Incidence and location of deep vein thrombosis in the lower extremities: what do we know?
and Rigshospitalet,
University of Copenhagen,
Denmark
Abstract
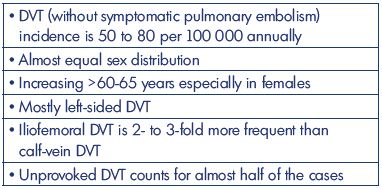
In the last century, deep vein thrombosis (DVT) plus pulmonary embolism was one of the most frequent causes of death in hospitalized patients. However, the incidence of DVT alone has been difficult to calculate from the entity of venous thromboembolism because the incidence of DVT is provided as either DVT without pulmonary embolism or pulmonary embolism ± DVT, where the latter group does not provide the precise rate of DVT. Therefore, our epidemiological review will focus on the first group, with an emphasis on first-time DVT. In the western world, the incidence of DVT (without pulmonary embolism) is currently around 50 to 80 per 100 000 per year, which increases during the winter, and the incidence increases with age and in females >60-65 years. Until recently, terms, such as distal and proximal DVT, have been used widely, now with suggestions of the anatomical descriptions for proximal DVT. Above-knee DVT is 2- to 3-fold more frequent than is calf-vein DVT, and left-sided DVT is the most frequent. The results from studies and lifetime risk estimates show that more emphasis needs to be placed on prophylaxis with risk assessments in the daily work environment. Prospective, large-scale, epidemiologic studies of patients with DVT verified by ultrasonography are needed with a clear presentation of location and extension. This basic information may reveal location-related rates of postthrombotic syndrome based on well-defined follow-up regimens.
Introduction
Deep vein thrombosis (DVT) is a serious disease, and it is often painful and potentially life threatening due to the occurrence of pulmonary embolisms in the acute phase. Later, DVT carries a varied risk for developing complications, such as postthrombotic syndrome, and surviving a pulmonary embolism event can lead to pulmonary hypertension and cardiac-respiratory failure and collapse. Both diseases greatly affect a patient’s quality of life (QOL), and postthrombotic syndrome, the most frequent complication, is responsible for tremendous costs in national health care systems worldwide.1,2
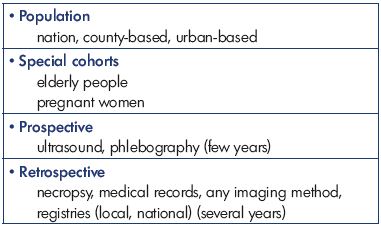
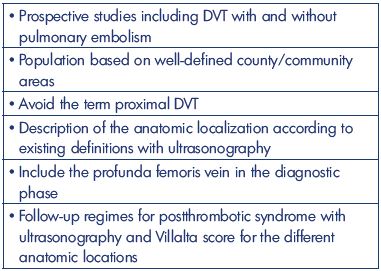
This review highlights the development of symptomatic and mainly first-time DVT and the incidence over a period that is balanced between increasing focus on prevention strategies and increased diagnostic accuracy and effectiveness. This review also explores the location and extension of the venous thrombosis, which is strongly connected to the frequency and severity of postthrombotic syndrome.3 Furthermore, this review presents the most important epidemiologic large-scale studies published in the last 25 years on mainly first-time DVT in the lower extremities, with some materials even collected back in the 1950’s and 1960’s for comparison. The entity of venous thromboembolism is mentioned when needed (Table I).
History
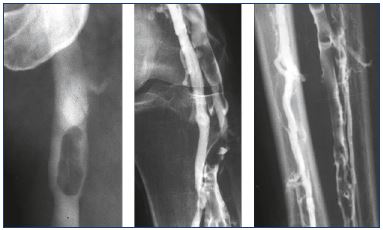
The recognition of a vein thrombosis probably goes back to the middle ages.4 Later, the disease gained attention as the most feared disease during pregnancy, and it was treated with bloodletting in the 1700’s.4 In 1793, Hunter hypothesized that the disease was due to veins occluded by clots. He ligated the veins proximally to avoid propagation and, as a consequence, pulmonary embolism, which was unknown at that time.4 Many years later, the method for vein interruption had been executed in different ways, and, in 1934, it was reinvented by Homans for preventing pulmonary embolisms.5,6 Virchow demonstrated that there was a connection between DVT and pulmonary embolisms, and, in 1856, his idea of a triad interaction between decreased vein flow, vein wall injury, and blood abnormalities was published as the pathophysiological background of the disease. At the same time, the recognition of the inflammatory response in the vein wall as the pathogenic mechanism was discovered.4 The clinical condition with phlegmasia alba dolens was described in 1784 and phlegmasia cerulea dolens in 1857 (Figure 1); the latter is identical with blockage of both deep and superficial veins.7,8 The sign of Homans from 1944 described dorsal flexion of the foot resulting in calf pain, which was an indication of DVT, but this sign was later questioned.9,10 For many years, phlebography (Figure 2), plethysmography, and radioactive fibrinogen were used as diagnostic tools until continuous-wave Doppler was applied in the 1960’s, and, 10-to-20 years later, continuous-wave Doppler combined with duplex technology became the procedure of choice (Figure 3).11
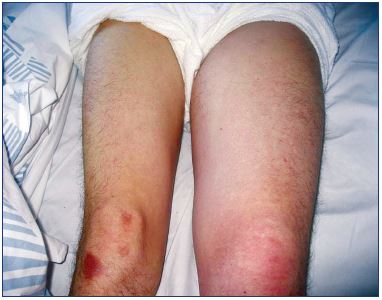
Figure. 1. Left-sided deep vein thrombosis.
When the swelling involves the entire limb, then the deep vein
thrombosis involves the pelvis.
Epidemiology studies
Large-scale necropsy data from the area of Malmö, Sweden emerged in 1991 with DVT being diagnosed via necropsy.12 Malmö had around 250 000 people in the period from 1957 to 1987 with few yearly variations; this study focused on 4 specific years–1957, 1964, 1975, and 1987. All necropsy reports from the departments of general surgery, infectious disease, internal medicine, oncology, and orthopedics were reviewed, and, of the 26 078 admissions, there were 1293 deaths. Of these 1293 deaths, necropsy was identified in 994, with 347 who had had a venous thromboembolism. While the rate of venous thromboembolisms occurred at a constant rate over the 4 years analyzed, there was a significant decrease in 1987 in patients from the orthopedic departments. This decrease was explained, especially by a fall in DVT, due to more preventive care and mobilization. The rate of necropsy was determined using a uniform necropsy protocol over the entire period to improve the validity of the analysis.
In 1992, a prospective study on the incidence of DVT was published on the 1987 urban population from Malmö). At that time, all patients suspected of DVT were referred to one hospital for phlebography. One-third of the suspected patients were positive for DVT: 170 males and 196 females with a mean age 66 years (range, 28-89 years) and 72 years (range, 13-95 years), respectively. The incidence was similar for men and women and increased considerably in the older groups. The incidence of DVT was 160 per 100 000, and only 5 of these patients had upperlimb thrombosis. Above-knee DVT was 2-fold higher than was below-knee DVT. Malignancy was diagnosed in 20%, a past-history of DVT was found in 26%, and DVT was diagnosed postoperatively in 24% of the men and 31% of the women. Half of the cases in women occurred after surgery for a lower-extremity fracture. The incidence in this study is one of the highest registered, which may be due to a high proportion of previous and asymptomatic DVT.
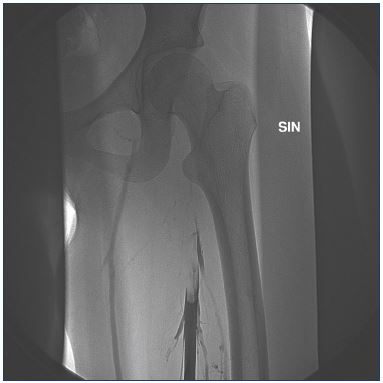
Figure 4. Venogram showing an acute deep vein thrombosis
(less than 14 days) from midthigh and upward to the groin and
pelvis.
No collaterals are seen pointing out the acute phase.
The results from the Olmsted County study (MN, USA) was published in 1998.14 Residents presenting with venous thromboembolism diagnosed by any method (n=2218) were retrospectively identified in a 25-year period from 1966 to 1990 (both years included; population in 1990 was 106 470). The mean age of onset was 61.7±20.4 years, and 56% of the patients were female. The incidence of DVT was stable for males in the period and increased for women >60 years old. The overall age- and sex-adjusted annual incidence of DVT alone was 48 per 100 000 and pulmonary embolism with or without DVT was 69 per 100 000. However, the incidence of pulmonary embolism was almost reduced by half during the last 15 years of the period to the same level as DVT without pulmonary embolism. Silverstein et al concluded that a more accurate risk identification of patients was needed for effective prevention.14
In a population of 342 000 inhabitants from Brittany, France (the Brest district), the yearly incidence of DVT (verified by ultrasonography) was 124 per 100 000, which was collected prospectively from April 1998 to March 1999, and published in 2000. Almost 63% of the patients were not hospitalized at the time of diagnosis, but 16% had been hospitalized within 3 months before the diagnosis.
The incidence increased in both sexes with age, and around 1000 people were >75 years old. Oger et al highly recommended more attention be given to DVT prevention.15
A few years later, another study from Olmsted County, Minnesota, USA compared two groups of residents (n=100 000) with venous thromboembolism in a 10-year period from 1980-1990. Of the 911 residents with venous thromboembolism, 253 were hospitalized for a reason other than DVT or pulmonary embolism and 658 residents were not hospitalized. The average annual age- and sex-adjusted incidence of in-hospital venous thromboembolism was 960.5 (95% CI, 795.1-1125.9) per 10 000 person years, which was 100 times greater than the incidence of 7.1 (95% CI, 6.5-7.6) in the nonhospitalized group. The incidence rates of DVT and pulmonary embolism in the two groups changed little over the period despite a reduction in the average hospital stay. Heit et al concluded that there is a strong need for better risk stratification and an intensive prevention program for hospitalized patients.16
Results from the Olmsted study were published in 2005 on the incidence of DVT during pregnancy and the postpartum period. Between 1966 and 1996, there were 105 cases of venous thromboembolism, including 32 cases of DVT during pregnancy and 44 during the postpartum period (defined as 3 months post-delivery).17 The incidence of DVT remained relatively constant during pregnancy, but decreased in the postpartum period. The incidence was high, especially in the third trimester and the first week of the postpartum period, with a relative risk for DVT postpartum vs during pregnancy being 4.12 (95% CI, 2.62-6.50; P<0.001). The relative risk of venous thromboembolism was 4-times higher for pregnant woman than for nonpregnant women of the same age. Pulmonary embolisms were especially high during the early postpartum period, but they decreased more than 2-fold by the end of the period.
In 2007, a study from Nord-Trøndelag, Norway, which was based on all residents ≥20 years (n=94 194), identified the incidence of venous thromboembolism between 1995 and 2001 from diagnosis characteristics retrieved from medical records.18 A total of 740 patients with a first-time venous thromboembolism event were identified (incidence rate, 1.43 per 1000 person-years; 95% CI, 1.33-1.54), with a DVT incidence rate of 0.93 per 1000 person-years (95% CI, 0.85-1.02). More women were overweight than were men (411 women vs 329 men; mean age, 75 and 71 years for women and men, respectively). Proximal DVT was 3-fold more frequent than was distal DVT, and it was mostly located on the left side. The incidence increased exponentially with age and it was higher in cancer patients. The ratio of idiopathic and secondary thrombosis was 1:1. The risk of dying was highest in the first month; after which, the mortality rate approached the rate in the general population.
A prospective community-based study from Perth, Australia investigated multiple overlapping retrospective sources, such as searching hospital morbidity and mortality databases using the ICD-10 codes (“cold pursuit”) published in 2008. The study period was 13 months and included 151 923 residents with a mean age of 64.4 years, of whom 1.4% were Indigenous Australians. The annual DVT incidence was 52 per 100 000 residents (95% CI, 0.41-0.61) with a minor male preponderance, and an almost exponential increase was noted in patients >50 years old. Ho et al concluded that 17 000 Australians would suffer from venous thromboembolism annually, calling for an effective prevention program.19
Data published in 2008 concerning venous thromboembolism in the elderly population from the Worcester metropolitan area (MA, USA) in 1999, 2001, and 2003 (n=477 800) showed that, of the 1897 validated events included in the study, 55% occurred in patients who were >65 years old. The DVT incidence in this category of patients increased from 59 per 100 000 in the population <65 years to 334 per 100 000 in patients between 64 and 74 years. The same tendency was observed for pulmonary embolism, which was found in one-third as many patients. The events in this elderly group were less unprovoked than in younger patients. The rate of recurrence did not differ significantly between the age classes. However, major bleeding from anticoagulation increased 2-fold in older patients. While age is not a predictor of recurrent venous thromboembolism, major bleeding is increased in older patients; therefore, treatment decisions should be tempered by this observation.
In 2009, another prospective study between 1998 and 2006 from Malmö, Sweden (n=280 000 inhabitants) was published.20,21 Patients >18 years, who were recruited from the only hospital in the region to treat venous thromboembolisms, were included after an objective diagnosis of (probably first time) DVT or pulmonary embolism. Mean age was 61 years in both sexes, with slightly more women included. DVT occurred in 71% of the patients without a pulmonary embolism and in 6% with a pulmonary embolism. The annual risk was 51 per 100 000 for DVT and 19 per 100 000 for pulmonary embolism. The left leg was the most affected limb, and the femoropopliteal location was the most frequent site involved. Iliac DVT occurred in only 13% of the cases. Hormone therapy, immobilization, previous surgery, and malignancy were the most acquired risk factors. A positive family history for venous thromboembolism was obtained from 25% of the DVT patients.
A nationwide, Swedish, large-scale, family study was performed in 2013 to assess the age-and sex-specific seasonal variation of venous thromboembolism in hospitalized patients.22 The study included 150 416 individuals who were registered with a first-time event in 288 months between 1987 and 2010, and, of these, 4.9% had at least one first-degree relative with a venous thromboembolism. The incidence of DVT peaked in February in individuals without a family history of venous thromboembolism (peak-to-low ratio, 1.24). In patients >50 years old, the seasonality was more prominent. These results support the data from a prospective study published 9 years earlier on 1154 Italian patients over a 6-year period.23 The seasonal analysis showed a significantly reduced frequency of DVT events in the summer and an increased frequency in the winter, for the total population (P0.0001), for men (P=0.003), and for women (P=0.007). The exact mechanisms of this variability are not well understood, although seasonal changes in the coagulation activity may play a role.24
A Swedish, retrospective, population-based registry included all adults ≥18 years old residing in the Västerbotten County in northern Sweden during 2006 (n=204 836), and 22.6% of the population were ≥65 years old.25 The mean incidence of venous thromboembolism was 167 individuals per 100 000 person-years (155 for men and 180 for women), the incidence increased with age, which was highest among older women (≥85 years old), and a firsttime event occurred in 82% of the patients. The incidence of lower-extremity DVT without pulmonary embolism was 76.6 per 100 000 individuals per year. The most prevalent risk factors were a recent hospitalization and a concurrent malignancy. Only 3.9% of the patients with a first-time venous thromboembolism were taking pharmacological prophylaxis at the time of diagnosis.
Instead of looking for the incidence of venous disease per individuals per year, estimates of lifetime risk have recently been calculated.26 Participants in different age groups were recruited from two known prospective cohort studies from the US: (i) the CHS study (Cardiovascular Health Study) (n=5414 individuals >65 years old followed at baseline visits from 1989–1990 and 1992–1993); and (ii) the ARIC study (Atherosclerosis Risk in Communities) (n=14 185 individuals 45 to 64 years old followed at baseline visits from 1987–1989). An incident venous thromboembolism was defined as the first DVT or pulmonary embolism from baseline through the end of follow-up in 2011 for ARIC and 2001 for CHS. At 45 years, the remaining lifetime risk of venous thromboembolism was 8.1% in ARIC, which was 2-fold higher than the incidence rate in CHS. This result was explained by differences in the period of venous thromboembolism ascertainment, which became more frequent in recent years. As expected, the remaining lifetime risk decreased across increasing index ages. Obesity was identified as a high risk factor (lifetime risk, 10.9%; 95% CI, 8.7-12.3). Bell et al concluded that at least 1 in 12 middle aged adults will develop venous thromboembolism, which may be more useful than the annual incidence studies to promote awareness of the diseases and guide decisions at both clinical and policy levels. Unfortunately, this publication did not assess DVT and pulmonary embolism separately.
A prospective, 1-year study compared venous thromboembolisms from 2013 in the same area in Western France to the study performed in 1998, and the incidence of symptomatic DVT decreased significantly to 0.76 per 1000. The numbers concerned distal and proximal DVT, especially in patients >60 years. Delluc et al concluded that these results might be due to an easier access to pharmacological thromboprophylaxis, early mobilization, and a reduction in the length of the hospital stay. On the contrary, an increase was found in the incidence of pulmonary embolism, which may be due to the availability of more sophisticated diagnostics.27
Finally, the results from a 1-year, retrospective, Chinese study (2010–2011), which was based on 7.1 million Han Chinese people, showed that the incidence of DVT was 30 per 100 000 people. DVT occurred in up to 0.2% after intermediate and major surgery. While it is known that, in this region of the world, venous thromboembolisms occur less frequently than in western countries, the reasons for this are unknown.28
First-time DVT studies
An estimation of the location of DVT has been made from large discharge cohorts of patients from the two biggest urban areas in Denmark—Copenhagen and Aarhus.29 Almost 160 000 men and women aged 50 to 64 years were identified from the Diet, Cancer, and Health study that was conducted between 1993 and 1997. Patients from the Danish National Patient Registry were identified with the codes for venous thromboembolism at discharge diagnosis, and this diagnosis was confirmed from the medical records. In 358 patients, 12.3% had distal DVT, 36% proximal DVT, 7.1% pelvic DVT, and 2.6% upper-extremity DVT. Of the confirmed events, approximately 50% were idiopathic and the other 50% were nonidiopathic events. The authors concluded that data on venous thromboembolism obtained from administrative registries are a valuable source of information, but this must be used with some caution.
The above-mentioned Swedish, retrospective, population based registry presented numbers for the location of first time DVT in the lower extremities: 43% had iliac DVT, 36% femoropopliteal DVT, and 21% crural DVT. Classified by age, iliac DVT occurred more frequently in the oldest group, whereas femoropopliteal DVT occurred more frequently in the youngest group.25
A new, retrospective, single-center study on ultrasound verified DVT has illustrated the large diversity of thrombus distribution.30 The analysis concerned patients >18 years old presenting with unilateral DVT who were referred to one hospital in Antwerp between 1994 and 2012 (n=1338). The anatomical site and extension was registered into five segments: (i) calf veins (segment 1); (ii) popliteal vein (segment 2); (iii) femoral vein (segment 3); (iv) common femoral vein (segment 4); and (v) iliac veins with or without the inferior vena cava (segment 5). The median age was 62 years (range 18 to 89 years) and the male/female ratio was 50/50. There was a left-sided dominance (57%) in all segments that increased from segment 1 to segment 5, including the iliofemoral segment. The reason for this dominant side location is due to the left-sided iliac vein compression syndrome, which explains the starting point of DVT from this location (Figure 5).31 Calf-vein DVT (distal DVT) occurred in 28% of the cohort, femoropopliteal DVT in 33%, and iliofemoral DVT (proximal DVT) in 38%; data on 23 patients were excluded from the analysis due to nonadjacent segments. The femoropopliteal DVT involved segments 2 and/or 3; whereas, iliofemoral DVT involved segments 4 or 5.30 Prospective studies are needed with more attention about the thrombosis of the profunda femoris vein. It could be argued that many thrombosis events in the common femoral vein belonged to the femoral vein, which decreases the severity of postthrombotic syndrome.
Pulmonary embolism
From some of the papers in this review, it seems that the incidence of pulmonary embolism has fallen during the past 50 years. However, the number given is inconsistent, ranging from 20 to 50 per 100 000 annually. The number includes a high rate of asymptomatic pulmonary embolisms, which are found due to an increased screening for cancer. However, pulmonary embolisms occur more frequently in connection with iliofemoral DVT than with calf-vein DVT.32 In addition, the rate of DVT (50 to 80 per 100 000) is an underestimation because the presentation of venous thromboembolism includes pulmonary embolism ± DVT, where the rate of DVT is not presented individually.
Cost studies
A recent cost analysis from the Olmsted County study in an 18-year period from 1988–2005 showed that the adjusted mean predicted costs were 1.5-fold greater for venous thromboembolism incidents in 355 patients undergoing major operations vs controls and 2.5-fold higher for 286 patients with acute medical illness vs controls. Cost differences between cases and controls were the greatest within the first 3 months.33,34
Could the incidence be lower?
Heit et al examined the reasons for the trends in venous thromboembolism over the last years.35 The population based cohort study from Olmsted county (MN, USA) showed that, while the annual venous thromboembolism incidence did not change significantly, the prevalence of obesity, major surgery, active cancer, trauma, and paresis has increased. Obesity has the greatest influence on the risk of a venous thromboembolic event. These conditions have challenged the preventive anticoagulation strategies. We have the medicine and mechanical devices for prophylaxis, risk scores, and national and international guidelines, but we have to provide education pertaining to the entire available armamentarium.
Conclusion
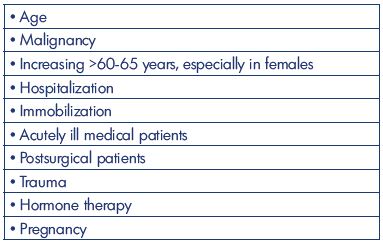
It seems that the incidence of DVT without pulmonary embolism during the past 50 years has been decreasing to about 50 to 80 per 100 000 annually (Table II). All studies are based on well-defined areas and populations, but with many different methods of registration. The 2-fold higher incidence from the Malmö study (year 1887) might be explained by patient referral from uptake areas outside the district of Malmö, and patients with a pulmonary embolism with simultaneously diagnosed asymptomatic DVT might be an explanation. Lower-extremity DVT is, in all studies, mostly left-sided, 2- to 3-fold more frequent for above-knee DVT, and, in general, occurs equally between men and women. The incidence increases with age, especially in people aged >60-65 years and in females. Other risk factors are malignancy, hospitalization, acutely ill medical patients, postsurgical condition, hormone therapy, and pregnancy. Descriptions are missing in older studies on the precise location and extent of the DVT (Table III). The constancy in the incidence emphasizes the need for an increased emphasis on prophylaxis based on education and the use of existing risk assessments and guidelines. Prospective studies to predict the precise location and extent of the thrombosis are warranted (Table IV).
REFERENCES
1. Guanella R, Ducruet T, Johri M, et al. Economic burden and cost determinants of deep venous thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost. 2011;9:2397-2405.
2. Kachroo S, Boyd D, Bookhart BK, et al. Quality of life and economic costs associated with postthrombotic syndrome. Am J Health Syst Pharm. 2012;69:567- 572.
3. Labropoulos N, Waggoner T, Sammis W, Samali S, Pappas PJ. The effect of venous thrombus location and extent on the development of post-thrombotic signs and symptoms. J Vasc Surg. 2008;48:407-412.
4. Galanaud JP, Laroche JP, Righini M. The history and historical treatment of deep vein thrombosis. J Thromb Haemost. 2013;11:402-411.
5. Homans J. Thrombosis of the deep veins of the lower leg, causing pulmonary embolism. N Engl J Med. 1934;211:993- 997.
6. Homans J. Exploration and division of the femoral and iliac veins in the treatment of thrombophlebitis of the leg. N Engl J Med. 1941;224:179-186.
7. Hodgson J, Breschet G. Traité des Maladies des Artères et des Veines. Paris, France: Gabon; 1819:279-283.
8. Caggiati A, Allegra C. Historical introduction. In: Bergan JJ, ed. The Vein Book. Oxford, UK: Elsevier; 2007:1-14.
9. Homans J. Diseases of the veins. N Engl J Med. 1944;231:51-60.
10. Barner HB, DeWeese JA. An evaluation of the sphygmomanometer cuff pain test in venous thrombosis. Surgery. 1960;48:915-924.
11. Illig KA, Rhodes JM, DeWeese JA. Venous and lymphatic disease: a historical review. In: Gloviczki P, ed. Handbook of Venous Disorders. 3rd ed. London, UK: Arnold; 2009:3-11.
12. Lindblad B, Sternby NH, Bergqvist D. Incidence of venous thromboembolism verified by necropsy over 30 years. BMJ. 1991;302:709-711.
13. Nordström M, Lindblad B, Bergqvist D, Kjeldström T. A prospective study of incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232:155-160.
14. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585-593.
15. Oger E; EPI-GETBP Study Group. Incidence of venous thromboembolism: a community-based study in Western France. Thromb Haemost. 2000;83:657- 660.
16. Heit JA, Melton LJ 3rd, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76:1102- 1110.
17. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697- 706.
18. Næss IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a populationbased study. J Thromb Haemost. 2007;5:692-699.
19. Ho WK, Hankey GJ, Eikelboom JW. The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust. 2008;189:144-147.
20. Spencer FA, Gore JM, Lessard D, et al. Venous thromboembolism in the elderly: a community-based perspective. Thromb Haemost. 2008;100:780-788.
21. Isma N, Svensson PJ, Gottsäter A, Lindblad B. Prospective analysis of risk factors and distribution of venous thromboembolism in the populationbased Malmö Thrombophilia Study (MATS). Thromb Res. 2009;124:663-666.
22. Zöller B, Li X, Ohlsson H, Sundquist K. Age- and sex-specific seasonal variation of venous thromboembolism in patients with and without family history: a nationwide family study in Sweden. Thromb Haemost. 2013;110:1164-1171.
23. Gallerani M, Boari B, deToma D, Salmi R, Manfredini R. Seasonal variation in the occurrence of deep vein thrombosis. Med Sci Monit. 2004;10:CR191-CR196.
24. Dentali F, Ageno W, Rancan E, et al. Seasonal and monthly variability in the incidence of venous thromboembolism. A systematic review and a meta-analysis of the literature. Thromb Haemost. 2011;106:439-447.
25. Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): a populationbased study. Thromb J. 2014;12:6.
26. Bell EJ, Lutzey PL, Basu S, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129:339. e19-e26.
27. Delluc A, Tromeur C, LeVen F, et al; EPIGETBO Study Group. Current incidence of venous thromboembolism and comparison with 1998: a community-based study in Western France. Thromb Haemost. 2016;116:967- 974.
28. Law Y, Chan YC, Cheng SW. Epidemiological updates of venous thromboembolism in a Chinese population. Asian J Surg. 2016 Dec 21. Epub ahead of print.
29. Severinsen MT, Kristensen SR, Overvad K, Dethlefsen C, Tjønneland A, Johnsen SP. Venous thromboembolism discharge diagnoses in the Danish National Patient Registry should be used with caution. J Clin Epidemiol. 2010;63:223-228.
30. De Maeseneer MG, Bochanen N, van Rooijen G, Neglén P. Analysis of 1,338 patients with acute lower deep venous thrombosis (DVT) supports the inadequacy of the term “proximal DVT.” Eur J Vasc Endovasc Surg. 2016;51:415- 420.
31. May R, Thurner J. The cause of predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
32. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22-I30.
33. Cahoon KP, Leibson CL, Ransom JE, et al. Costs of venous thromboembolism associated with hospitalization for medical illness. Am J Manag Care. 2015;21:e255-e263.
34. Cahoon KP, Leibson CL, Ransom JE, et al. Direct medical costs attributable to venous thromboembolism among persons hospitalized for major operation: a population-based longitudinal study. Surgery. 2015;157:423-431.
35. Heit JA, Ashrani AA, Crusan DJ, McBane RD, Petterson TM, Bailey KR. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost. 2017;117:390-400.