History of venous surgery (3)
Michel PERRIN
TREATMENT OF DEEP VEIN THROMBOSIS OF THE LOWER AND UPPER LIMBS DURING THE ACUTE PHASE.
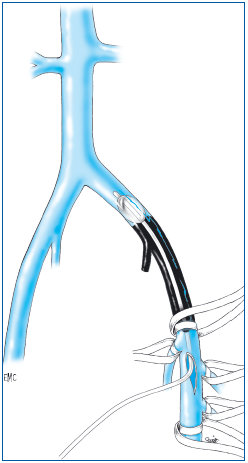
1. Thrombectomy
Thrombectomy is the resection of a blood clot and was the first surgical procedure performed in the treatment of acute deep vein thrombosis (Figure 21). In the lower limb, this procedure is attributed to the German surgeon Läwen in 1937. In principle, thrombectomy has three objectives: prevention of pulmonary embolism, treatment of the thrombosis itself, and prevention or limiting of sequelae, and postthrombotic syndrome. Combined with anticoagulant therapy, which made it possible, but also in competition with it, thrombectomy was recommended in France by Leriche, and then Fontaine, after the Second World War. It was favorably received by a few surgical teams, but did not enjoy total support by all vascular specialists. Subsequently, because of the availability of medical therapies and new techniques, the objectives of thrombectomy were called into question.
2. Fibrinolysis
In fibrinolysis, a fibrinolytic agent is administered to a patient with a thrombosis and activates plasminogen in the blood. The fibrinolytic agent converts fibrinogen into fibrin, which lyses blood clots in a process of fibrinolysis or thrombolysis.
In 1968, the first treatment was reported in Scandinavia (Robertson). The fibrinolytic agent was delivered by intravenous infusion, which had the disadvantage of delivering the fibrinolytic agent to the thrombus in a nontargeted manner and carried the risk of bleeding.
Fibrinolysis in situ was introduced in 1991 (Okrent, USA). Its principle consists of delivering the fibrinolytic agent with a catheter in contact with, or even in, the thrombus. This explains why fibrinolysis in situ is more effective with lower doses, thus decreasing the risk of bleeding.
3. Thrombectomy via an intravenous device inserted transcutaneously.
The principle is to insert into the venous lumen, at a distance from the thrombosis, a catheter with a specific mechanism so as to break up the clot and suck it out. This mechanical action can be combined with fibrinolysis (Figure 22).
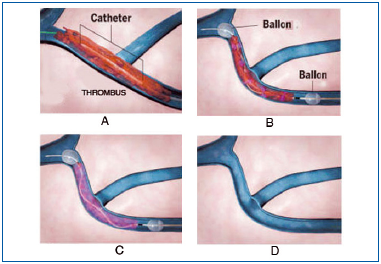
A. The catheter is inserted over a guide wire into the thrombotic deep vein. B. Two balloons are inflated upstream and downstream of the clot to prevent embolism during the following phase of surgery. C. A fibrinolytic agent is injected between the 2 balloons while a monitor produces an oscillating movement on the central guide wire to break up the clot. D. The catheter and the guide wire are removed at the end of the procedure.
4. Caval barriers
One of the major complications of a lower limb deep vein thrombosis is the migration of a blood clot from a lower limb vein into the pulmonary arteries. The result is a pulmonary embolism, of variable severity but whichcan be fatal. To prevent this type of complication, the first interventions in the early 20th century involved venous ligation downstream of the thrombus, generally of the inferior vena cava. Subsequently, pericaval clips were used to divide the venous lumen into several channels (Adams – De Weese, USA, 1958). This maintained the venous circulation but prevented the migration of large emboli (Figure 23).
Subsequently, clips were replaced by placement of an endovenous filter. The first such filter was based on the same therapeutic principle: it was inserted via a peripheral vein but without open surgery of the inferior vena cava, and thus was much less invasive. It was developed and used by L. Greenfield (USA) in 1972 (Figure 24 A). Since then, many such filters have beendeveloped. Using ultrasound guidance, they can be inserted during a bedside procedure. Lastly, insofar as the risk of a pulmonary embolism can be transient, temporary or removable filters (Figure 24 B) have been developed.
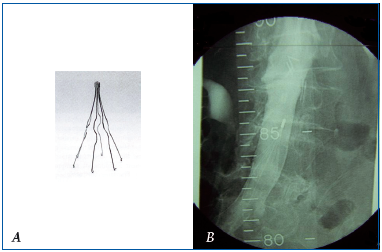
B. Venogram of an optional inferior vena cava filter.
Upper limbs
Even though a deep vein thrombosis in the arm is much less frequent than in the leg, the first venous thrombectomy was performed on the upper limb in 1910 by Schepelmann, a German surgeon. Only axillary and subclavian vein thromboses, that is, veins at the root of the upper limb, require surgery according to some authors, in particular US doctors. Just as in the lower limb, thrombolysis in situ has now replaced thrombectomy.
From the time of Paget (1866) and von Schrötter (1901), it has been known that a thrombosis of the subclavian vein can be associated with compression of vasculonervous structures at the junction of the thorax and the upper limb in the area between the clavicle and the first rib. Under these circumstances, an additional procedure is performed—when treatment of a venous thrombosis with thrombolysis has been chosen—removal of compression by partial resection of the clavicle (A. De Weese, USA, 1971) or removal of the first rib (Ross, USA, 1984).
SURGERY FOR TREATMENT OF REFLUX AND/OR OBSTRUCTION OF THE INTERNAL ILIAC AND GONADAL VEINS
It must be kept in mind that these abnormalities can be responsible for various disorders with a chronic course, for chronic venous disease, and gynecological, and urinary disorders (pelvic venous insufficiency syndrome).
Obstructive syndromes
Venous occlusion is defined as the existence of a complete blockage, while partial or total blockage of the venous lumen is referred to as obstruction. Only deep vein obstruction results in pathophysiological abnormalities, depending on its location. Generally, obstruction of a distal vein has no effect and, in particular, it is in the lower limb that obstruction of a proximal vein is harmful, in particular that of the iliac vein and the caval vein. Such obstruction may be related to a lesion of the venous lumen, most often postthrombotic syndrome, but may also be due to external compression of the vein by a tumor or an organ.
Initially, and according to the principles of arterial surgery, the bypass technique was used. The first venous bypass procedure was performed in 1948 by a Uruguayan surgeon, E. C. de Palma, who used the GSV as a vascular substitute to compensate for obstruction of the iliac vein. Subsequently, prosthetic materials have also been used.
Apart from obstruction related to cancer, where it may be necessary to resect the vein and to replace it, within the last 10 years, treatment with an endovenous stent has become the preferred technique. In fact, this technique involves dilatation of the stenotic area, or rechanneling in the case of an occlusion, performed by inflating a balloon catheter. This catheter is inserted over a guide wire using a transcutaneous approach by venipuncture of a distant vein. Once the obstruction site has been removed or the vein rechanneled by the balloon, the stent is positioned in the lumen of the vein to prevent repeat stenosis (Figures 25, 26 A, B). This type of endoluminal surgery is less invasive than open surgery such as bypass grafting.
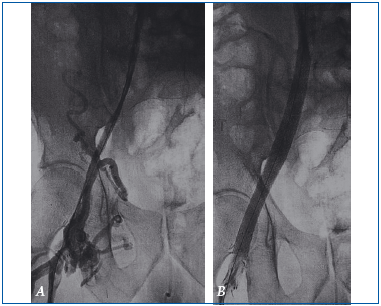
A. Venography in a patient who presented with postthrombotic right iliac vein obstruction. Note the irregular appearance and narrowing of the venous lumen.
B. The vein has resumed its normal diameter after balloon dilatation and stent placement. Both the balloon and stent are clearly visible in the postoperative venography.
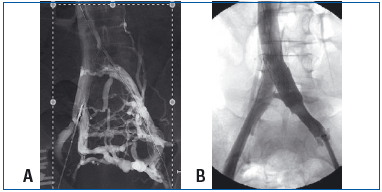
A. Shunt circulation was developed by the presacral venous plexus, the left paralumbar vein and the anastomotic network of the left iliac axis into the right iliac axis.
Source: Courtesy: J. Leal Monedero and S. Ezpeleta Zubicoa.
B. Same patient after stent placement.
Source: Courtesy: J. Leal Monedero and S. Ezpeleta Zubicoa.
We will only discuss the deep veins, since reflux into the superficial veins corresponds to varicose veins. This reflux can involve the lower limb veins and pelvic veins.
1. Reflux syndromes in the lower limb
When it extends from the groin to the calf, it produces a constant, major increase in venous pressure which is especially deleterious. As in the case of an obstruction, the etiology may be primary, secondary, or congenital.
Regarding primary etiology, the valve can be identified and the procedure is called a valvuloplasty. The first such procedure was performed in 1968 by R. Kistner (Hawaii, USA), the pioneer of deep vein reflux surgery. Different valvuloplasty techniques have subsequently been proposed. In internal valvuloplasty, the vein is opened and the valve is identified under direct visual control (Figure 27). In external valvuloplasty, the vein is repaired without opening it (Figure 28).
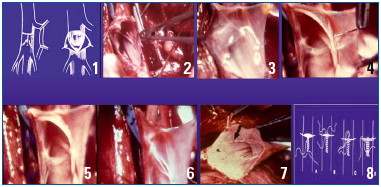
From left to right and from top to bottom: Dotted line tracing and opening of the vein by a T-incision (venotomy). (3) In the incised vein, the valve is identified and appears translucent. (4) Valvular repair is carried out by stretching its free borders with over-andover sutures. After the repair has been completed, the 2 free borders of the valves (6-7) are now in contact, and the valve is again competent. Closure of the vein with sutures.
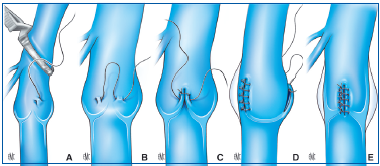
As in internal valvuloplasty, the procedure consists of stretching the 2 free borders of the valves. To do this, separate sutures are placed on the venous wall at the 2 commissures of the valve.
EMC (Elsevier Masson SAS, Paris), Techniques chirurgicales – Chirurgie vasculaire, 43-163, 2009.
Among secondary etiologies where the cause identified is postthrombotic syndrome, the valve is destroyed by the thrombosis and cannot be repaired. Among congenital causes, the valves may be absent or atrophied, and thus the same holds true. Therefore, other surgical techniques have to be used:
– Transplantation of a venous valvular segment. In 1982, Taheri (USA) and Raju (USA) proposed using the humeral and axillary veins which have a functional valve and can be collected undamaged and transplanted into the lower limb (Figure 29).
– Transposition consists of transposing the vein that is the site of reflux onto another lower limb vein, below its competent valve (Figure 30). R. Kistner (USA) invented this technique in 1982.
– The creation of a neovalve using venous tissue from the patient was proposed by P. Plagnol (France) in 1999 and by O. Maleti (Italy) in 2002 (Figures 31A, B). Bioprosthetic valves are currently being assessed.
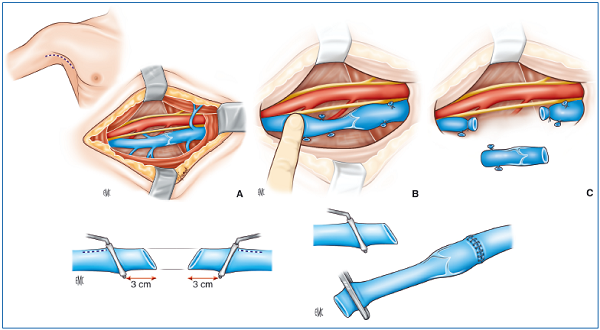
From top to bottom:
A, B, C: A segment of axillary vein is collected after verifying that it has a competent valve. An equivalent length of vein presenting reflux is resected. The venous valvular segment is transplanted. Here, only the proximal anastomosis has been performed; the distal anastomosis will restore continuity of the venous axis. Thus, a competent valve is placed in the venous axis that is the site of the reflux.
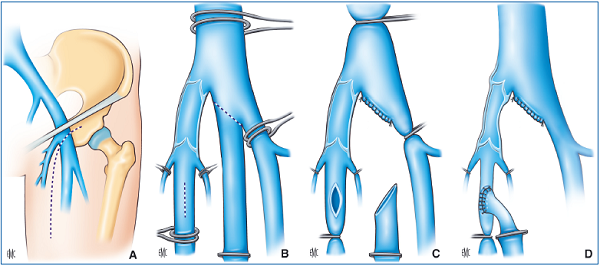
terminal and subterminal valves. The incompetent femoral vein is transposed below the competent valves of the great saphenous vein.
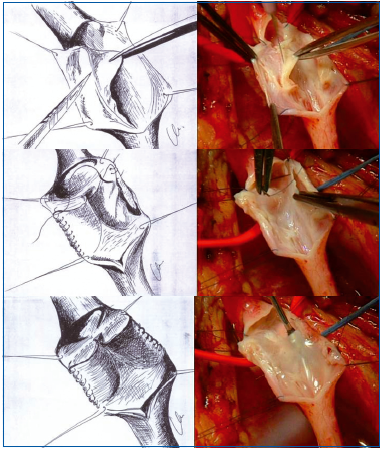
From top to bottom:
– After opening the vein a few centimeters along its axis, the operator divides its wall on one side into two layers.
– This detachment stopped in the middle allows construction of a sac which corresponds to a valve in a normal subject.
-The same technique is performed on the other side thus creating a valve with 2 valvular cusps.
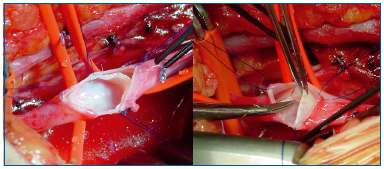
technique).
– On the left. Postthrombotic thickened venous wall.
– On the right. A monocuspid valve was created by separation from the wall.
2. Gonadal and/or pelvic vein reflux syndromes
In women, these can cause gynecological disorders such as pelvic congestion syndrome (H. Taylor, USA, 1949), vulvar or perineal varices, and lower limb varicose veins. In men, gonadal vein reflux causes dilatation of the testicular veins and can cause infertility. Such reflux can be treated with sclerotherapy, but in cases of major reflux, surgical ligation of the gonadal or pelvic veins is performed. Currently, coil embolization of refluxing veins and sclerotherapy are used in combination. This procedure, proposed by R. Edwards (USA) in 1993, obliterates the veins where reflux occurs (Figures 32 A, B).
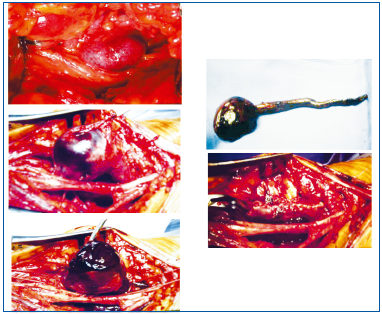
The aneurysm contains a large thrombus. After resecting the aneurysmal sac, continuity of the venous axis is restored by closing the vein with a suture.
SURGERY OF VENOUS ANEURYSMS
A venous aneurysm is defined as an increase in the size of a vein equal to at least twice the normal diameter of the vein considered. It is difficult to identify the date and the author of the first surgical treatment of a venousaneurysm. A rare disorder, venous aneurysm is most often located in the popliteal vein. It is agreed that aneurysms should be treated with open surgery depending on their morphology and on whether or not there are blood clots in the aneurysm sac. After resecting the aneurysm, venous continuity is restored whenever possible (Figures 33, 34).
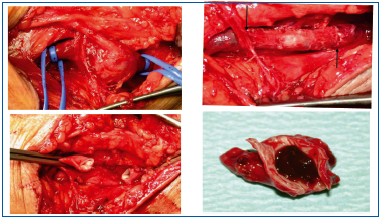
If the aneurysm occupies the entire circumference of the vein, the operator proceeds differently, but by suturing the vein end to end, continuity of the venous axis is also restored.
SURGERY TO TREAT THE “NUTCRACKER SYNDROME”
This term refers to disease resulting from compression of the left renal vein between the aorta and the superior mesenteric artery, in a nutcracker-like configuration, which accounts for its name (Figure 35). Such compression can cause lumbar pain, hematuria, and pelvic congestion syndrome by reflux of the left gonadal vein. Although surgical treatment is rarely indicated, many techniques have been proposed. First, open surgery techniques are used to eliminate compression, either by reimplanting the left renal vein or the kidney itself, or by performing a venous bypass. More recently, the nutcracker syndrome has been treated with endovenous stents (M. G. Neste USA, 1996) (Figure 36).
SURGERY FOR CONGENITAL VENOUS MALFORMATIONS
Congenital venous malformations in their severe form remain the most serious challenge in phlebology. Within the last 20 years, a relatively precise consensus has been reached regarding classification, thus making it possible to divide such malformations into two groups: venous and arteriovenous malformations, with the latter being most severe. Historically, surgery, sclerotherapy, and embolization have been used separately or in combination. Pioneers associated with advances in this field include (in alphabetical order) S. Belov (Bulgaria), P. O. Burrows (USA), J. Y. Kim (Korea), B. B. Lee (Korea), D. A. Loose (Germany), E. E. Scott (USA), D. E. Szilagy (USA), J. L. Villavicencio (USA), W. Yakes (USA). Currently, there is agreement on combined use of different surgical methods after multidisciplinary meetings.
SURGERY FOR VENOUS TUMORS
Primary venous tumors develop in the venous wall. They are rare and can be benign or malignant and aretreated by resection of the vein with possible restoration of venous continuity depending on tumor location. Secondary tumors are an extension of an adjacent cancer or metastatic spread of cancer or a distant cancer. Surgery is used to treat them in some cases. Historically, it has been observed that surgery to remove a tumor prolongs survival following traditional vascular reconstruction procedures.
SURGERY FOR VENOUS TRAUMA AND WOUNDS
This type of surgery has benefited from advances in intensive care and vascular reconstructive surgery, both in terms of survival as well as absence of sequelae. As a historical footnote, the French president Sadi Carnot died in Lyon in 1884 from a torn portal vein after he was stabbed in the abdomen by an immigrant anarchist, Sante Geronimo Cesario (Figure 37). A. Carrel, the French surgeon who trained in Lyon and then immigrated to the USA and who later received the Nobel Prize, wrote that if at that time it had been possible to repair blood vessels, a field in which he distinguished himself, the president would have survived. In 1947 the famous Spanish matador Manuel Laureano Rodríguez Sánchez, also known as Manolete, died of an injury to the femoral vein after having been impaled by the bull “Islero” from Don Eduardo II’s cattle ranch. His name was subsequently associated with a special type of high pass maneuver with the cape used in bullfighting known as the “manoletina” where the bull charges behind the bullfighter into his red “muleta” (Figure 38).
The Korean and Vietnam wars enabled military surgeons to better codify the veins that had to be reconstructed from those that had to be ligated (N. Rich, USA).


CONCLUSIONS AND FUTURE PERSPECTIVES
It is not within the scope of this paper on the history of venous surgery to discuss the advantages and disadvantages of the different methods, their results and their indications, all the more so since the speed with which new techniques are introduced would quickly make this document obsolete. A few comments are, however, warranted.
Surgery in the broader sense based on its etymological definition is increasingly less invasive, and this has transformed the quality of life of patients postoperatively.
It is likely that a certain number of venous disorders no longer require surgery insofar as their pathogenesis is better elucidated and because medical therapy will have an increasingly larger role, whether used separately or in combination with surgery.
Lastly, economic considerations will definitely have an impact on the future course of venous disease. The efficacy of treatment will have to take into account the cost-to-benefit ratio.

