Pathophysiology of pain in venous disease
Nicolas DANZIGER
Fédération de Neurophysiologie Clinique,
Faculté de Médecine Pitié-Salpêtrière, Pitié-
Salpêtrière Medical Center, Paris, France
SUMMARY
Pain is the chief complaint that leads to the diagnosis of venous disease and it has a significant impact on patients’ quality of life. But for the clinician as well as the researcher, pain in venous disease is difficult to assess, both because of its multifaceted nature and due to the absence of a close relationship between pain as a symptom and the severity of venous disease. Current hypotheses on the mechanisms of pain in venous disease emphasize a local inflammatory origin. However, although indicators suggesting an inflammatory reaction in varicose veins have accumulated dramatically over the last five years, the precise mechanisms governing the interaction between the mediators of inflammation and venous nociceptors, which may account for the variability of pain in venous disease, remain difficult to explain, both clinically and experimentally.
Pain, the chief complaint that leads to the diagnosis of venous disease1,2 has a significant impact on patients’ quality of life.3,4 But for the clinician as well as the researcher, pain in venous disease is difficult to assess. First, pain of venous origin is often multifaceted and is frequently associated with other unpleasant sensations, often difficult to describe, which do not belong to the range of symptoms of nociception, ie, a sensation of heaviness, cramps, and tension in the legs, or pruritus.1 Second, pain intensity in venous disease can vary considerably, both from one patient to another or in the same patient over the course of time, as venous disease continues to progress. Lastly, although the neurophysiological mechanisms of pain of venous origin are better understood,5 and although some biochemical and cellular processes involved in varicose vein remodeling have been elucidated by recent studies,6-8 the causal relationship between venous disease and pain of venous origin remains difficult to explain, both clinically and experimentally, in particular due to the absence of a close correlation between pain and the severity of venous disease.
ANATOMY OF VENOUS INNERVATION AND
PHYSIOLOGICAL PROPERTIES OF VENOUS AND
PERIVENOUS NOCICEPTORS
Data obtained by electron microscopy show that veins are innervated by sensory nerve fibers whose cell body is located in the dorsal root ganglia of the spinal cord.5 These sensory fibers are located along the venous wall and are subdivided into collaterals, which have two possible destinations. Some collaterals cross the tunica adventitia and end in the venous wall between endothelial cells and smooth muscle cells of the tunica media. Other collaterals reach the connective tissue of the perivenous space where they branch into unmyelinated free nerve endings, in close contact with the microcirculation. These subendothelial and perivascular nerve endings are nociceptors: they are the sole source for transmission of nociceptive afferent signals generated both in the venous wall itself and also in the perivenous connective tissue. The properties of venous and perivenous nociceptors account for the type of stimuli that can generate a painful sensation of venous origin. Clinical experience has shown that pain of venous origin can be induced by mechanical stimuli such as venipuncture and traction exerted on a vein or the existence of an indwelling catheter, as well as nonphysiological chemical stimuli such as the injection of hyperosmolar saline or a glucose solution, the injection of a frankly acidic (pH < 4), or alkaline (pH > 11) solution, or the injection of isotonic saline at low (<20°C) temperature.9 Furthermore, superficial venous inflammation or deep vein thrombosis is a source of acute pain of venous origin frequently observed in clinical practice. Experimentally, the properties of venous nociceptors have been studied in humans by applying different types of stimuli (mechanical, thermal, chemical) in an isolated venous segment and by asking the subject to qualify and grade the intensity of the sensation induced by these stimuli (Figure 1).
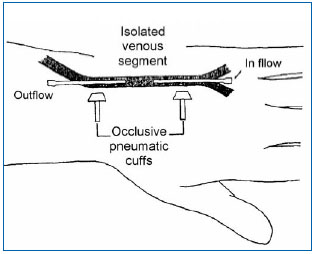
Figure 1. Experimental set-up to study painful sensations evoked by stimulation of an isolated venous segment in man (5). A venous segment in the back of the hand located between two Teflon canulas is isolated from the rest of the circulation by two occlusive pneumatic cuffs. Local anesthesia of the skin around the isolated venous segment ensures that sensations induced are specifically related to activation of venous afferent fibers, without the participation of cutaneous sensory fibers.
Concomitantly, electrophysiological tracings of nerve fibers that innervate the venous wall recorded in anesthetized animals have shown that two types of small-diameter afferent fibers can transmit nociceptive information of venous origin: type A_ myelinated afferent nerve fibers and type C unmyelinated afferent nerve fibers.10
The human venous pain model has made it possible to demonstrate that different types of nonphysiological endovenous stimuli, such as balloon dilation of the vein, the application of cold or heat, an electrical stimulus, and infusion of hyperosmolar saline produce a painful sensation, starting at a certain threshold, whose quality is the same whatever the method of stimulation used, and whose intensity increases exponentially with the intensity of the stimulus, and which totally disappears after injection of a local anesthetic in the isolated venous segment.5 Remarkably, the stimulus-sensation curve (intensity of the painful sensation according to the intensity of the applied stimulus) is superimposable from one stimulus to another. These results suggest that the different stimuli used activate the same venous nociceptors, which means that the vast majority of nociceptors located in the venous wall are polymodal nociceptors. These experiments show that mechanical venous balloon dilation starts to be experienced as painful only from the moment that the diameter of the vein reaches a value three times that of normal. If we add to this observation the fact that venous dilation generally is not perceived as painful when induced by pharmacological methods such as the local application of adenosine,11 it appears that venous dilation, even major, is not by itself a significant source of pain in normal subjects. Moreover, the painlessness of an arteriovenous fistula created for the purpose of hemodialysis is evidence in support of this hypothesis.
DISCREPANCY BETWEEN PAIN SYMPTOMS
AND CLINICAL SEVERITY OF VENOUS DISEASE
In light of recent clinical studies designed to identify the subpopulation of patients with chronic venous disease most likely to undergo surgical therapy, the first observation that should be made is that there is no close correlation between pain and the severity of venous disease. Quantitative assessment of the stage of chronic venous disease is based on the CEAP clinical classification,12 a system for classification of severity of clinical signs in seven classes (C0 to C6) (Table I).
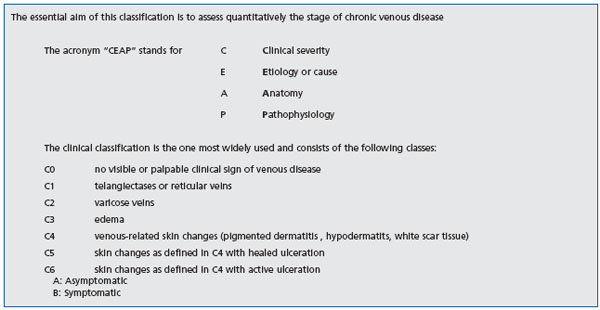
Table I: The CEAP classification12
Several epidemiological studies have shown that the existence or intensity or both of lower limb symptoms that can be associated with chronic venous disease are not correlated with the severity of clinically evaluated venous disease. In a population study of over 1500 subjects 18 to 64 years of age (Edinburgh Vein Study), Bradbury et al demonstrated a correlation between truncular varices seen in a clinical examination and three lower limb symptoms in women: pain, sensation of heaviness or tension, and pruritus.1 Although statistically significant, the correlations observed between each of these symptoms and truncular varices were too low to determine a causal relationship with the discomfort or pain associated with confirmed venous disease. In fact, in this study about 40% of asymptomatic women presented with varicose veins in the clinical examination while 45% of patients who complained of lower limb pain compatible with chronic venous disease did not have varicose veins. Furthermore, in male subjects, no significant correlation was found between pain and the existence of truncular varices. Lastly, none of the symptoms studied appeared to vary according to severity of the varicose veins, whatever the patient’s gender.
Many studies of patients with advanced chronic venous disease (classes C4 to C6) have demonstrated a relation between the degree of venous reflux identified with Doppler scanning and the severity of clinical signs and symptoms of venous origin. However, the search for such a correlation in the setting of the Edinburgh Vein Study, which focused primarily on patients presenting with early-stage venous disease, proved disappointing.13 In fact, only a low correlation was observed between pathologic superficial venous reflux (duration greater than or equal to 0.5 seconds) and sensation of swelling, heaviness, or tension. In addition, this correlation was limited either solely to men (sensation of swelling) or solely to women (sensation of heaviness or tension). No significant correlation was observed between superficial venous reflux and pain strictly speaking. Furthermore, there was no correlation between the studied symptoms and deep venous reflux (popliteal vein), whatever the patient’s gender. Similarly, in a study of over 120 patients presenting with mild to moderate venous disease,14 no correlation was observed between pain intensity and clinical severity of venous disease based on the CEAP classification. Lastly, in a cohort study of 132 patients, Howlader and Smith reported no statistical relation between pain score or heaviness score, evaluated on a 10-point visual analogue scale, and the clinical severity of venous disease.15 Thus, for example, the median pain score was 2.8 in the group of patients with class C2 venous disease, 4.5 in class C3, only 0.5 in class C4 and 0 in patients with class C5 venous disease. In addition, no difference was observed in pain scores between patients presenting with a superficial venous reflux and those presenting with deep venous reflux. These results totally confirm the observations made in the setting of a survey conducted in France focused on the frequency of clinical symptoms according to duration of venous disease.2 In fact, this survey demonstrated a very significant decrease in the frequency of functional signs of venous disease, in particular pain, over time. Thus, the frequency of the painful heaviness sensation declined from 71% in the group with symptoms of less than 5 years’ duration to only 50% in the group whose symptoms were of 30 years duration or longer. These results are in agreement with findings of an epidemiological study conducted in Switzerland, showing that the prevalence of varicose veins increases with age, while pain decreases with age.16
PAIN MECHANISMS IN VENOUS DISEASE:
INFLAMMATORY MARKERS
Current hypotheses on pain mechanisms in venous disease are focused on a local inflammatory origin, related to venous stasis. Interestingly, the same processes assumed to generate pain in venous disease also seem to be involved in the longer term in the process of varicose vein remodeling, defined as all of the qualitative and quantitative alterations in the cellular and matrix components of the venous wall.17 The starting point for these mechanisms probably is local hypoxia associated with capillary stasis. A significant fall in the partial pressure of oxygen after 30 minutes in the standing position has been demonstrated in lower limb veins in venous disease8 and several studies have demonstrated that hypoxia induced by capillary stasis has the effect of activating endothelial cells.7 Such activation is manifest by elevation of calcium concentrations in the cytoplasm of endothelial cells,18 which itself is responsible for an increase in phospholipase A2 activity.19 Activation of phospholipase A2, in turn, leads to the synthesis and local release of proinflammatory mediators such as bradykinin, prostaglandins E2 and D2, platelet-activating factor (PAF), and leukotriene B4.20,21 PAF seems to play a pivotal role: first, it enhances local release of serotonin and histamine, and, second, it produces abnormal adherence of neutrophils to the venous endothelium, prior to their infiltration of the venous wall itself, and stimulates the synthesis of leukotriene B4 by activated neutrophils. Evidence for such an inflammatory reaction in patients with varicose veins has accumulated dramatically over the last five years,22 and the biochemical changes identified suggest that endothelial cells and neutrophils are the source of this local inflammation (Figure 2).8,23-26 The presence of neutrophils, monocytes, and activated T lymphocytes, the accumulation of macrophages and mast cells, the expression of adhesion molecules on the surface of leukocytes and endothelial cells (LFA-1, VLA-4, ELAM- 1, ICAM-1, VCAM-1), the synthesis of cytokines (IL-1 beta, IL-6, TNF alpha) and prothrombotic factors (von Willebrand factor) are all indicators of inflammation in venous disease.12,27
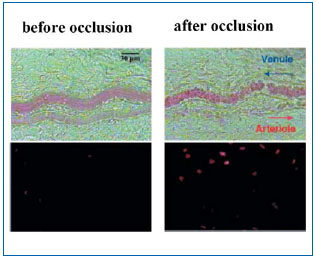
Figure 2. Inflammatory reaction observed in the microcirculation after one hour of venous hypertension induced by acute occlusion of a venule in the rat. The tissue adjacent to the venule shows signs of extensive cell apoptosis identified by fluorescent marking of parenchymal cell nuclei with propidium iodide (lower right), representing an advanced stage of inflammation.
Figure taken from Takase S, Lerond L, Bergan JJ, Schmid-Schönbein GW. The inflammatory reaction in
venous hypertension in the rat. Microcirculation. 2000;7:1-11.
Some proinflammatory mediators released locally as the result of hypoxia can activate nociceptors located in the venous wall (between endothelial cells and smooth muscle cells of the media) and in the connective tissue that forms the perivenous space, in close contact with the microcirculation. Therefore, the study of the painful sensation evoked in healthy subjects by the intravenous or perivenous application of bradykinin unambiguously demonstrates the role of this neuromediator in venous pain.28 Moreover, several studies suggest that the algogenic action of bradykinin in and around the vein depends on the release of nitric oxide (NO) by endothelial cells or by smooth muscle cells in the wall of the vein or by both,29 and the subsequent activation of cyclic GMP synthesis.30 This algogenic action of bradykinin is potentiated by the local administration of prostaglandin E2.31 Prostaglandin E2, whose application is by itself painless, thus has a sensitizing effect on venous nociceptors. Based on these data, the hypothesis can be formulated that such a cascade of reactions, by an auto-amplification process, can lead to the local release of a true “inflammatory mixture”, which can activate venous and perivenous nociceptors, as well as extravasation of plasma with transmural and tissue edema. As time passes, this process also results in varicose vein remodeling characterized by cellular and matrix alterations that lead to the loss of structural integrity of the venous wall and of its elastic properties.17 In agreement with this hypothesis, Howlader and Smith have previously demonstrated that nitric oxide concentrations measured in blood collected in the saphenous vein or in a vein in the dorsal aspect of the foot were significantly higher in patients with the most severe stage of venous disease.32 Similarly, some studies have reported a higher levels of markers of endothelial activation in experimental venous hypertension in the most advanced stages of venous disease.33 Considering the key role played by these inflammatory processes, both in pain as well as in varicose vein remodeling, it would have been expected that levels of some inflammatory markers are correlated with the intensity of pain in venous disease.
But, following the example of clinical estimation and evaluation by venous Doppler scanning, this search proved negative.15 In fact, no significant correlation was found between levels of the twelve inflammatory markers (measured in a vein on the dorsal aspect of the foot) and pain intensity score on a visual analogue scale in a population of 132 patients with chronic venous disease ranging from class C2 to C5. In spite of the solid physiological basis explained in the above, the assumed relationship between the inflammatory processes generated in the venous wall and pain associated with venous disease thus seems difficult to demonstrate formally.
POSSIBLE HYPOTHESES TO EXPLAIN DISCREPANCIES BETWEEN THE PAIN SYMPTOM AND OBJECTIVE MARKERS OF VENOUS DISEASE
Pain, clinical severity, and inflammatory markers
The intensity of pain of venous origin is not correlated with the extent of truncular varices observed in clinical examination, the severity of reflux measured with Doppler scan, or levels of inflammatory markers measured in a lower limb vein. Therefore, if hypoxia is indeed the major factor that triggers pain of venous origin, it is entirely possible that many painful hypoxiarelated conditions can occur transiently in a given patient, for example, solely at the end of the day, after standing for a prolonged period, or during certain periods of the menstrual cycle. In other words, the cascade of chemical reactions that activate venous and perivenous nociceptors can occur before any significant remodeling of large venous vessels arises. This could explain the frequency of functional signs such as pain or heaviness in the legs in patients who do not have varicose veins in the clinical examination and no abnormal reflux seen in a Doppler scan, as was observed in the Edinburgh Vein Study. In this regard, it is obvious that if pain and varicose vein remodeling involve biochemical and cellular processes to a similar extent, essentially inflammatory, the time line of these pathological mechanisms is basically different. In fact, pain occurs as the short-term consequence of venous hypoxia, while varicose vein remodeling only participates at a much later stage of venous disease.
The fact that pain is not closely correlated with objective parameters of varicose vein remodeling, incompetent venous valves, and inflammation suggests that the primary activation site of venous and/or perivenous nociceptors may not be in the large venous vessels. In this regard, the hypothesis of local activation of nociceptors in the microcirculation, where contact between nerve endings, the arteriole, the vein, and the capillary is probably much closer than on the macrovascular level, seems entirely plausible.
Consequently, several studies recently have focused on the search for microcirculatory parameters of venous disease.34,35 Furthermore, several recent studies using an experimental model of acute venous occlusion in the rat have shown the specific role of the increase in microvascular pressure in triggering an inflammatory reaction characterized by infiltration of neutrophils in the endothelium and adjacent tissues.24 The alteration of friction forces on the endothelium (shear stress) produced by blood flow is another essential factor that can promote local inflammation of the venous wall.22 In fact, several experimental studies have shown that shear stress, through integrins anchored in the endothelial cell membrane, can influence many intracellular biochemical processes, such as protein G phosphorylation, activation of tyrosine kinases, free radical production, and the synthesis of different nuclear transcription factors.36-38 In the light of available data, it seems that a physiologically normal shear stress produces a potent, local inflammatory effect, while a reduction or an increase in this force below or above a given physiological threshold can lead to overexpression of proinflammatory genes.22,27
How to explain the diminution of pain in the most advanced stages of venous disease?
The main hypothesis that can explain a significant decrease in the frequency and intensity of pain in the most advanced stages of venous disease is based on alteration of innervation of the venous wall and the perivenous tissue. This alteration of nerve fibers may reflect sensory peripheral neuropathy, possibly related to ischemia secondary to venous microangiopathy, and an increase in endoneural pressure.39 Consequently, several studies have demonstrated a significant elevation of the threshold of tactile, vibrational, and thermal sensation in the extremities in patients with chronic venous disease, suggesting the loss of sensory axons.39-41 Interestingly, sensory threshold elevation was significantly more pronounced in class C5 than in class C2 disease.41 It can be seen that such a reduction in the number of venous and perivenous nociceptors can account for a diminution of pain in the most advanced stages of venous disease.
Other factors that may explain inter-individual variability of pain in venous disease?
The complexity and diversity of mechanisms that can be activated in the pathogenesis of pain in venous disease are a considerable source of interindividual variability. Such variability involves both the reactivity of the cellular components involved (endothelial cells, neutrophils, venous, and perivenous nociceptors) and the mechanisms of integration of nociceptive stimuli in the brain. On the cellular level, for example, experimental studies of human umbilical cord venous endothelial cells have demonstrated that the quantity of different prostaglandins released as a result of the effect of hypoxia can vary by a factor of 10 depending on the donor.7 Similarly, neutrophil reactivity varies with age and previous sensitization (“priming”) to other inflammatory signals. Furthermore, the density of venous and perivenous innervation as well as the density of nociceptors’ ion channels, which allow conversion of the chemical message into a nerve impulse coding for nociceptive information, can also vary considerably from one subject to another. Lastly, at the other end of the chain of these algogenic processes, the intensity of brain modulation of nociceptive sensation resulting from the effect of endogenous opioids, whose variations from one subject to another are partly due to genetic factors, is also likely to partly determine pain sensitivity in a given subject with respect to venous nociceptive stimuli. As an example, recently it was demonstrated that the genotype of the enzyme catechol-O-methyl-transferase, on which depends the quantity of endogenous opioids released during a pain stimulus, significantly affects pain sensitivity in healthy subjects.42 But all these variable factors are only relative obstacles to the elucidation of the pain mechanisms in venous disease. In the absence of a correlation between the condition of the large venous vessels and the degree of pain reported by patients, the essential point perhaps consists of lessons learned by questioning not necessarily the validity of this complaint, but rather the primary site of interaction between the mediators of inflammation and venous nociceptors, with a view to developing a method to test the nociceptive function of the microcirculation in venous disease.
CONCLUSION
• Epidemiological studies demonstrate a discrepancy between pain severity in venous disease based on clinical findings and the degree of pain reported by patients.
• Such a discrepancy complicates the objective evaluation of analgesic therapies in venous disease.
• The neurophysiological mechanisms involved in venous pain are now better understood, but the causal relationship between venous disease and pain remains difficult to explain by experimental methods.
• The localized release of proinflammatory mediators seems to play a decisive role in the activation of venous and perivenous nociceptors and may account for the occurrence of pain starting at the early stages of venous disease.
• Diminution of pain in the advanced stages of venous disease may be related to peripheral sensory neuropathy induced by venous microangiopathy.

REFERENCES
2. Allaert FA. Evolution des tableaux cliniques de l’insuffisance veineuse chronique en fonction de son ancienneté. Angéiologie. 2002;54:1.
3. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539-554.
4. Andreozzi GM, Cordova RM, Scomparin A, Martini R, D’Eri A, Andreozzi F. Quality of life in chronic venous insufficiency. An Italian pilot study of the Triveneto Region. Int Angiol. 2005;24:272-277.
5. Arndt JO, Klement W. Pain evoked by polymodal stimulation of hand veins in humans. J Physiol. 1991;440:467.
6. Michiels C, Arnould T, Thibaut- Vercruyssen R, Bouaziz N, Janssens D, Remacle J. Perfused human saphenous veins for the study of the origin of varicose veins: role of the endothelium and of hypoxia. Int Angiol. 1997;16:134-141.
7. Michiels C, Bouaziz N, Remacle J. Role of the endothelium and blood stasis in the development of varicose veins. Int Angiol. 2002;21:18-25.
8. Jacob MP, Cazaubon M, Scemama A, et al. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation. 2002;106:535-538. 9. Klement W, Arndt JO. Pain but no temperature sensations are evoked by thermal stimulation of cutaneous veins in man. Neurosci Lett. 1991;123:119-122.
10. Michaelis M, Goder R, Habler HJ, Janig W. Properties of afferent nerve fibres supplying the saphenous vein in the cat. J Physiol. 1994;474:233-243.
11. Klement W, Arndt JO. Adenosine does not evoke pain from venous and paravascular nociceptors in the human. Cardiovasc Res. 1992;26:186-189.
12. Eklof B, Rutherford RB, Bergan JJ, et al. American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004; 40:1248-1252.
13. Bradbury A, Evans CJ, Allan P, Lee AJ, Ruckley CV, Fowkes FG. The relationship between lower limb symptoms and superficial and deep venous reflux on duplex ultrasonography: The Edinburgh Vein Study. J Vasc Surg. 2000;32:921-931.
14. Duque MI, Yosipovitch G, Chan YH, Smith R, Levy P. Itch, pain, and burning sensation are common symptoms in mild to moderate chronic venous insufficiency with an impact on quality of life. J Am Acad Dermatol. 2005;53:504-508.
15. Howlader MH, Smith PD. Symptoms of chronic venous disease and association with systemic inflammatory markers. J Vasc Surg. 2003;38:950-954.
16. Widmer LK, Zemp E. Diagnosis and treatment of varicose veins. Deductions from on a Basel prospective epidemiological study. Helv Chir Acta. 1988;54:531-539.
17. Badier-Commandier C, Jacob MP, Michel JB. Le remodelage variqueux. Médecine Thérapeutique. 2000;6:718-723.
18. Arnould T, Janssens D, Michiels C, Remacle J. Effect of aescine on hypoxiainduced activation of human endothelial cells. Eur J Pharmacol. 1996;315:227-233.
19. Michiels C, Renard P, Bouaziz N, et al. Identification of the phospholipase A(2) isoforms that contribute to arachidonic acid release in hypoxic endothelial cells: limits of phospholipase A(2) inhibitors. Biochem Pharmacol. 2002;63:321-332.
20. Michiels C, Arnould T, Knott I, Dieu M, Remacle J. Stimulation of prostaglandin synthesis by human endothelial cells exposed to hypoxia. Am J Physiol. 1993;264:C866-C874.
21. Michiels C, Arnould T, Remacle J. Hypoxia-induced activation of endothelial cells as a possible cause of venous diseases: hypothesis. Angiology. 1993;44:639-646.
22. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
23. Smith PD. Update on chronic-venousinsufficiency- induced inflammatory processes. Angiology. 2001;52:S35-S42. Review.
24. Takase S, Schmid-Schonbein GW, Bergan JJ. Leukocyte activation in patients with venous insufficiency. J Vasc Surg. 1999;30:148-156.
25. Junger M, Steins A, Hahn M, Hafner HM. Microcirculatory dysfunction in chronic venous insufficiency (CVI). Microcirculation. 2000;7:S3-S12.
26. Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge Smith PD. Leucocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265-273.
27. Schmid-Schönbein GW. Inflammation and the pathophysiology of chronic venous insufficiency. Phlebolymphology. 2003;39:95-99.
28. Kindgen-Milles D, Klement W, Arndt JO. The nociceptive systems of skin, paravascular tissue and hand veins of humans and their sensitivity to bradykinin. Neurosci Lett. 1994;181:39-42.
29. Holthusen H , Arndt JO. Nitric oxide evokes pain at nociceptors of the paravascular tissue and veins in humans. J Physiol. 1995;487:253-258.
30. Holthusen H. Involvement of the NO/cyclic GMP pathway in bradykininevoked pain from veins in humans. Pain. 1997;69:87-92.
31. Kindgen-Milles DW. Effects of prostaglandin E2 on the intensity of bradykinin-evoked pain from skin and veins of humans. Eur J Pharmacol. 1995;294:491-496.
32. Howlader MH, Smith PD. Increased plasma total nitric oxide among patients with severe chronic venous disease. Int Angiol. 2002;21:180-186.
33. Saharay M, Shields DA, Porter JB, Scurr JH, Coleridge Smith PD. Leukocyte activity in the microcirculation of the leg in patients with chronic venous disease. J Vasc Surg. 1997;26:265-273.
34. Howlader MH, Smith PD. Correlation of severity of chronic venous disease with capillary morphology assessed by capillary microscopy. J Vasc Surg. 2006;43:563-569.
35. Virgini-Magalhaes CE, Porto CL, Fernandes FF, Dorigo DM, Bottino DA, Bouskela E. Use of microcirculatory parameters to evaluate chronic venous insufficiency. J Vasc Surg. 2006;43:1037- 1044.
36. Resnick N, Yahav H, Khachigian LM, et al. Endothelial gene regulation by laminar shear stress. Adv Exp Med Biol. 1997;430:155-164.
37. Shyy JY, Li YS, Lin MC, et al. Multiple ciselements mediate shear stress-induced gene expression. J Biomech. 1995;28:1451- 1457.
38. Chen KD, Li YS, Kim M, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393-183400.
39. Reinhardt F, Wetzel T, Vetten S, et al. Peripheral neuropathy in chronic venous insufficiency. Muscle Nerve. 2000;23:883- 887.
40. Shami SK, Shields DA, Farrah J, Scurr JH, Coleridge Smith PD. Peripheral nerve function in chronic venous insufficiency. Eur J Vasc Surg. 1993;7:195-200.
41. Padberg FT, Jr., Maniker AH, Carmel G, Pappas PJ, Silva MB, Jr., Hobson RW, 2nd. Sensory impairment: a feature of chronic venous insufficiency. J Vasc Surg. 1999;30:836-842.
42. Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects muopioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240- 1243.
