Incompetent venous valves: ultrasound imaging and exo-stent repair
Joseph Anthony GRAICHE1
Michael Luciano CUZZILLA1
John Christopher CORONEOS1-3
2 Mater Private Hospital
3 North Shore Private Hospital
Sydney, AUSTRALIA
SUMMARY
Background: Lower limb venous disease remains a significant problem in our community today. The condition has been treated mainly with ablative procedures such as stripping and/or sclerotherapy. The aim of this study was to assess external valvular stenting (EVS) of incompetent venous valves as a reparative alternative to the management of patients with varicose veins. In addition, ultrasound examination of the superficial venous valves prior to surgery was also assessed for its ability to predict success with EVS.
Methods: Valves considered for EVS were assessed with brightness-mode (B-mode), spectral pulsed Doppler (PD), color Doppler imaging (CDI) and brightness-flow (B-flow). The ultrasonic features of the great saphenous vein (GSV), terminal valve (TV) and sub-terminal valves (STV) were considered. Inclusion criteria were valvular ring dilation <12 mm in diameter, internal diameter (ID) <12 mm along the entire length of the trunk, symmetry of the valve sinuses, positive identification of two valve cusps, and symmetrical reflux flow patterns through the incompetent valve. There were 69 limbs included in the study. All repaired TVs were tested intraoperatively for competence after application of the EVS. If there was evidence of residual reflux, the STV was also repaired. The operated limbs were assessed clinically 3 months after the procedure at which time ultrasound was also used to test the repaired valves.
Results: Of the 69 TVs that were examined preoperatively, a total of 50 were considered repairable by ultrasonic features. At operation, 44 of these valves were successfully repaired. In the 6 limbs that had residual TV reflux, the STV was repaired. All 6 had competence in the GSV trunk following the STV EVS. Of the 19 TVs that were considered by ultrasonic features to be unrepairable, 18 had gross reflux following EVS with 1 only being repaired successful. All limbs that were successfully repaired at operation were followed up 3 months later, and re-examined with diagnostic ultrasound. Of this group, 3 GSVs had residual reflux at the TV and STV, 1 GSV had major reflux and 1 GSV developed thrombophlebitis. The overall figures for the predictability of successful EVS based on ultrasonic features of the valve were sensitivity 97.8% (95% CI, 88.2 – 99.6), specificity 75% (95% CI 53.3 – 90.2) and accuracy 90.4%. Conclusions: In the treatment of varicose veins, a combination of ultrasound modalities accurately predicts EVS outcomes at the TV and STV of the GSV.
INTRODUCTION
The consensus among vascular surgeons is that high ligation of the great saphenous vein (GSV) without stripping makes it available for use as a bypass conduit, produces greater patient postoperative satisfaction, and minimies postoperative neuralgia.1 Unfortunately, this procedure results in a high long-term recurrence rate.2,3 Similarly, the use of ultrasound-guided sclerotherapy (UGS) to treat reflux in the GSV while cost effective and less invasive, at present has a high incidence of residual saphenofemoral junction (SFJ) and GSV reflux with subsequent recurrences.4-6 For treatment to be successfully reflux must be significantly abolished. Most available treatment options have achieved this however; all obstruct the normal upward flow of blood in the GSV. This results in neovascularization, usually at the groin, and the development of haphazard collateralization that presents as recurrent varicose veins. The most spectacular example of venous neovascularization is seen in obstruction of the inferior vena cava. Collateral pathways will always develop with obstruction to any part of the vascular system, arterial or venous. In the venous system, these collateral veins have no valves and therefore reflux returns.
The ideal surgical solution is a minor procedure, like high ligation, which abolishes the source of reflux without stimulating collateral development. External valvular stenting (EVS) has been performed in a large number of animals and man with encouraging results.7-10 The premise of this treatment is based upon the initial pathology being venous valve ring dilation with normal cusps that progressively become atrophied, avulsed, and resorbed.11-13 The physiology of the GSV is restored by repairing the terminal valve (TV) and/or subterminal valve (STV) at the SFJ because repair inhibits reflux without producing obstruction. The key to success in EVS lies in the identification of valves that are suitable for repair. The original criteria for determining valve suitability for EVS were based upon the preoperative brightness-mode ultrasound (B-mode) measurements of the internal diameter (ID) of the GSV at the SFJ and STV.8-10 Over time, imaging of the valve cusps has become more consistent as ultrasound technology has improved. In recent times, there has been significant improvement in the axial and lateral B-mode resolution, the development of color doppler imaging (CDI) and more recently the addition of brightness- flow (B-flow) technology.14-18
METHODS
Patients
Forty-two patients or 69 limbs were included in this study. A standard history was taken and clinical examination performed on all patients with the management options in mind being both to repair the SFJ and preserve the GSV or, conversely, ablation of the GSV. Patients with a history of above-knee thrombophlebitis or obvious advanced disease (gross dilatation of the GSV trunk itself or ID.12 mm) were excluded from the study. Good indicators of a successful restorative procedure were mild to moderate varicose veins in a young patient, particularly with an incompetent anterior or lateral accessory system with minimal involvement of the GSV trunk.8 Males with a strong personal or family history of degenerative arterial disease or patients with underlying primary or secondary deep venous disease were a further relative inclusive category. Future pregnancy aspirations were also considered, as EVS at the SFJ should be performed prior to the next conception if possible. This is in stark comparison to conventional management practice where it is recommended that surgery is delayed until after the final pregnancy.
Ultrasound equipment
A General Electric, Logic 700 Expert Series (General Electric, Milwaukee, Wisconsin, USA) with 5-10 MHz and 6-13 MHz carrier frequency probes were utilized in this study. This system also includes a B-flow module that allows simultaneous imaging of tissue and blood flow.
Scanning technique
a. B-mode
In a sagittal plane, the valve mechanism is usually identified as a pear-shaped dilatation extending over approximately 1-1.5 cm. This is accentuated with a Valsalva maneuver. The cusps of a normal venous valve are semilunar in shape and directly opposed (Figure 1).
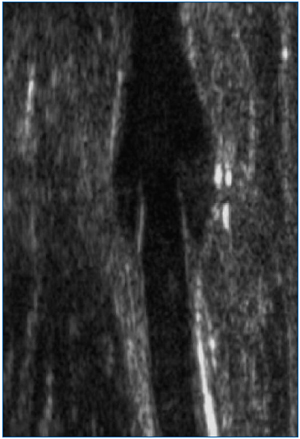
Figure 1. Open venous valve (B-mode). Sagittal image of an open STV at the SFJ. Following flow augmentation, separation of the two cusps is clearly demonstrated as flow.
The most common location of any valve is immediately distal to the point of entry of a major tributary. The valve cusps themselves project directly into the lumen and, being specular reflectors, produce brighter or stronger echoes than the surrounding blood. The curvature of the cusps can often be seen and normal cusps may be seen to move or flutter with respiration. During the Valsalva maneuver, with normal competent valves the cusps can be seen to move towards each other. If the cusps are diseased, movement may be decreased and occasionally the cusps themselves move discernibly slower. In the diseased vein, dilatation of the valve ring may also be evident. The cusps may look normal in the early stages with progressive thickening, atrophy and eventually resorption with advanced disease. Movement is decreased and there may be asymmetry of the valve sinus. Indeed it is important to scan the whole system to make sure there is no thrombophlebitis or that the remainder of the GSV trunk is too diseased to consider repair even if the valves appear suitable. In advanced disease the GSV is >10 mm ID in females and >11.0 mm ID in males.7,8 It is interesting to note, however, that occasionally competence may be found in systems that are quite dilated and in these cases, the valve cusps themselves are usually quite long. Therefore, the measurements are a rough guide only. It is important that perforating systems, particularly the reentry perforating systems, are detected and removed at surgery. Also, an understanding of pathology involving the short saphenous vein and the underlying deep system is critical.
b. Color duplex imaging
This is extremely helpful when applied to venous valve localization assessment, particularly in the deep veins of the thigh where the resolution of the B-mode may be inadequate. When reflux is present, retrograde flow produces a change in the color flow above the level of the valve sinus, turbulence is readily seen immediately superior to the valve cusp indicated by multidirectional flow and frequency aliasing in some cases. This usually occurs in the angle between the valve leaf and the venous wall. Depending on the valve dilatation and subsequent valve cusp misalignment, a high flow is seen originating from the center of the vessel between the valve cusps. The use of this technique needs to be combined with the other two as confusion can arise when there are incompetent tributaries and varicosities near the valve under investigation.
c. B-flow This technique uses the unique ultrasonic reflectivity of aggregates of moving red cells. The higher the velocity of the red cells the greater the return signal and the brighter the real-time echo. Streaming of red cells is demonstrated and, the laminae between relatively high and low flows can often be discerned. Turbulence can be detected as an interruption of the laminae. In other words, B-flow looks and acts like a radiological contrast agent.
Optimally, B-flow images should be assessed using cineloop video so that the playback speed can be adjusted. This allows more detailed examination of the various blood flow patterns that develop in and around the valve cusps. A normal open valve is demonstrated in Figure 2. The location of the valve cusps is implied by the hypoechoic areas on either side of the vein lumen where the streaming red cells produce an “hour-glass” appearance. As the valve opens with flow augmentation via compression of the distal muscle groups, the intrusions decrease in size and may completely disappear (Figure 3).
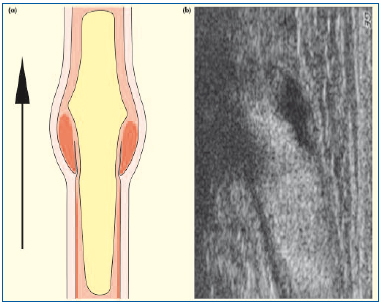
Figure 2. Competent venous valve (open). Sagittal section of a normal venous valve demonstrated on B-flow. There is central streaming, with a decrease in the “hourglass” appearance following flow augmentation with distal limb compression: (a) schematic and (b) B-flow ultrasound images.
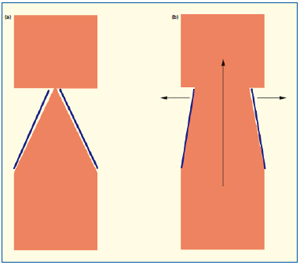
Figure 3. When the valve is closed (a), the B-flow image is comparable to that given by descending phlebography during the Valsalva maneuver. When the valve is open (b), the lumen widens because of the passage of blood.
With a cross-section image, the central streaming and subsequently an increase in velocity of red blood cell rouleaux density produces an increased ultrasonic return echo as it passes through the valve. In assessing retrograde flow, either a Valsalva maneuver or digital compression produces no streaming beyond the valve. Figure 4 is a typical representation of a closed or competent valve following a Valsalva maneuver. The valve sinus is symmetrical, the valve cusps come together and as flow has ceased there is no B-flow information.
An abnormal finding is shown in Figure 5.
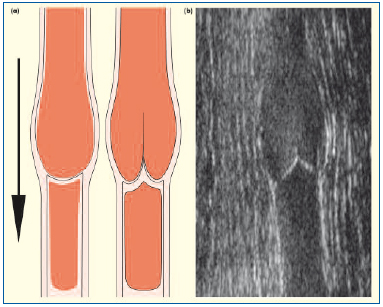
Figure 4. Competent venous valve (closed). The valve sinus is symmetrical, the flow defect is seen either side of the flow stream and the valve cusps approximate following a Valsalva maneuver. No B-flow is seen as blood flow has ceased: (a) schematic and (b) B-flow ultrasound images.
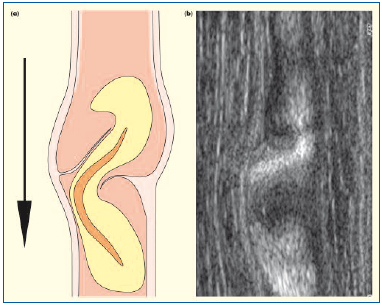
Figure 5. Incompetent abnormal venous valve. The valve sinus is distorted. The cusp above the dilatation is frozen and the adjacent cusp is prolapsed. The high-velocity retrograde streaming deviates laterally above a prolapsing cusp: (a) schematic and (b) B-flow ultrasound images.
In the longitudinal plane, the “hourglass” appearance is asymmetrical. When the valve cusp is fixed and immobile, the central streaming does not move out to the vein wall and there may be a high-velocity flow as shown by increasing density of the returning echoes. On cross-section, irregular streaming and asymmetrical vortices associated with asymmetrical areas of low echoes indicate low flow.
A repairable valve is demonstrated in Figure 6. There is symmetrical streaming but retrograde flow indicating incompetence. There are symmetrical hypoechoic areas in the valve sinus and inferior to the cusps themselves. The central streaming broadens, however, with digital pressure inferior to the valve. Figure 7 demonstrates prolapsing valve cusps, which have been christened the “Dagger Sign” by the sonographer and co-author. Two valve cusps are implied by the symmetrical flow defect and appear to be flattened against the wall with upward flow. This valve also repairs well. Figure 8 is of a SFJ with no demonstrable valve where there is clear retrograde flow, ie, reflux and no apparent eddies with a uniform return of echoes across the entire lumen.
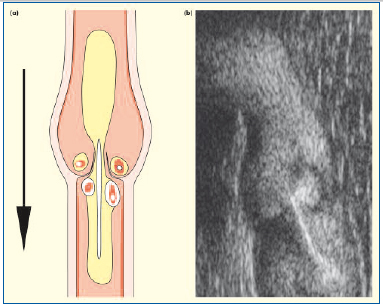
Figure 6. Incompetent repairable venous valve. Retrograde high velocity central streaming is seen with turbulence above the upturned valve cusps and decrease flow below. Flow distribution is symmetrical: (a) schematic and (b) B-flow ultrasound images.
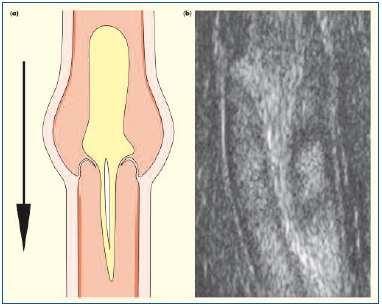
Figure 7. The “Dagger Sign.” Retrograde flow is seen through and over the downward-facing, prolapsed valve cusps with a curved tapering stream as flow velocity decreases distally: (a) schematic and (b) B-flow ultrasound images.
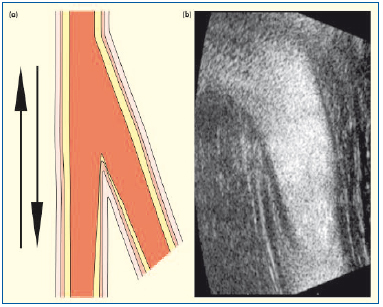
Figure 8. Avalvular vein. No cusps were visible on B-mode. As the lumen is empty, no laminar flow separation is seen on B-flow: (a) schematic and (b) B-flow ultrasound images of the terminal and subterminal valves sites, respectively.
d. Ultrasound data collation
All examinations were performed by the same sonographer with many years of extensive experience in the diagnosis of venous disease. Ultrasound findings were documented on a “Venous Map”, a worksheet with a schematic representation of the deep and superficial veins of the lower limb (Figure 9). This provided graphic information on the size and condition of the GSV and any associated tributaries or perforators. Particular emphasis was placed on determining the effects of previous surgical intervention, the maximum ID of the GSV and any significant tortuosity, and any evidence of superficial thrombophlebitis. Abnormalities within the deep venous system and/or the short saphenous vein were also recorded. For clarity, an enlarged section representing the terminal segment of the GSV was incorporated into the worksheet to detail the condition of the TV and STV and their relationship to the common femoral vein (Figure 10).
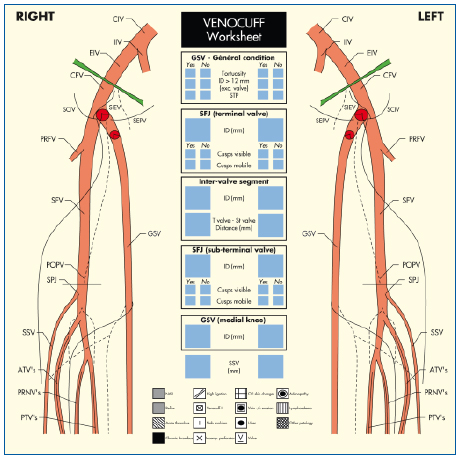
Figure 9. Venous ultrasound examination worksheet, used to record findings including the anatomy of the deep and superficial veins, previous surgical intervention, and any evidence of deep vein thrombosis or superficial thrombophlebitis. An enlarged schematic representation of the terminal segment of the great saphenous vein allows for more precise documentation of the terminal and subterminal valves.
CIV = common iliac vein
IIV = internal iliac vein
EIV = external iliac vein
CFV = common femoral vein
PRFV = profunda femoris vein
SCIV = superficial circumflex iliac vein
SIEV = superficial inferior epigastric vein
SEPV = superficial external pudendal vein
SFV = superficial femoral vein
SFJ = saphenofemoral junction
GSV = great saphenous vein
POPV = popliteal vein
SPJ = saphenopopliteal vein
SSV = small saphenous vein
ATV’s = anterior tibial veins
PRNV’s = peroneal veins
PTV’s = posterior tibial veins
ID = internal diameter T valve = terminal valve
ST valve = subterminal valve
NAD = no abnormality detected
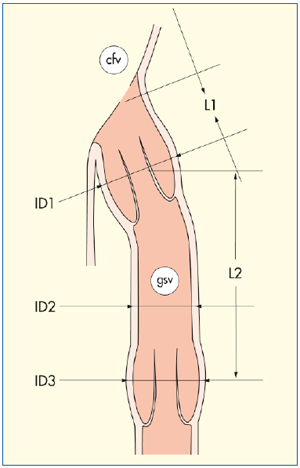
Figure 10. Venous mapping after ultrasound examination: schematic representation of the terminal and subterminal valves.
ID1 = internal diameter of the great saphenous vein at the level of the terminal valve (ostial valve)
ID2 = internal diameter of the great saphenous vein between the terminal and subterminal valves
ID3 = internal diameter of the great saphenous vein at the level of the subterminal valve
L1 = distance between the saphenofemoral junction and the terminal valve
L2 = distance between the terminal and subterminal valves
cfv = common femoral vein
gsv = great saphenous vein
MATERIALS
The usual configuration of the materials supplied in a Venocuff II‘ Kit, (Imthage, Sydney, New South Wales, Australia) include a designated “L” stent, which is for repairing the left SFJ, a designated “R” for the right SFJ, and an un-notched stent, “D”. The “D” is used for deep venous valve reconstruction, but can also be used to repair to the ST valve of the left or right GSV. Holes have been placed in the belt of the Venocuff II‘ act as a guide indicating the stent ID. The first hole in the belt indicates a stent ID of 5.5 mm equating to an GSV ID of 4.5 mm when the wall thickness of the GSV is considered. From experience this is the most appropriate size of the TV ring for a small woman. The next hole on the belt indicates a stent ID of 6.5 or GSV ID of 5.5 mm. The third hole indicates a stent ID of 7.5 mm or GSV ID of 6.5 mm, which is most appropriate for a larger male. The shape of the notch has also been modified to improve the symmetrical reduction of the diameter of the valve ring of the SFJ. Also, the belt buckle has been widened to allow the device to take on an elliptical shape as the diameter is decreased.
Operative management
In patients taking hormonal medication, medication was stopped and treatment was suspended for a minimum of 3 weeks prior to operative intervention. Infected lower limb lacerations were assessed, documented and the procedure was postponed if there appeared to be any chance of an infective complication occurring. The patients were instructed not to shave their legs or the groin region as the chance of developing an infection in the severed hair follicles is high. Shaving was performed at the time of induction. Immediately before operative intervention 5,000 units of heparin were given subcutaneously and 2,000 units intravenously. At this stage intravenous antibiotics were also given.
A standard groin incision was used dissect to and expose the SFJ. Tributaries were clipped for access only and the common femoral vein was exposed with at least 1/2 cm clearly visible in the operative field. A Vessiloop™ was placed around the terminal portion of the GSV 3 cm below to the SFJ and was used to control inflow while testing the competence of the SFJ after EVS. The exact location of the TV was identified based on preoperative ultrasound measurements and with a right-angle forceps; the stent was introduced around the valve. The end of the stent was inserted into the buckle and tightened. By determining the ID of the SFJ preoperatively, it was often possible to predict the ID required at operation to achieve competence as previously described. A mosquito clip was used to temporarily fix the diameter of the stent and then to position the device as high as possible onto the common femoral vein by using the notch in the belt to cover the valve ring.
The valve repair was then tested. The head of the bed was elevated maximally in order to increase the venous pressure, and if the patient was under general anesthesia, the anesthetist assisted the patient to perform an operative Valsalva maneuver. If the operation is performed under local anesthetic, the patient may perform the manoeuver. If no reflux was seen, the diameter of the stent was fixed by using a 5.0 Prolene suture through the buckle, the belt, and the common femoral vein. A further suture was also used distally in order to maintain the diameter of the stent. If required, the stent could be made conical by reducing the lower diameter of the stent. Operative management is summarized in Figure 10.
Testing maneuvers
The most reliable and reproducible test to determine competence was to leave an untied tributary distal to the valve repair. It was important to ensure that the inflow was blocked by using a Vessiloop™ and, after the anesthetist assists the patient to perform the Valsalva maneuver, there should be no bleeding. However, there should free bleeding from the tributary if the Vessiloop™ is loosened and upward flow is reestablished.
• The “Milking Test”: When the inflow was blocked, the segment of SFJ between the Vessiloop™and stent was milked free of blood. The segment remained empty if the valve was competent.
• Intraoperative duplex ultrasound and continuous wave Doppler were also reliable modalities for testing and recording incompetence.
Immediate postoperative strategy
The patients were mobilized as soon as possible. Compression of the limb was achieved by applying rolled wool and simple crepe bandages in the operating room. Prior to discharge from hospital, compression bandages were used to encompass the whole lower limb. All patients were given antiplatelet agent for 2 to 3 weeks postoperatively, eg, salicylic acid or an equivalent. Surgical clips used to close stab avulsions were removed approximately 3 days postoperatively and replaced with “Steri-Strips” or a similar adhesive material. At that time, the underlying compressive bandages were discarded and only the overlying self-elasticized bandages were used. Compression was continued for a further 4 days. The patients were then reviewed at 3 months and a duplex scan was performed to assess the competence and size of the GSV.
Data management
All data were evaluated using receiver operator characteristics (ROC) curve analysis (Metz, 1978; Zwieg & Campbell, 1993).
MATERIALS
The results of intraoperative competence testing following EVS are summarized in Table Ia. Of the 69 TV’s that were examined preoperatively, 50 (50/69, 72%) were considered repairable in view of their ultrasonic features. At operation, 44 (44/50, 88%) of these valves were successfully repaired. In the 6 limbs that had an unrepairable TV, repair of the STV was attempted at operation. All 6 (100%) STV’s were successfully repaired with EVS and competence of the corresponding SFJ’s was restored intraoperatively. Of the 19 (19/69, 28%) TVs whose ultrasonic features indicated they were unrepairable, 18 (18/19, 95%) had gross reflux but 1 (1/19, 6%) was repaired successfully. All STV deemed unrepairable remained incompetent following EVS (18/18, 100%). At 3 months after the procedure, only 3 (3/44, 7%) GSV’s demonstrated residual reflux at the TV and STV, 1 (1/44, 2.3%) GSV demonstrated major reflux and 1 (1/44, 2.3%) LSV developed thrombophlebitis.
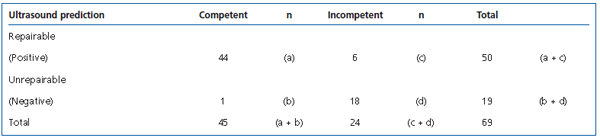
Table Ia. Results at operation following EVS.
Intraoperative data and the findings are summarized in Table Ib. The predictability of successful EVS based on ultrasonic features of the valve were sensitivity 97.8% (95% CI, 88.2 – 99.6), specificity 75% (95% CI 53.3 – 90.2), and accuracy 90.4%. Of the 24 (24/69, 35%) limbs where competence was not restored at operation by the EVS procedure, the GSV was tied and stripped in the usual manner.
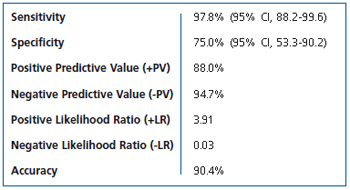
Table Ib. Ultrasound EVS prediction test statistical analysis.
DISCUSSION
The advantages of GSV preservation with valvular stenting relate to correcting reflux and subsequent physiological normalization. With little stimulus for the development of collateralization, the incidence of recurrent varicose veins following EVS is approximately nine times less than stripping in a long-term, prospective, controlled, multi-center trial.8 The initial results with external valvular stenting were based upon the size of the GSV as a predictor of early varicose veins and minimal disruption of venous valve function.7 However, minor dilatation of the GSV can occasionally be associated with severe valve atrophy. Corcos has shown that atrophy of the cusps is important, confirming previous work by Cotton and Edwards & Edward.11-13 All of these pathological findings have usually been associated with advanced disease while in earlier cases the cusps appeared intact.13 External stenting can only produce competence and patency where the valve cusps are essentially nondiseased. The method of EVS to the TV and/or STV often produces descending competence so that the entire GSV functions normally. The GSV resumes its normal size and, in many cases of associated incompetent lateral accessory disease, the GSV remains normal.7,8
Better preoperative understanding of the architecture of the TV and STV at the SFJ should produce better results. The ability of ultrasound to predict a successful valvular stent repair of the TV or SFJ valve was emphasized by a sensitivity of 97.8% and specificity of 75%. However, in many cases, it is possible to repair the STV as well, due to the fact that, if present, the valve cusps were almost always readily identifiable on ultrasound. The more reliable imaging of the STV relates to the angle of incidence of the ultrasound beam. At the SFJ one or both of the cusps may be parallel to the axis of ultrasound beam producing poor return echoes. This is in contrast to the return echoes produced by the STV, which lies closer to the skin and the cusps are usually perpendicular to the beam. In the case of advanced disease, the STV should be repaired also. The tests for competence commonly used at operation are the “Milking-Test” and the test involving blocking of the inflow while leaving a tributary untied so that free egress blood can be seen with increasing central venous pressure. The hydrostatic pressures produced do not completely equate to those produced when tested at 3 months using ultrasound, which explains the difference between operative findings and postoperative ultrasound results. In this series, 4 (4/45, 8.8%) cases of residual reflux were identified 3 months postoperatively. Previous experience has shown that minor reflux rarely progresses when the valve ring diameter is fixed by the external stent and recurrences in this situation are distinctly uncommon. 8 The single case of major reflux identified at 3 months postoperation represented a technical failure. Though the stent was placed at the terminal segment of the GSV, the TV was, in fact, another 1 cm distal to the stent and junction. This anatomical variation is not uncommon.
In every case there was a dramatic reduction in ID of the GSV.7-9 In addition, incompetent tributaries are removed by stab avulsion. Therefore, the postoperative ultrasound usually indicates that the GSV is “normal”.
One complication of venous valve repairs using external stenting is thrombophlebitis. This often occurs when the diseased valve ring is quite dilated (ID>12). Although the ultrasonic appearance of the TV cusps may suggest the valve is repairable, stenting produces folding within the valve ring, which subsequently renders the valve and vein susceptible to thrombosis. It is worth noting that this is a not an uncommon outcome in UGS and may also occur following simple high-ligation of the GSV.4-6 Reflux is obviously abolished but stimulation of collateral veins may occur. Occasionally a localized plug of thrombus occurs immediately distal to the stent and in the short term this will usually recanalize and, in most cases, the SFJ remains competent.
The imaging quality of the valve cusps depends upon the axial and lateral resolution of the B-mode image. Although this has improved dramatically over the past decade, the fine details of the valve cusps and their motion, even in the superficial venous valves, can be challenging in obese patients. To obtain better penetration, a lower frequency probe is required, but this is associated with a dramatic decrease in resolution. Color duplex images are a combination of color-coded Doppler information superimposed over a B-mode image. Although it proves to be highly accurate in locating the site of valves and the presence of reflux, it is usually impossible to completely discern streaming, localized turbulence, laminar flow characteristics, and the relationship of the venous valve structure to venous flow. In comparison, B-flow imaging can demonstrate all of these flow characteristics. Further, B-flow images and the resultant flow patterns can be used to infer morphological detail. For example in Figure 6, the actual cusps can’t be seen directly but prolapsing cusps are strongly suggested. Turbulence, which does not change with the forward and backward flow, strongly suggests thickening and nonmovement of the valve cusps. These cusps themselves may be very difficult to discern with B-mode alone. A similar finding occurs when forward flow is used to assess valve movement. Flattening of the cusps against the wall is a very good sign for EVS success. Conversely asymmetrical funneling or irregular vortices are a reliable predictor for a poor outcome following EVS. These findings have been confirmed by examining the valves of veins that were removed at operation by high ligation and stripping.
The mechanism and action of “B-flow” relates to the reflectivity of red cells at low venous flow. Large numbers of red cells aggregate and rouleaux which produce back scattering proportional to the fourth power of the carrier frequency of the transducer (Rayleigh scattering).19 With increasing velocity, the number of pixels activated per unit time by the same group of red cells is increased which intensifies a return signal. Pre- and postsignal processing can magnify the effects. In other words, “B-flow” acts and looks like a radiographic contrast agent.
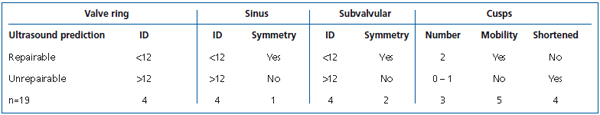
Table II. Preoperative diagnostic ultrasound criteria for EVS.
B-mode imaging alone may be used to reliably predict repairability.7 The basic requirement is the presence of two discernible cusps which move and are not thickened in the presence of reflux. The valves sinus should be symmetrical and the vein below not aneurysmal. Good signs are symmetrical widening with prolapse, long thin cusps, and valve ring dilatation of up to 10 mm in females and 12 mm in males (Table II). Also, having both the TV and STV deemed suitable for EVS is a good indication for success as the STV valve acts as a backup. The best outcomes were seen in patients presenting with TV incompetence and an associated large incompetent anterior or lateral accessory system and the STV had minimal reflux or none at all.7
CONCLUSION
High quality ultrasound with B-flow provides new and valuable preoperative information on the status of the venous valve mechanism at the SFJ, negating the need for invasive diagnostic procedures. The combination of advances in ultrasound technology and improvements to the stent device itself can produce very favorable results when repairing venous valves using EVS in the treatment of varicose veins. The results of this series indicate that venous valves can be repaired provided that two cusps are present and have not been significantly damaged. Compared with conventional stripping operations, EVS is a less invasive procedure, more physiologically acceptable, and more often preferred by patients.8 Table III summarizes the differences between EVS and stripping.20 It is recommended that high-resolution ultrasound and, if available, B-flow, be used to assess venous valve function as an essential preliminary investigation for patients being considered for EVS of the GSV in the treatment of varicose veins.
This article is a translation of the original published in the journal Phlébologie: Graiche JA, Lane RJ, Cuzzilla ML, Coroneos JC, Berney CR. Insuffisance valvulaire veineuse : imagerie par ultrasonographie et réparation par manchonnage. Phlébologie. 2004;57:237-252. Published here with the kind permission of Jean-Paul Henriet.

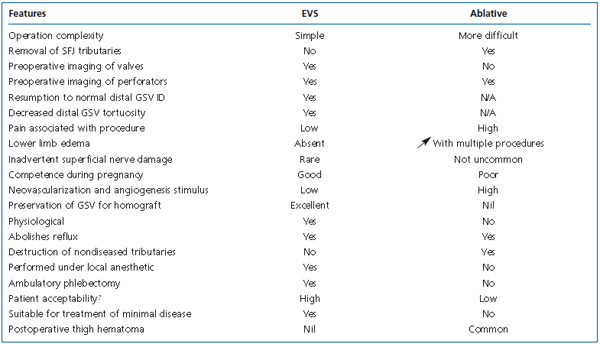
Table III. Summary of comparisons between EVS and stripping of the GSV.
REFERENCES
2. Stonebridge PA, Chalmers N, Beggs I, Bradbury AW, Ruckley CV. Recurrent varicose veins: a varicographic analysis leading to a new practical classification. Br J Surg. 1995;82:60-62.
3. Quigley FG, Raptis S, Cashman M. Duplex ultrasonography of recurrent varicose veins. Cardiovasc Surg. 1994;2775-2777.
4. Pichot O, Sessa C, Chandler JG, et al. Role of duplex imaging in endovenous obliteration for primary venous insufficiency. J Endovasc Ther. 2000;7:451-459.
5. Bishop CC, Fronek HS, Fronek A, et al. Real-time colour duplex scanning after sclerotherapy of the greater saphenous vein. J Vasc Surg. 1991;14:505-508.
6. Myers KA, Wood SR, Lee V. Early results for objective follow-up by duplex ultrasound scanning after echosclerotherapy or surgery for varicose veins. ANZ J Phleb. 2000;4:71-74.
7. Lane RJ, McMahon C, Cuzzilla M. The treatment of varicose veins using the venous valve cuff. Phlebology. 1994;9:3-11.
8. Lane RJ, Cuzzilla ML, Coroneos JC. The treatment of varicose veins with external stenting to the saphenofemoral junction. Vasc Surg. 2002;36:179-192.
9. Corcos L, De Anna D, Zamboni P, et al. Reparative surgery of valves in the treatment of superficial venous insufficiency. External banding valvuloplasty versus high ligation or disconnection. A prospective multi centric trial. J Mal Vasc. 1997;22: 128-136.
10. Donini I, Corcos L, De Anna D. Preliminary results of external sapheno-femoral valvuloplasty: a trial by the Italian Society of Phlebolymphology. Phlebology. 1991;6: 167-179.
11. Corcos L, Procacci T, Peruzzi G, et al. Sapheno-femoral valves. Histopathological observations and diagnostic approach before surgery. Dermatol Surg. 1996;22: 873-880.
12. Akesson H, Risberg B, Bjorgell O. External support valvuloplasty in the treatment of chronic deep vein incompetence of the legs. Int. Angiol. 1998;18:233-238.
13. Edwards JE, Edwards EA. The saphenous valves in varicose veins. AM Heart J. 1940; 19:338-351.
14. Pellerito JS. Current approach to peripheral arterial sonography. Radiol Clin North Am. 2001;39:553-567.
15. Furuse J, Maru Y, Mera K, et al. Visualisation of blood flow in hepatic vessels and hepatocellular carcinoma using B-flow sonography. J Clin Ultrasound. 2001;29:1-6.
16. Henri P, Tranquart F. B-flow ultrasonographic imaging of circulating blood. J Radiol. 2000;81:465-467.
17. Pooh RK. New application of B-flow sonoangiography in perinatology. Ultrasound Obstet Gynecol. 2000;15:163.
18. Deane C. Extended field-of-view and Bflow ultrasound: fashion or future? Ultrasound Obstet Gynecol. 2000;15:96-97.
19. Sigel B, Machi J, Beitler JC, et al. Variable ultrasound echogenicity in flowing blood. Science. 1982;218:1321-1323.
20. Lane RJ, Graiche JA, Coroneos JC, Cuzzilla ML. Long-term comparison of external valvular stenting and stripping of varicose veins. Aust NZ J Surg. 2003;73: 605-609.
