II. First meeting of the Deep Venous Reconstructive Surgery in Chronic Venous Insufficiency (DVRS-CVI) CLUB
As the President of the 13th meeting of the European Venous Forum held in Florence, it was a pleasure and honor to host the first meeting of the Deep Venous Reconstructive Surgery for Chronic Venous Insufficiency (DVRS-CVI). The idea for this meeting came about a few years ago, when Michel Perrin and Oscar Maleti dreamed of a DVRS Club open to everyone interested in this surgical challenge. It has now become a reality with the blessing of Andrew Nicolaides, General Secretary of the EVF.
It was a great privilege to listen to the presentations made by the pioneers of DVRS: Bob Kistner, who was the first surgeon to perform a valve plasty and later on, a valve transposition at the groin, and Seshradi Raju who performed the first axillary valve transplantation and the first stent for vein obstruction. European surgeons owe Michel Perrin for the promotion of DVRS in Western Europe and for encouraging new talents to emerge in deep vein surgery. Let’s not forget Oscar Maleti and Marzia Lugli from Modena who successfully used the thickened wall of a post- thrombotic vein to construct a neovalve. Such findings promote progress in venous surgery. Surgeons from both sides of the Atlantic Ocean met in Florence and delivered seventeen short presentations on all fields of DVRS, thereby giving a comprehensive review of the topic.
A selection of their abstracts is presented in this special issue. We hope you will enjoy reading it.
OVERVIEW OF THE SESSION ON DEEP VENOUS RECONSTRUCTIVE SURGERY IN CHRONIC VENOUS INSUFFICIENCY
Robert L. Kistner (Honolulu, HI, USA)
It is a great honor to open this session devoted to deep vein surgical procedures for this distinguished audience. Dr Maleti and Dr Perrin have organized a series of rapid-fire presentations from authorities in different aspects of deep vein disease and during the next 2.5 hours we will be treated to a review of this whole field. My task is to set the stage for what we are about to hear.
The discussions that follow will describe surgical procedures that the majority of surgeons have never performed and that many believe to be seldom, if ever, indicated. The fact is that many of these techniques have been available for over 3 decades and have not been widely adopted so they have either failed the test of time or they have some other defect that makes them unpalatable. In response to this state of affairs a new “club” made up of persons who have actual experience in deep reconstruction called the DVRS-CVI club—you will recognize that this acronym stands for the title of this talk: Deep Venous Reconstructive Surgery for Chronic Venous Insufficiency. It is pronounced: ‘Deevers-Civi Club’. This presentation today is the initial function of that group and your critical input following the session is urgently welcome.
The concept of deep vein reconstruction flies in the face of the taboos that dominated surgical thinking when I entered the surgical disciplines in the 1960s. The gospel at that time was to handle the veins with a “no-touch” technique— reminiscent of the no-touch technique of the “laying on of hands” used by some advocates of semi-spiritual healing. The concern was that the veins could not tolerate manipulation without developing thromboembolic complications. In retrospect, this was a state of irrational reaction to earlier experiences where the veins were traumatized without anticoagulation and without attention to already established principles of vascular surgery—these include provision for adequate inflow and outflow to the operative site and handling the vessels with vascular clamping, avoiding excessive stretching and torsion, and employing minimally clamping of the vessels under therapeutic levels of intraoperative anticoagulation. It is not that others had not experimented with surgery of the veins earlier—one can recall the description of venous transplantation in 1906 by Carrel and Guthrie, Trendelenburg in 1906, and Homans in 1934 with ligations of inferior vena cava and femoral veins, Lawen’s description of thrombectomy in 1938, followed by Warren and Thayer’s description of a sapheno-popliteal bypass in 1954, Palma’s cross-femoral bypass in 1958, and Eiseman and Malette’s folding of the veins to produce a competent valve in the 1950s. But all of these were whispers in the wind that failed to influence surgical practice immediately. The signal contribution of Bauer in the 1940s describing idiopathic (nonthrombotic) venous valve reflux was also largely ignored. All of this changed in the period from 1960 to 2000 when the earlier techniques of operating gently with proper instruments under anticoagulation using principles of vascular repair learned from arterial surgery were employed. By this time venography had been improved with better fluoroscopy techniques and the biggest boon of all to diagnosis and knowledge of the veins—the non-invasive ultrasound—came on the scene. A notable flurry of activity followed in many places around the globe during these years.
Surgical procedures to repair primary valvular reflux and others to manage post-thrombotic disease by transposing or transplanting venous segments were devised, reported, and evaluated with small series of patients. Some of these proved to be reproducible by different surgeons in different parts of the world and the question of which patients should be candidates for the procedures became subject for debate. I believe the wider interest in deep vein surgery became stalled at that point in favor of the search for less invasive techniques and the romance of the interventional approach. Progress in the deep veins has continued with the description of the creation of the Maleti/Lugli neovalve and with deeper focus on the importance of restoring patency in the iliac vein by thrombectomy, angioplasty, and stenting. All of this you will hear in the next couple of hours.
So, where are we and Quo Vadis—where do we go from here? Is there a place for deep vein reconstruction? How should we evaluate the procedures to justify their risk and expense? Do we need a rash of prospective randomized trials costing millions of dollars and years of development? Is it possible to design these trials? Who would do them? Or is this even advisable now? Is the fact that there are sufferers from venous disease where clinical improvement provides the justification to perform relatively safe procedures while firm evidence is being accumulated?
Some things we know are needed are: basic science investigations into molecular, hematologic, and inflammatory aspects of venous and ulcer disease; safe thrombolytics to dissolve clots before they destroy the inside of the veins; a better understanding of the physiology of venous flow and the role of the venous valve; a much better awareness in the profession and in the public about venous disease; and the emergence of venous specialists who are experts in the natural history of chronic venous disease and can manage its medical and surgical aspects.
It is with these thoughts in mind that I am anxious to hear the papers that will follow. My hope is that their presentation in a concentrated session will stimulate some among the audience to use the present accomplishments as a prelude to their own future efforts to push the frontiers forward. We know that the presence of axial venous reflux and critical sites of obstruction in the veins will result in a progressive loss of quality of life. We know that the end stage of chronic venous disease is not the loss of the limb—rather it is the loss of the use of the limb, which amounts to a near-image of amputation.
Simply stated, the surgical intent is to interrupt the progressive development of disability from chronic venous disease in the safest and most effective way to preserve normal activity, free of discomfort and disability. The surgery of deep vein disease is properly done to adapt the venous system to the individual’s way of life rather than having the person limit activities to meet the restrictions imposed by chronic venous disease.
A STRATEGY FOR THROMBUS REMOVAL FOR ACUTE ILIOFEMORAL DVT REDUCES POSTTHROMBOTIC SYNDROME
Anthony J. Comerota (Toledo, OH, USA)
Iliofemoral deep venous thrombosis is associated with severe postthrombotic morbidity when treated with anticoagulation alone. Catheter-directed thrombolysis (CDT), with or without the addition of mechanical techniques, is increasingly recommended for patients with iliofemoral DVT, although its effect on postthrombotic syndrome has not been well established. Recently, clinical observations from nonrandomized studies,1 the report of a small randomized trial by Elsarawy et al,2 and the recent publication of the CaVenT trial3 all strongly support the concept that successful catheter-directed thrombolysis reduces post-thrombotic morbidity.
Comerota et al1 studied 71 consecutive patients with iliofemoral DVT who were treated with CDT. Pretreatment and posttreatment phlebograms were evaluated for quantity of residual thrombus by physicians blinded to clinical patient outcomes. Post-thrombotic syndrome (PTS) was assessed using the Villalta score by examiners blinded to the phlebographic results. Patients who had ≥50% lysis had significantly lower Villalta scores (2.21) compared with those with <50% lysis (7.13; P=0.011). There was a direct and significant correlation of the CEAP class with the amount of residual thrombus at the completion of CDT and a direct linear correlation of Villalta score with residual thrombus. Elsharawy et al2 demonstrated that there was significantly better patency and significantly better preservation of valve function when patients with iliofemoral DVT were randomized to CDT compared with those randomized to anticoagulation alone.
In their report of the CaVenT trial,3 Enden and colleagues reported that there was significantly better iliofemoral venous segment patency with catheter-directed thrombolysis and significantly less postthrombotic syndrome in patients randomized to CDT.
The ATTRACT trial is ongoing, and when completed, it will be the largest study to date evaluating a strategy of thrombus removal with catheter-based techniques compared with anticoagulation alone in patients with acute iliofemoral DVT and those with acute femoral popliteal DVT.
References
1. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55:768-773.
2. Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209-214.
3. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomized trial. Lancet. 2012;379:31-38.
ULTRASOUND EVALUATION WITH B-FLOW DURING VALVE REPAIR
Fedor Lurie (Honolulu, HI, USA)
Duplex and triplex ultrasound scans are sufficient for addressing the most important questions prior to and after valve reconstruction surgery.
These include detection of significant reflux and/or obstruction, presence of valves, and their location. The limitations of conventional ultrasound techniques are:
1. Low reliability of valve visualization,
2. Low sensitivity in detection of limited reflux
3. Inability to detect valvular function in physiological conditions without forced reflux-provocation.
B-flow modality is angle-independent grayscale Doppler coding, which allows blood flow visualization without obstruction of the B-mode echo image. Use of B-flow improves valve detection and visualization, increases sensitivity of detection of limited reflux, and allows the study of valve function during physiologic flow.
During this presentation, we will demonstrate examples of identification of otherwise invisible-to-ultrasound valves, examples of valvular dysfunction that is difficult to diagnose by conventional techniques, and an example of intraoperative detection of residual limited reflux, its location and correction by guided stitching.
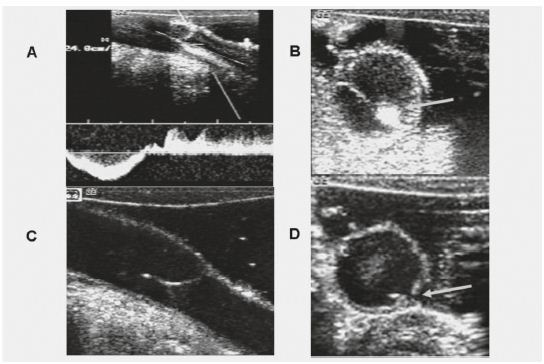
Figure 1. Intra-operative scan immediately after valvuloplasty shows remaining reflux (A). Transverse B-flow image (B) shows exact location of the gap between the cusps, allowing precise external placement of a stitch. B-flow images after the placement of an additional stitsch show absence of reflux (C,D).
DEEP VENOUS RECONSTRUCTIVE SURGERY FOR REFLUX IN PRIMARY VENOUS INSUFFICIENCY
Michel Perrin (Lyon, France)
Introduction. The effectiveness and usefulness of deep venous reconstructive surgery (DVRS) for primary deep reflux remain controversial.
Etiology and physiopathology. venous reflux is the postthrombotic syndrome, but primary deep vein insufficiency is frequently overshadowed. Clinical examination frequently does not allow the distinction between superficial and deep venous insufficiency. In addition, primary reflux is difficult to identify from secondary deep reflux.
Investigations. Duplex scanning provides etiologic, anatomic, and hemodynamic information. Plethysmography gives information on the overall severity of venous disease, but not on its etiology and is not reliable for identifying the predominant component when superficial and deep insufficiencies are combined. It would seem logical to go beyond these investigations only in those patients in whom surgery for deep reflux may be considered. In the absence of contraindication, such as an ineffective calf pump, the following complementary investigations must be carried out: ambulatory venous pressure measurement and venography including ascending and descending phlebography.
Aim of DVRS. The goal of DVRS for reflux is to correct the reflux related to deep venous insufficiency at the subinguinal level. However, we must keep in mind that deep reflux is frequently combined with superficial and perforator reflux; consequently, all these mechanisms have to be corrected in order to reduce ambulatory venous pressure. Besides, primary deep venous obstruction should be associated with primary deep venous reflux
Surgical techniques can be classified into 3 groups
• Procedures with phlebotomy: internal valvuloplasty, transposition, transplantation, neovalve construction, and allografts.
• Procedures without phlebotomy, including banding and external valvuloplasty.
• Artificial valves inserted through vascular access.
Results
Outcomes of deep venous reconstructive surgery for primary deep reflux are difficult to assess as this surgery is frequently combined with superficial and perforator vein surgery, but both have usually been performed before as a first step.
In primary deep venous reflux the most frequent procedure is valvuloplasty, which is credited with achieving a good result in 70% of cases in terms of clinical outcome, defined as freedom from ulcer recurrence and reduction in pain, valve competence, and hemodynamic improvement over a follow-up period of more than 5 years. Internal valvuloplasty is credited with better results than external valvuloplasty.
The indication for DVRS in deep reflux relies on clinical, hemodynamic, and imaging criteria.
• Clinical criterion: C4b and C6 patients.
• Hemodynamic criterion: Axial reflux: uninterrupted retrograde venous flow from the groin to the calf. Axial reflux is important, segmental reflux is not.
• Imaging criterion: Venography is crucial to identify primary reflux and the station to be chosen.
When deep primary reflux is combined with superficial and perforator reflux they must be treated first, but we know from several studies that approximately half of the patients with deep axial reflux are not improved in the presence of chronic venous insufficiency.
When deep primary reflux is combined with obstruction, there is a large consensus for treating the obstruction first. What remains controversial is the need to treat reflux as a second step.
Conclusion
• General practitioners as well as angiologists and vascular surgeons underdiagnose primary deep venous reflux.
• When identified, patients are treated by conservative treatment as very few doctors are aware of the possibilities offered by DVRS.
• Valve reconstruction for primary reflux is underused in selected cases, especially since it deserves a Grade 1A recommendation according to the 3rd edition of the Handbook of Venous Disorders.
• DVRS for primary reflux is not, however, often indicated and has to be performed in highly-specialized units.
References
Camilli S, Guarnera G. External banding valvuloplasty of the superficial femoral vein in the treatment of primary deep valvular incompetence. Int Angiol. 1994;13:218-222.
Kistner RL. Surgical repair of a venous valve. Straub Clin Proc.1968;24: 41-43.
Kistner RL. Surgical repair of the incompetent femoral vein valve. Arch Surg. 1975; 110:1636-1642.
Kistner RL. Primary venous valve incompetence of the leg. Am J Surg. 1980;140:218-222.
Kistner RL, Eklöf B, Masuda EM. Deep venous valve reconstruction. Cardiovasc Surg. 1995;3:129-140.
Lane RJ, Cuzilla ML, McMahon CG. Intermediate to long-term results of repairing incompetent multiple deep venous valves using external stenting. ANZ J Surg. 2003;73:267-274.
Lethola A, Oinonen A, Sugano N, Alback A, Lepantalo M. Deep venous reconstructions: long-term outcome in patients with primary or post-thrombotic deep venous incompetence. Eur J Vasc Endovasc Surg. 2008;35:487-493.
Maleti O, Perrin M. Reconstructive Surgery for Deep Vein Reflux in the Lower Limbs: Techniques, Results andIndication. Eur J Vasc Endovasc Surg. 2011 ;41 :837- 848.
Masuda EM, Kistner RL. Long term results of venous valve reconstruction: a four to twenty-one year follow-up. J Vasc Surg. 1994;19:391-403.
Perrin M. Reconstructive surgery for deep venous reflux. Cardiovasc Surg. 2000;8:246-255.
Raju S, Fredericks R, Neglen P, Bass JD. Durability of venous valve reconstruction for primary and post-thrombotic reflux. J Vasc Surg. 1996;23:357-367.
Raju S, Berry MA, Neglen P. Transcommissural valvuloplasty: technique and results. J Vasc Surg. 2000;32:969-976.
Rosales A, Slagsvold CE, Kroese AJ, Stranden E, Risum O, Jorgensen JJ. External venous valveplasty (EVVp) in patients with primary chronic venous insufficiency (PVCI). Eur J Vasc Endovasc Surg. 2006:32: 570-576.
Sottiurai VS. Technique in direct venous valvuloplasty. J Vasc Surg. 1988;8:646- 648. Sottiurai VS. Current surgical approaches to venous hypertension and valvular reflux. Int J Angiol. 1996;5:49-54.
Tripathi R, Ktenidis KD. Trapdoor internal valvuloplasty: a new technique for primary deep vein valvular incompetence. Eur J Vasc Endovasc Surg. 2001;22: 86-89.
Tripathi R, Sieunarine K, Abbas M, Duranni N. Deep venous valve reconstruction for non-healing ulcers: techniques and results. ANZ J Surg. 2004;74: 34-39.
Wang SM, Hu ZJ, Li SQ, Huang XL, Ye CS. Effect of external valvuloplasty of the deep vein in the treatment of chronic venous insufficiency of the lower extremity. J Vasc Surg. 2006;44:1296-1300.
THE NEOVALVE: TECHNIQUES AND INDICATIONS
Oscar Maleti (Modena, Italy)
Neovalve construction is a technique aimed at creating an antireflux mechanism in patients affected by deep venous reflux whenever valvuloplasty is not feasible (postthrombotic syndrome and valve agenesis).
After attempting to restore the leg’s hemodynamic equilibrium by correcting any proximal obstruction and/or superficial reflux that may be present, the failure of such conservative treatment should lead us to evaluate whether deep venous reconstruction is opportune.
Neovalve construction is one of three leading techniques: vein transposition, vein transplant, and neovalve construction itself. Neovalve construction can also be associated with endophlebectomy where necessary. Neovalve creation has undergone technical change over the years, from the first parietal dissection that created a bicuspid valve up to the most recent technical modification aimed at achieving a fluctuating flap.
Bicuspid parietal dissection aimed at mimicking the physiological human valve; however, given that it requires homogeneous wall thickness, it was not widely applicable due to wall fibrosis, which was frequently asymmetrical. For this reason constructing a monocuspid valve is the most feasible technique and it has demonstrated a performance comparable to that of a bicuspid valve.
The crucial difference is that the pocket is deeper in the monocuspid valve compared with the bicuspid valve, and this may increase the risk of readhesion. In order to avoid this eventuality, we introduced a modified technique in which the neovalve was fixed in a semi-open position to reproduce the physiological configuration of the valve.
Another challenge was the fact that particularly thin walls could impede parietal dissection. In selected cases we solved this problem by invaginating the entire vessel wall and reconstructing the pocket using a bovine pericardium patch. Subsequently, we paid particular attention to obtaining a thin flap in order to achieve greater mobility and thus efficacy.
This thin flap or leaflet was progressively increased from half of the circumference length to the entire circumference length, once again reconstructing the vein by means of a bovine pericardium or autologous venous tissue patch.
Finally, in another attempt to obtain a mobile leaflet that could work as a valve, we performed a parietal dissection on the opposite wall at the level of a venous collateral, which could create a competing flow inside the valve sinus.
Admittedly, since the venous collateral is inside the pocket of the valve, this technical solution does not reproduce the physiology of a human valve. However, there are two significant advantages: an extremely mobile flap and a washing-out action that prevents flap readhesion, venous stasis, and therefore also thrombosis.
The outcomes of the neovalve technique are excellent in the short term, good in the medium term, and similar to the other techniques in the long term. A progressive loss of function is reported.
However, the neovalve technique has shown that we can treat the deep venous system directly by open surgery without any fear of pulmonary embolism and deep venous thrombosis. Working as an antireflux mechanism, the neovalve allows the patient not only to benefit from a protracted ulcer-free period but also, principally, to maintain a protracted C4b-free period or at least gain a significant improvement in symptoms.
The downside of constructing a neovalve is that it is not an easy operation to perform and it cannot be standardized. Fortunately, currently available technology is already increasing the chances of performing this operation and a transcutaneous device is being developed that will simplify the procedure, making it easier to standardize.
From a clinical perspective, the indication to create a neovalve is broadly the same as the indication to perform deep venous reconstructive surgery in patients affected by PTS or valve agenesia whenever the following conditions are present:
• Failure to maintain the equilibrium of the leg by means of medical and compression therapy.
• Limited compliance with compression therapy.
• Inadequate results obtained by means of surgical correction or proximal obstruction correction.
• It is preferable to perform the operation in an active patient with efficient ambulation.
From a technical point of view, neovalve construction is indicated when a valvuloplasty or a valve transposition cannot be performed. Between vein transplant and neovalve construction, it is a question of choice.
References
Maleti O, Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794-799.
Kistner RL. Surgical repair of a venous valve. Straub Clin Proc. 1968;24:41-43.
Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401-409.
Maleti O, Lugli M, Perrin M. Chirurgie du reflux veineux profond. Encyclopédie Médico Chirurgicale (Elsevier Masson SAS, Paris), Techniques chirurgicales – Chirurgie vasculaire, 43 -163, 2009.
Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of postthrombotic deep veins – endophlebectomy – is feasible. J Vasc Surg. 2004;39:1048-1052.
Lugli M, Guerzoni S, Garofalo M, Smedile G, Maleti O. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49:156-162.
THE ROLE OF ENDOPHLEBECTOMY
Marzia Lugli (Modena, Italy)
The main characteristic alterations of post-thrombotic syndrome are obstructive lesions, which determine obstacles to blood flow, and valve destruction, which causes reflux. These lesions are frequently associated. However, when combined, the respective roles and hierarchical relationship of these lesions have not been clearly established. The correction of obstruction provides clinical results such as the improvement of symptoms and restoration of the hemodynamic equilibrium of the leg.
Obstructive lesions above the inguinal ligament are commonly treated by means of venoplasty and stenting. This procedure is rarely employed below the ligament, even if in some situations stenting needs to be extended from the iliac segment to the common femoral vein. Indeed, the common femoral vein is a crucial area in determining the outflow of the leg.
The presence of a large amount of fibrotic tissue and the involvement of collateral vessel confluence, especially that of the deep femoral vein, suggest that open access surgery is the best surgical option. This intervention, known as “endophlebectomy,” involves the disobliteration of chronically obstructed vein segments.
Endophlebectomy, which was first mentioned by Raju in association with a valve transplant, was later described as a stand-alone method by Puggioni and Kistner. Unlike endoarterectomy, endophlebectomy leaves intact a large part of the endothelium, thanks to the specific anatomo-pathological characteristics of endovenous fibrosis. For this reason, endophlebectomy has a low rate of thrombotic complications.
Although post-thrombotic disease usually affects an entire venous segment, the strategic treatment of selected crucial areas may lead to restoring the hemodynamic equilibrium of the leg, which is the objective of endophlebectomy.
Since obstruction is usually concomitant with deep venous reflux, endophlebectomy can be associated with neovalve construction. These two surgical options are frequently integrated and the concomitant neovalve construction obviates the need for any subsequent intervention that may have been required in the event of persistent symptomatic reflux.
The indication criteria for endophlebectomy are difficult to define. Preoperative instrumental evaluation—both ultrasound and venography—usually underestimate the amount of endovenous fibrotic tissue and there are no parameters to quantify its impact on venous inflow. Compared with common ultrasound, B-flow yields more information. Only intravascular ultrasonography can precisely evaluate the extent of the lesions but it is not easily performable at the above-mentioned sites.
Endophlebectomy is an operation that should be more widely indicated, both as a stand-alone technique and in association with angioplasty/stenting of proximal lesions or with deep venous reconstructive surgery.
Currently, there are no codified criteria for indicating the extent and location of the disobliteration to be performed, particularly below the inguinal ligament. As a result, it is essential to identify those areas that are crucial in restoring the leg to hemodynamic equilibrium.
Such codification will logically lead to the standardization of the endophlebectomy technique.
References
Gloviczki P, Kaira M, Duncan AA, Oderich GS, Vrtiska TJ, Bower TC. Open and hybrid deep vein reconstructions: to do or not to do? Phlebology. 2012;27S:103- 106.
Maleti O, Perrin M. Reconstructive surgery for Deep Vein Reflux in the lower limbs: techniques, results and indications. Eur J Vasc Endovasc Surg. 2011;41:837-848.
Puggioni A, Kistner RL, Eklof B, Lurie F. Surgical disobliteration of post thrombotic deep vein – endophlebectomy – is feasible. J Vasc Surg. 2004;39:1048-1052.
Raju S, Neglén P, Doolittle J, Meydrech EF. Axillary vein transfer: trabeculated postthrombotic veins. J Vasc Surg. 1999;29:1050-1062
Vogel D, Comerota AJ, Al-Jabouri M, Assi ZI. Common femoral endovenectomy with iliocaval endoluminal recanalization improves symptoms and quality of life in patients with postthrombotic iliofemoral obstruction. J Vasc Surg. 2012;55:129- 135.
VENOUS STENTING IN CHRONIC ILIAC VEIN OBSTRUCTION
Seshadri Raju (Flowood, MS, USA)
Venous stenting has emerged as a major modality of treatment for chronic venous disease. Routine use of intravascular ultrasonography for diagnostics has shown that obstructive lesions are often present in the iliac venous system at arterial crossover points in patients with advanced primary disease. The presence of reflux in association with postthrombotic disease is well established. Thus, iliac venous stenting appears to have a role in a wide spectrum of pathologies. More interestingly, substantial symptom relief including healing of venous ulceration occurs with iliac vein stenting alone in combined obstruction/reflux cases. In an analysis of 528 limbs with combined pathology, 42% had axial reflux and 58% had reflux segment score ≥3. Nevertheless, clinical relief from the stent procedure alone was satisfactory with relief of pain, swelling, and ulcer healing of (cumulative) 74%, 62%, and 61%, respectively. Further correction of reflux was not necessary. The durable results from stenting alone, despite persistent uncorrected reflux, opens fundamental questions regarding pathophysiology in chronic venous disease.
A common pathway for venous pathology, be it obstruction, reflux, or both may be through oxygenation of the skin. Recently, it has become clear that a severe hypoxemia of about 50% compared with normal supine values occurs when assuming the erect position. Studies in a mechanical venous model suggest that reflux impacts the duration of arterial inflow occurring with calf pump action.
IVUS: SHOULD IT BE USED MORE FREQUENTLY?
Peter Neglén (Trimiklini, Cyprus)
The simple reply to the question stated in the title is: Yes. There is no doubt that intravascular ultrasonography (IVUS) best delineates intraluminal structures such as webs and trabeculations, thrombus, wall structure, and external compression (Figure1). It is the gold standard for imaging the extent and severity of femoroilio- caval venous outflow obstruction, which makes it the premier diagnostic tool without any need to inject contrast dye. IVUS can also easily identify the anatomical location of the outflow by identifying the confluence of large tributaries.
There is no noninvasive or invasive test to detect a hemodynamically significant femoro-ilio-caval outflow obstruction. The pathophysiology of an outflow obstruction and the influence of length and degree of obstruction on venous hemodynamics are poorly understood. A >50% morphological obstruction of the cross-cut area in the pelvic outflow is presently considered significant and warrants stenting. This is an arbitrary number based on favorable clinical results after venoplasty and stent placement. Since there is no noninvasive accurate test, more or less invasive morphological investigations have to be utilized, such as venography, computed tomography venography, magnetic resonance venography and preferably IVUS (Figure2).
Classic anterior-posterior venographic imaging of the ilio-femoral venous outflow has been shown to be inadequate; actually 25% of the venograms (n=304) were reported as “normal.” Compared with IVUS findings, venograms underestimate the degree of stenosis by 30%. The sensitivity and negative predictive value to identify >70% diameter stenosis was 45% and 49%, respectively. In a group of 104 patients with >50% diameter stenosis detected by IVUS, the venogram revealed no stenosis in 17% and in an additional 41% an inaccurate location or extent of the stenosis was shown.
The sensitivity and negative predictive value was only 43% and 56%, respectively. By performing oblique views, the sensitivity and negative predictive value improved substantially to 63% and 68%, respectively (unpublished data), but are still insufficient.
There is no doubt that IVUS is superior to venogram. There are no comparative studies performed between CT-V/MR-V and IVUS.
IVUS is used not only to diagnose the severity, type, and extent of a venous outflow obstruction but also to guide the subsequent stent placement. The longterm patency of a stent is primarily dependent on an unobstructed outflow and brisk inflow of the stent module. IVUS is the only modality to identify segments free of significant occlusive postthrombotic disease, thus ensuring an adequate landing zone and patent outflow and inflow vessels. In a similar fashion, IVUS can differentiate between residual intraluminal thrombus and true underlying stenosis following early mechanical or lytic clot removal of the ilio-femoral segment. IVUS will ensure sufficient lysis and adequate stenting of the reveal chronic obstruction. Most IVC filters can now be placed bedside under IVUS guidance.
The major obstacle to a more frequent use of IVUS has been the cost of the catheters. When the manufacturers realize the increased need for IVUS investigations (a larger market), less expensive, simpler devices with fewer unnecessary bells and whistles will be produced, possibly based on laptop technology.
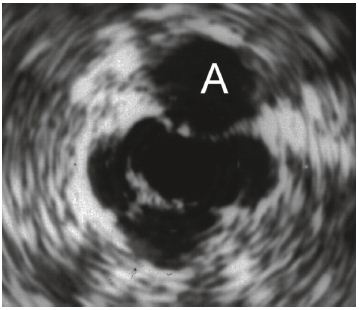
Figure 1. The IVUS image shows the postthrombotic vein
below the artery (A). The irregular vein is shown with
surrounding fibrosis (increased echogenicity) and
intraluminal trabeculations.
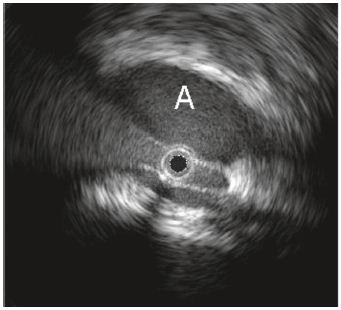
Figure 2. The right common iliac artery (A) clearly
markedly compresses the left common iliac vein (below).
The black hole is the IVUS catheter.
