The history of catheter-directed thrombolysis of deep venous thrombosis
thrombolysis of deep venous
thrombosis
Rigshospitalet, University of Copenhagen,
Denmark
bDepartment of Clinical Physiology and
Nuclear Medicine, Herlev Hospital,
University of Copenhagen, Denmark
Abstract
Catheter-directed thrombolysis (CDT) is a relatively new treatment modality that actively removes a venous thrombosis. CDT has been chiefly practiced for acute iliofemoral deep venous thrombosis because this vein segment has a poor rate of spontaneous recanalization compared with more distal vein segments. This can be explained by the frequent occurrence of the May-Thurner syndrome (also known as Cockett’s syndrome or iliac vein compression syndrome), which was described more than 50 years ago. CDT works with different guide-wires, catheters, and delivery systems to allow a stenting procedure for any residual iliac obstruction that remains after thrombolysis. CDT is a simple technique that, with the right inclusion and exclusion criteria, can obtain results superior to conventional treatment with anticoagulation and compression stockings and reduces the development of postthrombotic syndrome. These results are based on many single-center studies and a few randomized controlled trials. CDT has been modified over the years with a combination of mechanical devices to shorten the treatment time from days to hours. The main purpose of this review is to describe the development of the basic method of CDT and provide general considerations for completing this intrathrombus-removal strategy.
Introduction
Thrombolysis, as a method for removing deep venous thrombosis (DVT), has been used for many years; first it was systemically used, even in the trials against anticoagulation.1,2 Thrombolysis had serious side effects and resulted in inadequate restoration of the iliac lumen; however, it was better than anticoagulation. Without a doubt, the next step was to administer thrombolysis into the thrombus area (ie, regional thrombolysis) by injecting the solution into the pedal vein; however, no further benefit was observed.3 After these attempts, with suboptimal results, it was logical to deliver the lytic fluid directly into the thrombus itself. Catheterdirected thrombolysis was defined and described for the first time by Okrent et al in a case story from 1991,4 and seems to be an extremely logical strategy for intrathrombus removal. CDT requires guide wires, catheters, delivery systems, and stenting procedures to treat uncovered persistent obstructive iliac lesions.
Stenting is the most conspicuous advantage in working with the wire-systems. Many studies have addressed CDT in the “pure form,” and later, in combination with techniques using mechanical devices and aspiration to speed up the treatment time. This paper will address many aspects of basic CDT and highlight the most important results with recommendations based on the existing literature. The article will follow the terms, which are recommended as reporting standards.5
Why use catheter-directed
thrombolysis?
The rationale for CDT is the lack of sufficient recanalization after DVT, which leads to obstruction, as either occlusion or stenosis, and is sometimes found in combination with valve incompetence. The femoral vein segments are able to recanalize in 80% of cases after 3 months, but the iliofemoral outflow tract will, especially on the left side, only recanalize in 20% to 25% of cases.6 The consequences are pathophysiological changes that include ambulatory venous hypertension and postthrombotic syndrome, where the later occurs more frequently after iliofemoral DVT and accounts for almost 50% of cases.6,7 According to the definition, the iliofemoral segment includes the common femoral vein, external iliac vein, and common iliac vein.5 Obstruction of these vein segments will negatively influence the outflow. Fortunately, iliofemoral DVT is only a minor part of the total DVT population, but is observed in one quarter to one-third of the patients.8 CDT may overcome the problem of DVT, which seriously affects health and quality of life.9
Which patients can be considered for catheter-directed thrombolysis?
Only a small set of patients with iliofemoral DVT can be considered for CDT. Patients excluded from CDT due to a higher risk of bleeding include patients with severe hypertension, hepatic insufficiency, renal insufficiency, bleeding disorders, previous cerebral hemorrhage, surgery within the last 7 to 10 days, pregnancy, delivery within the last 7 days, and an international normalized ratio (INR) >2.5,10 The most debated criterion is whether CDT is suitable for patients with DVT and cancer. Most studies have excluded patients with active cancer due to a risk of bleeding and rethombosis, but have accepted patients that have been cured of cancer or have been cancer free for at least 1 to 2 years. Another questionable issue is the duration of symptoms. Based on a few animal experiments and duplex findings, it seems that thrombus material may probably irreversibly damage the vein wall after 2 weeks, leading to chronic changes.11 Several international guidelines highlight that the maximum efficacy for CDT occurs within the time limit of 2 weeks.12,13
What are the pharmacological principles involved in catheter-directed thrombolysis?
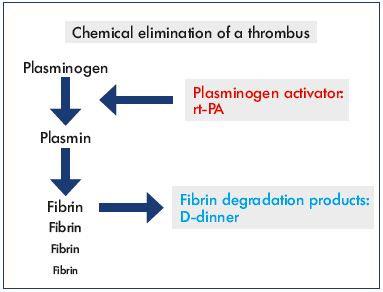
The direct pathway to degrade the fibrin component of a thrombus occurs via proteolytic cleavage of plasminogen to plasmin. An increase in D-dimer, a fibrin degradation product, signals that fibrin has been degraded. Several plasminogen activators are known, including streptokinase, urokinase, and recombinant human tissue-type plasminogen activator (rt-PA). Previously, streptokinase was systemically administered, but it was abandoned due to massive allergic reactions and side effects. Urokinase is still used, but rt-PA is the most utilized fibrinolytic drug with the shortest half-life (≈5 minutes) (Figure 1). The clearance of rt-PA is more than 90% effective in the first pass via the liver. In 2004, a study compared rt-PA (0.5 mg/hour) with urokinase (120 000 U/hour) in CDT and no differences were observed concerning infusion time, success rate, and complication rate, but rt-PA was less expensive.14
May-Thurner Syndrome
Understanding the May-Thurner syndrome (also known as Cockett’s syndrome or iliac vein compression syndrome) is necessary before describing CDT. In 1957, May and Thurner (both from Austria) published the results of a largescale study that described how the right common iliac artery compresses the left common iliac vein against the fifth lumbar vertebra.15 They investigated cadavers and embryos and the findings were analyzed 10 years later by Cockett et al related to a possible explanation for DVT.16 In later studies, iliac compression was diagnosed using computed tomographic venography, and 66% of “normal” subjects had a >25% reduction in the vein lumen and this was more predominant in females.17 Often the iliac vein is widened and flattened with translucency to be seen on an image (Figure 2 and 3). Patients with left-sided iliofemoral DVT revealed compression in 74% of cases compared with only 28% in a control group (P=0.05),18 and one-half to two-thirds of these patients had perivenous fibrosis, causing webs and spurs inside the thrombus lumen due to repetitive mechanical compression and arterial pulsations.19
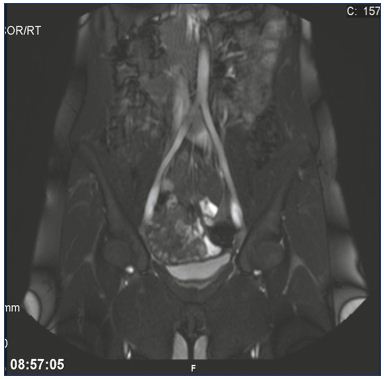
Figure 2. May-Thurner syndrome.
Illustration of the translucency and widened left common iliac
vein due to the compression from the right common iliac artery
using magnetic resonance venography.
From reference 25: Bækgaard et al. Phlebology. 2014;29(suppl 1):118-118.
© 2014, SAGE Publications.
In addition, compression can sometimes occur along the entire length of the iliac vein, both the left and right side. Collateral veins can be seen depending on the grade of obstruction. It is believed that the DVT process originates in the diseased iliac vein and propagates in a descending direction, resulting in a fully occluded vein segment. In the initial DVT process, no collateral veins are seen. During thrombolysis, the previously created and now thrombosed collaterals can be cleared and a preexisting obstruction is then documented.
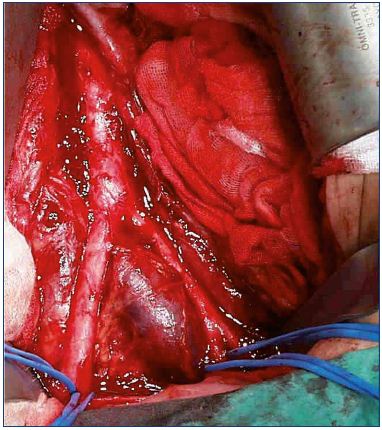
Figure 3. Iliac vein compression seen from the outside.
From reference 25: Bækgaard et al. Phlebology. 2014;29(suppl 1):118-118.
© 2014, SAGE Publications.
Technique of catheter-directed thrombolysis
After diagnosing DVT using duplex ultrasonography, computed tomographic venography, or magnetic resonance venography, an access for puncture has to be chosen. An open popliteal vein is the most commonly used access site, and the puncture is performed using a micropuncture technique in the prone position under local anesthesia and with ultrasound guidance. The distal posterior tibial vein at the ankle can be used in the event of crural involvement as well. A publication has used the popliteal access, even with a concomitant thrombosis in this vein or more distally, and the follow-up revealed a patent popliteal vein in 90% of cases after 8 months.20 Different lengths of sheaths could be inserted at this point. A hydrophilic guide-wire is manipulated through the thrombus and can sometimes be used with a looping technique, especially in the area of the occlusive lesion at the iliac level. During the procedure, fluoroscopy and repeated venograms in several planes are necessary to secure the right intraluminal placement with a final destination in the inferior vena cava or at least in a thrombus-free vein segment. Different guide wires, with a higher or lower stiffness, can be used and is user dependent. In special cases, it is possible to use a contralateral femoral and jugular access. A daily venogram control is necessary to monitor the treatment and to reposition the thrombolysis catheter, if necessary.
Thrombolytic composition and thrombolysis procedure
For thrombolysis, five components are important and include: (i) thrombolytic drug choice; (ii) heparin; (iii) volume of the infusion; (iv) type of infusion; and (v) intermittent pneumatic compression. The thrombolytic drug with the highest recommendation is rt-PA due to its short half-life (≈5 minutes), which is essential when bleeding occurs. The short half-life means that when the infusion is stopped, further influence of the drug is neutralized immediately. With reference to arterial thrombolysis, an older recommendation suggests using 1 to 2 mg rt-PA per hour daily.21 In Copenhagen, 1.2 mg rt-PA per hour is used without an upper total limit.10 The large-scale ATTRACT trial (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) set the maximal dose at 35 mg.22
The infusion acts optimally in combination with heparin as either unfractionated heparin or low-molecular-weight heparin to keep the lysed vein segments open. The dose of unfractionated heparin was adjusted to keep the activated partial thromboplastin time (aPTT) between 50 and 60 seconds, and upwards of 90 seconds.10,23 A weight adjusted dose of low-molecular-weight heparin is given according to general recommendations. A continuous dripinfusion can be used23; however, the pulse-spray technique using a multiple side-hole catheter with tip occlusion seems to be more efficient, suggesting that there is a mechanical effect on the thrombus.10 The total amount of infusion liquid can be up to 3 L per day.10 Use of intermittent pneumatic compression on the legs is recommended based on a Japanese randomized controlled trial,24 as it was shown to facilitate inflow, probably by increasing endogenous fibrinolytic activity.
Stenting
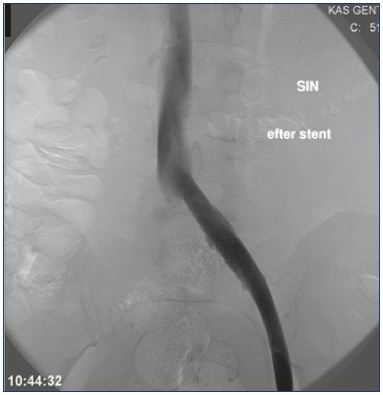
Unlike arteries, veins react differently to stenting, as veins tolerate extensive dilations without rupturing, and despite extrinsic compression, the affected vein wall retains some elasticity. Therefore, any uncovered obstructive lesions after thrombolysis in the iliac vein segment must be dilated and stented with self-expandable stents. Balloon angioplasty alone is insufficient due to relapse or recoiling of the vein wall, but pre- and postdilatation is necessary in combination with stenting. Kissing stents are not necessary in the iliac confluence unless both veins are affected. Success criteria after CDT and stent insertion include an unobstructed vein with spontaneous flow (rapid clearance of contrast medium) and disappearance of collateral veins (Figures 4 and 5).25
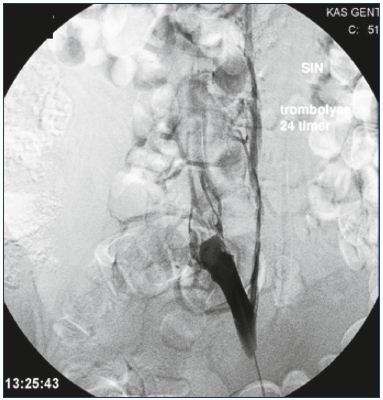
Figure 4. Persistent obstruction after CDT.
All thrombus material has been removed, but the obstruction
has to be stented.
Abbreviations: CDT, catheter-directed thrombolysis
The most commonly used stent has been the Wallstent (Boston Scientific, Inc), which was originally constructed for the arterial system. The stent is characterized by pronounced radial force, but without sufficient flexible properties after insertion. One disadvantage of the Wallstent is shortening during placement due to its braided construction. This stent is the only stent made of stainless steel, as other stents are made of nitinol with a closed-cell design developed for sufficient attachment to the vein wall.25 New stent designs with an open-cell structure have emerged, demonstrating more flexibility, while maintaining a high radial force suitable for the curved iliac vein, especially on the left side. Several designs are currently being tested. The rate of stenting varies. CDT with a treatment duration of a couple of days has been associated with a stenting rate of 50% to 60%, but much lower rates have been reported.23,26
Intravascular ultrasound
Intravascular ultrasound has not found its place in CDT compared with its recommended use in chronic venous disease. A possible explanation may be due to the remaining obstructive changes in the iliac vein segment that often have a shorter extension in patients with iliofemoral DVT. Therefore, it may be easier to visualize the length of the diseased vein segment using multiplane venograms. Only one publication has used intravascular ultrasound to identify residual thrombus, resulting in continued CDT.27 Another paper had previously shown that residual thrombus is associated with an increased risk of postthrombotic syndrome.28
Inferior vena cava filter
The use of an inferior vena cava filter (Günther Temporary Vena Cava Filter, Cook Medical) during CDT has been described mostly for cases where a floating thrombus was identified in the inferior vena cava.23,26 The value of protecting against pulmonary emboli has often been questioned, as insertion and withdrawal of the filter can be accompanied by additional difficulties and problems. If inserted, the filter must be removed immediately after the CDT procedure. A forgotten filter itself can cause an occluding thrombosis in the inferior vena cava, and a few groups have successfully stented such occlusions.29 Due to these concerns, prophylactic inferior vena cava filter placement is not routinely performed.5
Complications
Very few fatal episodes have been published.30 The most frequent complication is either major or minor bleeding. Minor bleeding can be managed with simple compression at the puncture site, sheath upsizing, or dose alteration.5 Major bleeding is defined as intracranial bleeding or bleeding severe enough to result in death, surgery, cessation of therapy, or blood transfusion. The frequency of minor bleeding episodes seldom exceeds 10% to 20%, whereas the frequency of major bleeding episodes varies in the literature, but normally does not surpass more than a few percent.31 During CDT, transient hematuria is frequent and pulmonary emboli are rare.26
Biochemical monitoring
Due to fibrinolysis, D-dimer will increase during CDT. A significant increase in D-dimer indicates a new and large thrombus, whereas a minor increase may indicate an older thrombus. During our experiences in Copenhagen, we measured the levels of D-dimer daily during treatment, and successful treatment was defined as a continuous decline in D-dimer levels. In patients with a restored lumen on a venogram, but whose levels of D-dimer were still elevated, we continued the lytic infusion for another 6 hours, which successfully eliminated the thrombus. We have not published separate data on this strategy, but it has been incorporated in the results.26 However, there is a new publication presenting results on this specific monitoring tool. D-dimer above 18.4 μg/mL at the 12 hours had a high predictive rate of more than 50 % lysis at the end of CDT in 24 patients.32
A marked decrease in fibrinogen and hemoglobin may indicate a risk of bleeding or actual bleeding and requires a careful physical examination. The greatest risk of bleeding occurs during the final stage of CDT when the lumen is restored to an almost normal state, with more run-off from the infusion.
Posttreatment and follow-up
It is recommended to use compression stockings (up to 2 years) and anticoagulation therapy (6 to 12 months) after CDT without any evidence of recurrence. In some studies, patients with severe thrombophilia, which is observed five times more frequently in patients with thrombi, are kept on life-long anticoagulation therapy. To monitor these patients properly, a close follow-up is necessary to identify patients who need a reintervention and to determine patency, valve function, the clinical, etiological, anatomical, and physiological (CEAP) score, and signs of postthrombotic syndrome. An assessment of the patient’s health-related quality of life is recommended.10,23
Results and discussion
The first review on CDT was published in 1998 after collecting 15 studies with 263 patients with iliofemoral DVT.33 Many valuable conclusions were drawn from this review. Short-term success varied from 68% to 100%, even without knowing the exact meaning of success. Patients with clots older than 4 weeks had inferior results compared with patients with younger clots. Blood transfusion was only required in 5% of patients. Inferior vena cava filters were used in 49 patients, of which 31 were retrievable filters. Only a minority of patients were stented, 1 patient died, and only 2 patients had a nonfatal pulmonary embolism.
Until now, the largest study conducted was a multicenter venous registry from the US in 1999.30 A total of 221 patients with iliofemoral DVT were treated. Urokinase was used as the lytic agent, duration of symptoms before treatment was accepted for up to 3 weeks, and almost onethird had a previous DVT. Stenting was done when needed and accounted for one-third of patients. The mortality rate was <1%. The most important result from this study was that iliac patency was significantly superior 1 year after stenting compared with the group of patients without stenting (74% vs 53%, P=0.001), meaning that stenting positively influenced the outcome after CDT. Another lesson learned was the disappointing results after stenting of the femoral vein, which has been abandoned ever since.
Only two randomized controlled trials have been published. One included only a small number of patients, and the results showed an advantage of CDT vs anticoagulation.34 To date, the second trial from Oslo–the CaVenT study (Catheter-directed Venous thrombolysis in acute iliofemoral vein Thrombosis)–is the most comprehensive randomized controlled trial on CDT. A total of 90 patients were randomized to CDT and 99 patients to anticoagulation.35 The occurrence of postthrombotic syndrome was significantly lower in the CDT group compared with the anticoagulation group (P=0.047), but quality of life was equal in both groups after 2 years. The results were less favorable than expected. Several factors might explain these results: (i) only half of the included patients had iliac involvement; (ii) symptom duration before treatment was tolerated up to 3 weeks; (iii) the iliac stenting rate was only 17%; and (iv) balloon angioplasty was performed in some cases. This study demonstrated the importance of using strict inclusion criteria, especially the involvement of the pelvic vein segment, which will benefit from CDT, including a sufficient rate of stenting without balloon expansion alone.36
In 2010, we reported on 103 lower extremities, which were diagnosed for the first time with iliofemoral DVT and were treated using a pulse-spray infusion technique with 1.2 mg rt-PA per hour, 120 mL infusion volume per hour, and intermittent pneumatic compression during treatment.26 More than 50% of the patients had stenting mostly on the left side (84%). The treatment time was 2.5 days on average. Kaplan-Meier analysis showed that 82% had competent veins (patent veins in the entire treated segment, including normal valve function) at 6 years with a median follow-up of 52 months. A total of 16% of the patients had postthrombotic syndrome (half of which were mild) after a median follow-up of 71 months.9 The technique has also been successfully used for patients with inferior vena cava atresia and DVT in the pelvic area.37
Residual thrombus material after CDT is a predictor of an increased rate of postthrombotic syndrome, as was shown in a single-center study.28 This observation was highlighted in the CaVenT study. Both reflux and lack of patency at 6 months were independent predictors for development of postthrombotic syndrome after 24 months.38 The authors of this study are in support of the “open vein hypothesis,” which states that effective removal of an acute venous thrombus will reduce the risk of postthrombotic syndrome. Another issue is the fact that the Villalta score, a global score system for determining the severity of postthrombotic syndrome, may overestimate symptoms from the superficial system and underestimate symptoms, such as venous claudication, caused by pelvic venous obstruction. Furthermore, quality of life score systems cannot sufficiently identify or estimate outcomes.
A meta-analysis on four studies (some of which have been mentioned above) from 2012 concluded that there is a significant increase in patency (risk ratio, 0.38; 95% CI, 0.18-0.37) after CDT combined with stenting.39 It strengthens the necessity for reporting on the restoration and function of the venous anatomy after CDT.
Catheter-directed thrombolysis combined with other treatment modalities
A criticism against CDT has been the length of treatment time, which is mostly because patients in many countries had to be placed in the intensive care unit with a considerable increase in cost. In Copenhagen, patients are treated in the ordinary ward with dedicated nurses.26 One reason for adding “mechanical” devices to CDT is the desire for a more rapid treatment in general. Two very different methods are available–suction and ultrasound enhanced CDT. The suction method uses the Venturi effect to create a backward jet stream, but it can also be performed with a simple syringe technique. The other method has the purpose to create permeability of the thrombus.40-42 In addition to the shorter treatment time, a positive consequence is that a lower amount of lytic infusion is required; therefore, reducing the risk for bleeding, which is essential for many patients, eg, cancer patients. However, it seems that the rate of stenting is higher using shorter treatment times, which must be addressed in the future. Newly designed stents demand great durability, in patency and physical properties, concerning possible kinking, migration, and fracture.
Conclusion
CDT is a very simple technique. The optimal patients to treat are those with acute iliofemoral DVT. Optimal treatment involves using rt-PA combined with heparin in a high-volume infusion per hour and intermittent pneumatic compression. Biochemical control and daily multiplane venograms, with stenting of all residual obstructive lesions, even minor lesions, is mandatory. Intravascular ultrasound may also be considered. The results are promising, even in the long term. Some mechanical devices can be added to CDT to shorten the treatment time, and these devices are currently being validated in ongoing trials.22,43

1. Browse NL, Thomas ML, Pim HP. Streptokinase and deep venous thrombosis. Br Med J. 1968;3:717-720.
2. Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med. 1990;88:235-240.
3. Schwieder G, Grimm W, Siemens HJ, et al. Intermittent regional therapy with rt-PA is not superior to systemic thrombolysis in deep vein thrombosis (DVT)–a German multicenter study. Thromb Haemost. 1995;74:1240-1243.
4. Okrent D, Messersmith R, Buckman J. Transcatheter fibrinolytic therapy and angioplasty for left iliofemoral venous thrombosis. J Vasc Interv Radiol. 1991;2:195-197.
5. Vedanthan S, Sista AK, Klein SJ, et al. Quality improvenment guidelines for treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25:1317-1325.
6. Akesson H, Brudin L, Dahlström JA, Eklöf B, Ohlin P, Plate G. Venous function assessed during a 5 year period after acute ilio-femoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990;4:43-48.
7. K ahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698-707.
8. Strijkers RH, Arnoldussen CW, Wittens CH. Validation of the LET classification. Phlebology. 2015;30(suppl 1):14-19.
9. Broholm R, Sillesen H, Damsgaard MT, et al. Postthrombotic syndrome and quality of life in patients with iliofemoral venous thrombosis treated with catheter-directed thrombolysis. J Vasc Surg. 2011;54(suppl 6):18S-25S.
10. Sillesen H, Just S, Jørgensen M, Baekgaard N. Catheter directed thrombolysis of ilio-femoral deep venous thrombosis is durable, preserves venous valve function and may prevent chronic venous insufficiency. Eur J Vasc Endovasc Surg. 2005;30:556-562.
11. Bækgaard N, Foegh P, Wittens CH, Arnoldussen C. Thrombus age is ideally measured by history or MRV prior to thrombus removal. Phlebology. 2015;30(suppl 1):20-26.
12. Meissner MH, Gloviczki P, Comerota AJ, et al; Society for Vascular Surgery, American Venous Forum. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55:1449-1462.
13. K ahn SR, Comerota AJ, Cushman M, et al. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636- 1661.
14. Grunwald MR, Hofmann LV. Comparison of urokinase, alteplase, and reteplase for catheter-directed thrombolysis of deep venous thrombosis. J Vasc Interv Radiol. 2004;15:347-352.
15. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
16. Cockett FB, Thomas ML, Negus D. Iliac vein compression: its relation to iliofemoral thrombosis and the postthrombotic syndrome. Br Med J. 1967;2:14-19.
17. K ibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937-943.
18. Oguzkurt L, Ozkan U, Uluson S, Koc Z, Tercan F. Compression of the left common iliac vein in asymptomatic subjects and patients with left iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2008;19:366-370.
19. Shebal ND, Whalen CC. Diagnosis and management of iliac vein compression syndrome. J Vasc Nurs. 2005;23:10-17.
20. Jeyabalan G, Marone L, Rhee R, et al. Inflow thrombosis does not adversely affect thrombolysis outcomes of symptomatic iliofemoral deep vein thrombosis. J Vasc Surg. 2011;54:448- 453.
21. Semba CP, Bakal CW, Calis KA, et al. Alteplase as an alternative to urokinase. Advisory panel on catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 2000;11:279-287.
22. Comerota AJ. The ATTRACT trial: rationale for early intervention for iliofemoral DVT. Perspect Vasc Surg Endovasc Ther. 2009;21:221-224.
23. Enden T, Kløw NE, Sandvik L, et al; CaVenT Study Group. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268-1275.
24. Ogawa T, Hoshino S, Midorikawa H, Sato K. Intermittent pneumatic compression of the foot and calf improves the outcome of catheterdirected thrombolysis using low-dose urokinase in patients with acute proximal venous thrombosis of the leg. J Vasc Surg. 2005;42:940-944.
25. Bækgaard N, Just S, Foegh P. Which criteria demand additive stenting during catheter-directed thrombolysis? Phlebology. 2014;29(suppl 1):118-118.
26. Bækgaard N, Broholm R, Just S, Jørgensen M, Jensen LP. Long-term results using catheter-directed thrombolysis in 103 lower limbs with acute iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:112-117.
27. Raju S, Martin A, Davis M. The importance of IVUS assessment in venous thrombolytic regimens. J Vasc Surg Venous Lymphat Disord. 2013;1:108.
28. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheterdirected thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55:768-773.
29. Neglen P, Oglesbee M, Olivier J, Raju S. Stenting of chronically obstructed inferior vena cava filters. J Vasc Surg. 2011;54:153-161.
30. Mewissen MW, Seabrook GR, Meissner MH, Cynamon J, Labropoulos N, Haughton SH. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39-49.
31. Bækgaard N, Klitfod L, Broholm R. Safety and efficacy of catheter-directed thrombolysis. Phlebology. 2012;27(suppl 1):149-154.
32. Luo CM, Wu IH, Chan CY, Chen YS, Yang WS, Wang SS. Dimerized plasmin fragment D as a potential biomarker to predict successful catheter-directed thrombolysis therapy in acute deep vein thrombosis. Phlebology. 2015;30:620- 626.
33. Grossman C, McPherson S. Safety and efficacy of catheter-directed thrombolysis for iliofemoral venous thrombosis. Am J Roentgenol. 1999;172:667-672.
34. Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209-214.
35. Enden T, Haig Y, Kløw NE, et al; CaVenT Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep venous thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31-38.
36. Bækgaard N. Benefit of catheter-directed thrombolysis for acute iliofemoral DVT: myth or reality? Eur J Vasc Endovasc Surg. 2014;48:361-362.
37. Broholm R, Jørgensen M, Just S, Jensen LP, Bækgaard N. Acute iliofemoral venous thrombosis in patients with atresia of the inferior vena cava can be treated successfully with catheter-directed thrombolysis. J Vasc Interv Radiol. 2011;22:801-805.
38. Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Kløw NE. Residual rates of reflux and obstruction and their correlation to post-thrombotic syndrome in a randomized study on catheterdirected thrombolysis for deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2014;2:123-130.
39. Casey ET, Hassan MH, Zumaeta-Garcia M, et al. Treatment of acute iliofemoral deep venous thrombosis. J Vasc Surg. 2012;55:1463-1473.
40. Lin PH, Zhou W, Dardik A, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192:782-788.
41. O’Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007;18:715-724.
42. Oğuzkurt L, Ozkan U, Gülcan O, Koca N, Gür S. Endovascular treatment of acute and subacute iliofemoral deep venous thrombosis by using manual aspiration thrombectomy: long-term results of 139 patients in a single center. Diagn Interv Radiol. 2012;18:410-416.
43. Engelberger RP, Fahrni J, Willenberg T, et al. Fixed low-dose ultrasound-assisted catheter-directed thrombolysis followed by routine stenting of residual stenosis for acute ilio-femoral deep-vein thrombosis. Tromb Haemost. 2014;111:1153-1160.