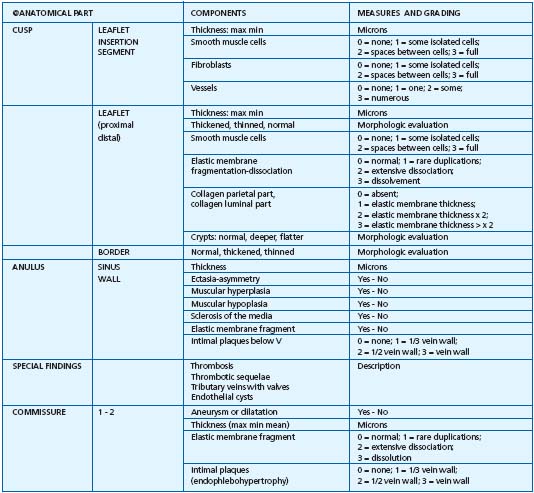
Table II. Histopathological examination of 65 proximal great saphenous vein valves: parameters examined and study protocol.
RESULTS
Nonsignifiant correlations are summarized in Table III. Flattened or absent crypts were mainly observed in the distal portion of thinned leaflets (P=0.075). Thickening of the border was detected in 71.8% of the samples. Endophlebohypertrophy in the anulus below the valve was observed in 21.4% of cases, and dilatation or aneurysm of one or both commissures in 74.4%. Some inflammatory cells were detected in the valve tissues of only six cases with thrombotic occlusion. No inflammatory cells or microthrombi were observed in any of the other cases.
Significant correlations are summarized in Table IV. The main results are the following. The mean thickness values of the valve leaflet insertion segment, valvular sinus, and proximal part of the leaflet undergo parallel variations (Figures 1 and 2). The thickening of the whole leaflet appeared to be mainly related to the collagen thickness in the parietal part (Figure 3). The number of smooth muscle cells in the leaflet insertion segment increases in the thickened proximal part of the leaflet (Figure 4). The fragmentation- dissociation of the elastic membrane and the increase in elastic fibrils are prevalent in the thickened leaflets (Figure 5). A thickened border may represent the result of retraction and crumpling of the thinned hypotrophic distal part of the leaflet (Figures 6 and 7). The ectasis-asymmetry of the vein wall in the valvular sinus and commissure are mainly due to muscular hypoplasia. Sclerosis of the media, elastic membrane fragmentation-dissociation, and intimal fibromuscular hypertrophy with plaques were found in 97% of the vein walls examined (Figure 8).

Table III. Histopathological examination of 65 proximal great saphenous vein valves: no significant correlations.
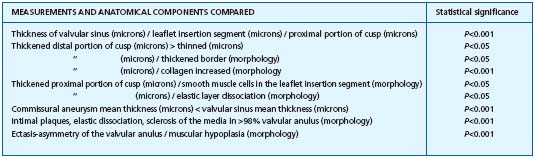
Table IV. Histopathological examination of 65 proximal great saphenous vein valves: significant correlations.
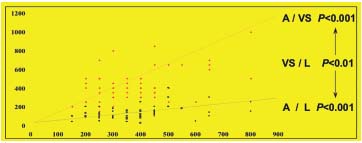
Figure 2. Cusp leaflet insertion segment,
leaflet proximal part, and valvular
sinus thickness evaluation and comparison.
Comparison between cusp leaflet insertion
segment (A)and valvular sinus (VS) thickness:
P<0.001.Comparison between cusp leaflet
insertion segment (A) and leaflet proximal
part (L) thickness: P<0.001.Comparison between
leaflet proximal part (L)and valvular
sinus (VS) thickness: P<0.01. The thickness of
the three structures seem to overlap
and undergo parallel variations.
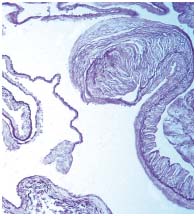
Figure 3. Low cross-section of a proximal valve. Two opposite cusps
are visible: one is thickened and the opposite is thinned.
The main difference is represented by the collagen thickness in the
parietal part of the leaflet. The two borders are thickened.
A whirled architecture is visible in the larger one. Weigert 150 x.
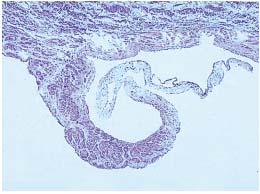
Figure 4. Longitudinal section of a prolapsed proximal valve.
The leaflet insertion segment and the proximal part of the leaflet
are thickened by the presence of smooth muscular cell bundles.
Hematoxilin-eosin 125 x.
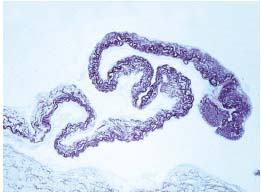
Figure 5. Cross-section of a proximal valve. A 3-degree elastic
membrane fragmentation and dissociation in the whole leaflet and
in the border are present with the elastic fibrils spreading
throughout the thickness of the leaflet. Weigert 300 x.
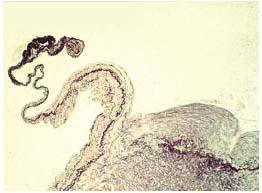
Figure 6. Longitudinal section of a terminal valve. The proximal
part of the leaflet is thickened by increased collagen in the parietal
side. The distal part is thinned and the elastic membrane appears to
be crumpled close to the end of the leaflet and in the border.
A thick fibromuscular plaque below the leaflet insertion segment is
visible. Weigert 125 x.
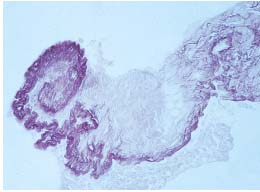
Figure 7. Cross-section of a proximal valve. The elastic membrane
in the thickened border is crumpled in a spiral. The thickened
border is the latest result of the crumpling of the distal part of the
leaflet. Weigert 150 x.
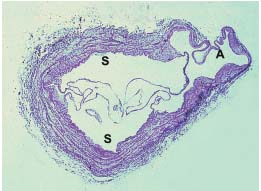
Figure 8. Cross-section of a proximal valve. Sclerosis of the media,
ectasis and asymmetry of the wall, fibromuscular hypertrophy of
the intimal layer. The minimum thickness in the commissural
aneurysm (A) is less than in the valvular sinus (S). Weigert 15 x.
DISCUSSION
The valvular sinus, valve leaflet insertion segment, and proximal part of the leaflet are of similar thickness in the same subject, and this confirms the overlapping of the two main pathophysiological theories of primary venous disease: the first based on the primary vein wall dilatation and the second on primary cusp degeneration. Cusp hypotrophic thinning (hypotrophy) was observed in the majority of the degenerated cusps in the distal part of the leaflet, and was usually represented by the reduction of the collagen and striking thinning to a minimum of 3 microns.
The increase in the vasa vasorum, connective tissue cells, smooth muscle cells, and elastic fiber in the leaflet insertion segment and leaflet of degenerated cusps seem to represent mainly a structural variation rather than the expression of the valvular pathology, except for the smooth muscle cells and the elastic membrane alterations.
The absence of inflammatory cells and microthrombi do not confirm the pathogenetic theory based on postthrombotic alterations.
The thickened borders seem to be composed of crumpled distal hypotrophic leaflets caused by some mechanical factors as the turbulence and cusp abnormal mobility due to the valvular reflux.
The intimal fibromuscular plaques (endophlebohypertrophy) below the valve were not frequent, nor they can be considered as one of the specific expressions of primary valvular alterations. It can be supposed that these findings may be due to a combination of biochemical and mechanical factors depending on the venous stasis caused by the reflux and its turbulence.
Muscular hypoplasia was found to be prevalent in the ectasic and asymmetric valvular anulus and mainly in the commissure aneurysm. The symmetric dilatation or aneurysm of both commissures in the valvular anulus seems to prevail in the pathological saphenofemoral valves of varicose subjects. In one third of cases the commissures were not dilated. In these subjects the valvular incompetence was probably due to primary hypotrophic cusp degeneration.
CONCLUSIONS
The concept of valvular hypotrophy seems to emerge from the prevalence of the structural thinning at the distal part of the leaflet, the valvular sinus, and the commissural aneurysm. Hypotrophy of these structures is mainly due to reduction in the collagen component in the leaflet and to the media smooth muscle tissue hypoplasia of the valvular anulus.
The histological study of saphenofemoral valves was able to detect the tissue changes due to three pathophysiological factors: primary wall dilatation, cusp tissue alterations, and the structural consequences of the hemodynamic disorder such as the thickening of the border and the endophlebohypertrophy below the valve. It can be now supposed that hemodynamic disorders may play an additional role in the already incompetent valves and lead to further injuries to the anulus and cusps. A modern combined theory for the explanation of primary venous insufficiency can be summarized in the concept of a vicious circle (Figure 9) basically due to the interaction between hypotrophic structural degeneration and hemodynamic mechanical injury due to reflux.
These new aspects of venous valve pathophysiology seem to lead to a plausible explanation for the controversial results obtained by reconstructive valve surgery. Their clinical implications are mainly represented by the need for a more careful instrumental assessment of venous valve conditions by ultrasound and/or angioscopic detailed examination before and during reconstructive surgical procedures in order to make the best technical choice.
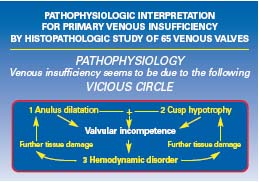
Figure 9. Three factors seem to concur in the onset and development
of primary venous insufficiency: (1) anulus dilatation with
hypotrophic parts, (2) cusp hypotrophy, and (3) hemodynamic
disorder which leads to a mechanical injury of both anulus and
cusps. One of the three factors can represent the beginning of the
vicious circle which leads to venous insufficiency. In the majority of
the cases studied they seem to overlap.
FURTHER READING
● Corcos L, De Anna D. Saphenous vein valvuloplasty: techniques and results. In: Kieffer E, Bahnini A, eds. Actualités de Chirurgie Vasculaire. Chirurgie des Veines des Membres Inferieures. Paris: Editions AERCV; 1996.
● Corcos L, De Anna D, Zamboni P, et al. Reparative surgery of valves in the treatment of superficial venous insufficiency. External banding valvuloplasty versus high ligation or disconnection. A prospective multicentric trial. J Mal Vasc. 1997;22:128-136.
● Alexander CJ. The theoretical basis of varicose veins formation. Med J Aust. 1972;1:258-261.
● Ludbrook J. Primary great saphenous vein revisited. World J Surg. 1986;10:94-98.
● Ewards JE, Edwards AE. The saphenous valves in varicose veins. Am Heart J. 1940;19:338-351.
● Gottlob R, May R. Venous Valves. Vienna, Austria: Springer-Verlag; 1986.
● Obitsu Y, Ishimaru S, Furokawa K, et al. Histopathological studies of the valves of varicose veins. Phlebology. 1990;5:245-254.
● Butterworth DM, Rose SS, Clark P, et al. Light microscopy, immunohistochemistry and electron microscopy of the valves of the lower limb veins and jugular veins. Phlebology. 1992;7:27-30.
● Marinov G, Minkov M, Knyazhev V. Specificités ultrastructurelles des cellules endotheliales valvulaires de la veine saphene interne variqueuse et non variqueuse. Phlebologie. 1994;47:145-150.
● Goldman MP. Sclerotherapy Treatment of Varicose and Telangiectatic Leg Veins. St Louis, Mo; Mosby-Year Book; 1995.
● Corcos L, Procacci T, Peruzzi GP, et al. Sapheno-femoral valves. Histopathological observations and diagnostic approach before surgery. Dermatol Surg. 1996;22:873-880.
● Corcos L, Peruzzi G, Romeo V, et al. Peripheral venous biopsy: significance, limitations, indications and clinical applications. Phlebology. 1989;4:271-274.
● Michiels C, Arnould TH, Thibault- Vercruyssen R, et al. Perfused human saphenous vein for the study of the origin of varicose veins: role of the endothelium and of hypoxia. Int Angiol. 1997;16:134-141.
COMMENT BY THE AUTHOR
At the time of the study, our interest was focalized on the main anatomical causes of valvular incompetence, and our conclusions made possible a better selection of patients to be subjected to the external banding of the proximal great saphenous vein.1 The characteristics of the proximal valves were carefully observed and measured by echography, and the cases affected with early and evident hypotrophy of the valve cusps were excluded. The number of the interventions decreased, but the quality of the clinical and hemodynamic outcome increased.2 At the present, these criteria are still taken into consideration before performing the procedure.
In the meantime, we were also interested in deep venous repair in cases affected with primary and secondary chronic venous insufficiency, and the topic was presented and discussed during several meetings in Rome (1998), Bremen, and Florence (1999).3-7 Some atypical intravenous valvuloplasties of the femoral or the popliteal veins were successfully performed in cases with unusual anatomical alterations by primary disease, such as venous aneurysms and a monocuspid valve, and in postthrombotic veins where the cusps appeared to be severely damaged. While performing such interventions, the histological knowledge of the valvular elementary alterations was of great help in the choice of the surgical strategy: single residual cusps were found suitable to be transposed in the vein lumen in order to create monocuspid valves. Others were divided into two cusps and tricuspid valves were obtained. The results of the new procedures were satisfactory at a mean follow-up of 5 years.7,8
By the interpretation of the histological findings, and on the basis of the recent results, the creation of autologous intimal flaps for monocuspid valvular reconstruction in cases with primary and secondary valveless syndromes was taken into consideration.9,10 The preliminary results are satisfactory and the follow-up of new cases is still in progress.
More recently, Crotty published an impressive article on a pathogenetic hypothesis for the explanation of valvular incompetence in primary chronic venous insufficiency.11 The study indicates that noradrenaline can regulate the tone of the vein as a venoconstrictor and can also act as a venodilator when it diffuses from the vein lumen into the media through the vasa venarum located in the valvular sinus. A malfunction of this feedback leads to the progressive dilatation of the vein wall by physiological reflux and turbulence. When the elastic sphincter of the valvular leaflet insertion segment is finally damaged, reflux and turbulence become stable and pathological. This vicious circle seems to confirm our histological hypothesis (Figure 9) and gives a satisfactory explanation for the progressive annulus dilatation. The simultaneous development of cusp hypotrophy and the significance of the intimal fibromuscular plaques below the valve are still to be better clarified.
The knowledge of vein valve pathophysiology, histology, and histopathology seems to represent the basis for the future of the pharmacological and surgical treatment of chronic venous insufficiency.
REFERENCES
2. Corcos L, Trignano M, De Anna D. External banding valvuloplasty of the proximal great saphenous vein: 10 years experience and follow-up. Acta Phlebologica. 2000;1:51-58.
3. Corcos L, De Anna D,Trignano M. Insufficienza venosa cronica profonda degli arti inferiori. Tecniche chirurgiche a confronto. Archivio ed Atti della Società Italiana di Chirurgia. Rome, Italy: Luigi Pozzi. 1998;4:128-151.
4. Corcos L, De Anna D, Dini M, Macchi C, Ferrari F, Dini S. Main histological alterations of venous valves in primary venous insufficiency. Pathophysiological and Diagnostic implications. Phlebology ’99. Rabe E, Gerlach H, Lechner W, eds. Selected Contributions of the European Congress of the Union Internationale de Phlébologie (UIP). 41. Annual Meeting of the German Society of Phlebology. 26th September – 1st October. 1999:12-14.
5. Corcos L, De Anna D, Trignano M. Chronic venous insufficiency of the lower limbs: combination and comparison of surgical techniques. Phlebology ’99. Rabe E, Gerlach H, Lechner W, eds. Selected Contributions of the European Congress of the Union Internationale de Phlébologie (UIP). 41. Annual Meeting of the German Society of Phlebology. 26th September – 1st October. 1999:191-194.
6. Corcos L, De Anna D, Dini M, Macchi C Ferrari PA, Dini S. Primary venous insufficiency: main histological alterations of venous valves and pathophysiological implications. Phlebolymphology. 2000; special issue:21.
7. Corcos L, De Anna D, Trignano M. The combination of surgical techniques for the treatment of chronic venous insufficiency of the lower limbs. Comparison of the results and special cases report. Phlebolymphology. 2000;Special issue:58.
8. Corcos L, Cavina C, Peruzzi G, Procacci T, Spina T, De Anna D. Deep intravenous atypical valvuloplasties: four case reports. Acta Phlebol. 2002;3:39-48.
9. Corcos L, De Anna D. La valvuloplastica interna. In: G. Genovese, ed. Chirurgia venosa. Masson; 2003;123-131.
10. Corcos L, Peruzzi G, Procacci T, Spina T, Cavina C, De Anna D. A new autologous venous valve by intimal flap: one cases report. Minerva Cardioangiol. 2003;51, 4:395-404.
11. Crotty TP. The corrupted feedback hypothesis. Med Hypotheses. 2003;61:605-616.

