Pelviperineal venous insufficiency and varicose veins of the lower limbs
Jean-Louis LASRY,
Gérard COPPÉ,
Hervé BORIE,
Agnès LEROUX,
Dominique BRYON,
Stéphane KOVARSKY
Dept. of Cardiology and Interventional
Radiology
Investigation and treatment of pelvic venous insufficiency (PVI) in women to date has primarily involved its most familiar clinical presentation, ie, pelvic congestion syndrome (PCS) accurately described by Hobbs.1 For the last few years,2 more attention has focused on involvement of PVI in the pathogenesis of primary varicose veins or recurring varicosities of the lower limbs. This finding has been confirmed by a national epidemiologic survey that evaluated the potential incidence of pelvic pain of venous origin in a targeted population of women, and its possible association with lower limb varicosities.3
While advances in the recognition of PCS have been made as the result of findings of laparoscopy and phlebography, currently its relationship with superficial varices of the lower limbs has been further elucidated by noninvasive imaging methods, primarily Doppler ultrasonography.
Treatment of this condition by interventional radiology procedures is an effective therapeutic alternative in ruling out pelvic varicosities, and complements surgical disconnection of incontinent anastomoses (sites of leakage), which supply varicose veins of the lower limbs.
CLINICAL PRESENTATION
PVI seems to be the most appropriate term to describe the hemodynamic findings in this disease and fits the description of its different clinical manifestations. Depending on the territory mainly involved, such manifestations predominate in the pelvis or the lower limbs: in PCS and/or varicose veins of the lower limbs.
The principal specialists involved in management of this condition, ie, gynecologists, vascular surgeons, and angiologists, should therefore be aware of PVI when they examine women presenting with pelvic pain and/or atypical or recurring varicose veins of the lower limbs, and should order the required tests, in particular, Doppler ultrasonography.
Pelvic congestion syndrome
PCS is characterized by chronic pain (of more than 6 months duration) manifest by pelvic heaviness, exacerbated by the standing position, and its presence at the end of the day and during the premenstrual period. Dysmenorrhea, dyspareunia, post-coital pain and dysuria are frequently present. Such pain is refractory to analgesics. Sometimes, hematuria associated with left low back pain is observed, suggesting tight extrinsic compression of the left renal vein between the aorta and the superior mesenteric artery, the so-called “nutcracker phenomenon.”4
Examination may reveal a retroverted uterus combined with varicose veins of the lower limbs, suggesting superficial venous insufficiency (SVI), hemorrhoids, vulvar and gluteal varicosities.
Varicose veins of the lower limbs
Lower limb varicosities can be manifest in three forms: perineal and/or gluteal varicosities, specific for SVI, territories of the long or short saphenous veins, and recurrence following invasive treatment. In these two populations, which often overlap, there is an increased incidence of multiparous women; and a family history of venous disease, gynecological disorders and surgery. Emotional disorders are often observed in such patients.
SCHEMATIC DESCRIPTION AND ANATOMICAL CHARACTERIZATION OF THE PELVIC VENOUS SYSTEM IN WOMEN
Anatomical characteristics
This complex and incompletely systematized network of intersecting veins centered around the uterus comprises many interconnected plexuses.5 The very dense collecting network is located as a shunt in the femoral iliocaval system, and disease in one plexus can affect another.
The paucity or total absence of venous valves allows twodirectional blood flow. Another essential feature of the pelvic venous system is its ability to adapt to certain conditions, in particular pregnancy (Figure 1). The physiological changes that it produces can become abnormal and continue after childbirth.
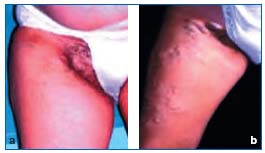
Figure 1. a and b: perineal varicose veins of pregnancy.
Anatomical description
The pelvic venous system consists of visceral and parietal networks.
• The visceral network: is highly variable, forms avalvular venous plexuses, with abundant interconnecting uteroovarian, uterovaginal, vesicovaginal, vesical and rectal anastomoses, which are partly drained by the visceroperineal venous trunk into the avalvular internal iliac vein, and partly into the ovarian veins. Lastly, an accessory pedicle, the vein of the round ligament, connects to the utero-ovarian system on one side and on the other with the superficial epigastric vein or the external iliac vein or both (Figure 2);
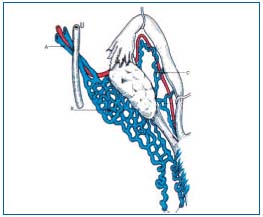
Figure 2 (Courtesy Dr Le Pennec). a) ovarian vein b) utero-ovarian venous plexus c) tubal veins.
• The parietal network is systematized, but nonetheless has many anatomical variants. Generally, the veins follow a pathway very similar to that of the arteries. This network consists of the superior gluteal, inferior gluteal, lateral sacral, iliolumbar, obturator, and internal pudendal veins (Figure 3).
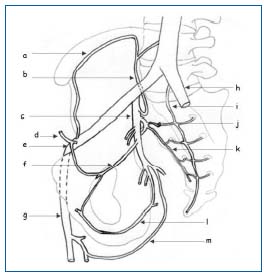
Figure 3.
a) ascending ramus of the deep circumflex iliac vein
b) iliolumbar vein
c) internal iliac vein
d) inferior epigastric vein
e) external iliac vein
f) obturator vein
g) femoral vein
h) common iliac vein
i) median sacral vein
j) superior lateral sacral branch
k) inferior lateral sacral branch
l) internal pudendal vein
m) femoral branch of inferior gluteal vein
These veins combine to form the anterior branch of the internal iliac vein, which is a single vein in 50% of cases, double in 36%, and plexiform in 14% (Figure 3).
These two networks are drained by three collecting veins: the internal iliac vein, which with the external iliac vein forms the common iliac vein, and arises from the inferior vena cava, the ovarian veins, which on the left side end in the renal vein in 99% of cases (a single vein in 79% of cases) and in the vena cava in 1%; and on the right side end in the inferior vena cava in 98% of cases (a single vein in 78% of cases) and in the renal vein in 2%, the superior rectal vein, which joins the inferior mesenteric vein.
The intra/extra-pelvic anastomoses with the lower limbs (Figure 4).
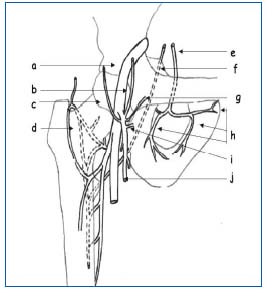
Figure 4 (Courtesy Dr Le Pennec).
a) avalvular femoral vein
b) superior epigastric vein
c) superficial circumflex iliac vein
d) median circumflex vein
e) obturator vein
f) inferior gluteal vein
g) superficial external pudendal vein
h) branches of obturator vein
i) deep external pudendal vein
j) long saphenous vein
There are two connecting networks, the gluteal-ischiatic and the internal pudendal venous.
The gluteal-ischiatic network:
• The gluteal vein, which drains the sacral plexus, anastomoses with the external circumflex iliac vein, a collateral of the internal saphenous vein,
• The ischiatic vein, which drains the posterior compartment of the thigh, empties into the internal pudendal vein and anastomoses with the deep femoral vein, its perforating veins and with the internal saphenous vein.
These two veins can anastomose with each other.
The internal pudendal venous network:
• It anastomoses with the long saphenous vein and with the external pudendal vein via the network of the superficial and deep dorsal veins of the clitoris, or directly by anastomosis between the internal pudendal and the external pudendal veins in the labia majora (which gives rise to vulvar varicosities during pregnancy),
• The internal pudendal vein forms an anastomosis with the perineal veins of the visceroperineal trunk (anterior branch) and the obturator branch.
PATHOPHYSIOLOGY
The ovarian veins, which drain in a vertical direction, frequently have valves (48% on the right side, 62% on the left) at their ends (84% on the right side, 77% on the left) and normally at the mouth of each branch.
The hypogastric veins, which drain in a horizontal direction, most often do not have valves and communicate largely between each other by different venous plexuses of the pelvis. Thus, they form a unique network that is physiologically incontinent.
The perineal, labial and clitoral superficial veins have valves, which prevent a reflux from the pelvis to the lower limbs.6
Some anatomical, hemodynamic and hormonal conditions, which can occur concomitantly, may, as a result of the excessive pressure they produce, generate or exacerbate venous disease with venous insufficiency, which causes varicosities of the lower limbs and/or pelvic varicoceles.
• The nutcracker syndrome: there is a variable degree of compression of the left renal vein in its pathway between the superior mesenteric artery and the aorta. Evaluation of the impact of this effect is difficult because it varies depending on the patient’s position, respiration, and is very likely enhanced by the standing position. It can have a specific clinical expression associating pelvic congestion syndrome with left flank pain and microscopic hematuria (nutcracker syndrome).4
• The natural very high blood flow in the renal veins;
• Pregnancy generates major hemodynamic changes: very high blood flow in arteries, and thus in the uterine veins (60 times normal), and compression of the major large veins which can produce pelvic and perineal vein dilation. These veins can become varicose and progress after childbirth. They produce venous reflux and varicosities in the superficial network of the perineum, vulva, and lower limbs. This risk increases with subsequent pregnancies.
These circumstances produce almost constant dilation of the left ovarian vein and increased blood flow into the uterine veins. Generally, the right ovarian vein is subject only to pregnancy-related changes, and often serves as a shunt, relieving the effects of incontinence of the left ovarian vein.
The effects of incontinence of the left ovarian vein can be compared with those of the long saphenous vein, because of its biomechanical consequences: the height of the column of blood is increased by the high blood flow in the renal vein.
• This incontinence becomes permanent in the uterine veins, which are dilated during pregnancy. Extension to the vaginal veins and then to the perineal veins occurs as a logical consequence
• Extension to other anastomoses of the uterine veins and a possible direct impact on the arch of the long saphenous vein are explained by the concept of very high blood flow. If indeed one of the pathways of physiological drainage is this arch, pregnancy or incontinence of the ovarian vein will divert blood flow towards this downward drainage.
• The long saphenous vein is not always able to accept this increased blood flow. This can then result in incontinence of the underlying long saphenous vein at the saphenofemoral junction. This abnormality is frequent and may account for at least 15% of cases of incontinence of the long saphenous vein.
• Another mechanism for incontinence of the saphenous veins can be the increased blood flow into the posterior communicating vein, the natural drainage pathway of the perineal veins.
• Subsequently, incontinence occurs below the area where it empties into the long saphenous vein, or even incontinence of the communicating vein and then of the short saphenous vein.
INVESTIGATIONS
Computed tomographic scan and magnetic resonance imaging
Computed tomographic (CT)-scan and magnetic resonance imaging (MRI) are rarely proposed as first-line methods for diagnosis of PVI.7 Yet, they do have an essential part to play in the assessment of abdominalpelvic pain. In this context, they can detect ovarian vein incontinence and varicocele, which can be asymptomatic (Figure 5).8 They can rule out other major causes of pelvic pain, eg, fibroids, endometriosis, adenomyosis, an ovarian mass, and disorders of the lumbar spine.
MRI with injection of gadolinium has advantages over CTscan: there is no associated radiation, it identifies the direction of blood flow, and has increased sensitivity and specificity (Figure 6).
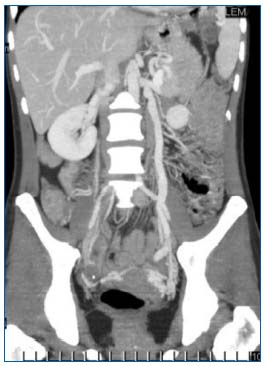
Figure 5. CT-scan: coronal reformatting (multiplanar reconstruction images): dilation of the ovarian vein and left para-uterine varicosities.
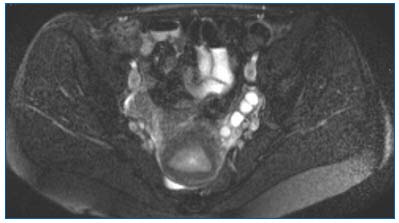
Figure 6. T2 Fat Sat axial MRI: left para-uterine varicocele.
Doppler ultrasound
Doppler ultrasound (Duplex scanning) is the first-line method of investigation in suspected PVI. Venous examination of the lower limbs (with a linear transducer of at least 7.5 MHz) is carried out prior to, or concomitantly with, examination of the pelvis when there are varicosities. The examination is performed opposite the anatomic zones and seeks to detect reflux, which if longer than 1s signifies incontinence. It is necessary to establish mapping of abnormalities and to assess the relative impact of pelvic hemodynamic abnormalities. In female patients who do not undergo surgery, varicosities are classified as saphenous or non-saphenous. When there is saphenous vein involvement, it is essential to define involvement of the saphenofemoral junction in the case of the long saphenous vein, and participation of the posterior communicating vein in incontinence of the short saphenous vein. The absence of involvement of the saphenofemoral junction, as well as incontinence of the posterior communicating vein, are findings in support of participation of the posterior communicating vein or of pelvic varices in this process. For nonsaphenous varicosities, pelvic origin is the conclusion in the case of gluteal and/or perineal varicosities.
Examination of patients presenting with varicose vein recurrence after surgery or effective sclerotherapy is extremely complicated and requires excellent knowledge of varicose venous disease. Features that suggest PVI may include the absence of a neo-arch, the stump of the arch or of an incontinent perforating vein, and the existence of veins of abdominal origin (superficial circumflex, superficial epigastric vein) or subinguinal origin (external pudendal vein, posterior communicating vein), emptying into the incontinent superficial venous network.9-11
In a recent article, possible involvement of two sites of systemic leakage were found, one opposite the inguinal orifice (where the leak may be attributable to the vein of the round ligament) and the other opposite the superficial aponeurosis of the perineum (where the leak may be attributable to branches of the internal pudendal vein).6 Examination of the abdomen (with a 5.3 or 5.2 MHz transducer depending on body weight) requires preparation: the patient must be fasting and have had a residue-free diet.
The ovarian veins (Figure 7):
• On the left side, the vein is identified as a compressible hypoechoic image, emptying into the renal vein seen on cross-section. The examination is then continued with longitudinal sections. The vein then ascends along the anterior aspect of the psoas muscle, from inward to outward, crossing the ureter at L4-L5 and then anterior to the iliac axis, practically opposite the arterial bifurcation.
• It is considered pathological when there is reflux that lasts longer than two seconds, and when venous diameter is greater than 8 mm. Reflux is sought after compression of the upper part of the inferior vena cava, in the standing position. When observed, it very frequently occurs spontaneously:
• On the right side, the right ovarian vein rarely empties into the renal vein. It is more difficult than the left ovarian vein to identify strictly.12 When pathological, it meets the same criteria as on the left.
• The left renal vein, the iliocaval veins: and the “nutcracker syndrome” should systematically be sought, but the evaluation is best performed by phlebography4 (Figure 8). Moreover, it is appropriate to look for Cockett’s syndrome or sequelae of iliac vein thrombosis.
Endocavitary examination(Figure 9) requires use of a 5 to 7.5 MHz endocavitary transducer. Its primary use is in visualizing the lateral uterine and lateral vaginal venous plexuses, the adnexal veins and the transuterine venous passages. Venous formations appear as anechoic cords, more or less dilated. A diameter greater than 8 mm, a sinuous appearance, and an abundance of veins are the features most often referred to when speaking of PVI.12,13 Venous stasis with reduced blood flow rate and the absence of change related to respiration should also be sought to posit PVI.13 Most of the time, a Valsalva maneuver (or compression of the vena cava) produces a reflux without it being systematically considered abnormal.
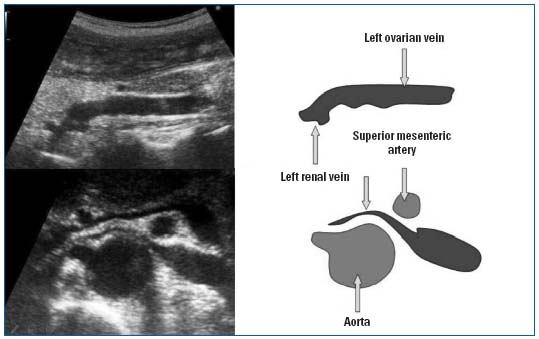
Figure 7. Doppler ultrasonography of pelvic varicosities, upper panel: dilation of the ovarian vein, mesoaortic compression.
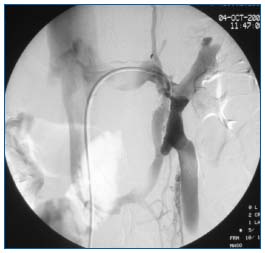
Figure 8. Phlebography of left renal vein. Tight extrinsic compression of the left renal vein between the aorta and the superior mesenteric artery with collateral shunts and reflux into the ovarian vein.
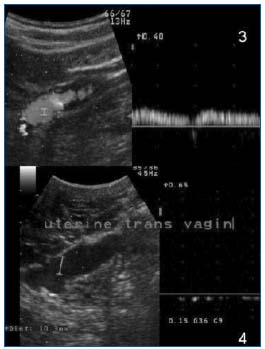
Figure 9. Transvaginal Doppler ultrasound. Upper: spontaneous incontinence of the ovarian vein / Lower: uterine varicosities, trans-cavitary pathway.
Phlebography
As the reference imaging method of the pelvic veins, phlebography should be performed only after confirmation by Doppler ultrasound of utero-ovarian vein incontinence, uterine varicosities, and as the first step in a therapeutic procedure.
After prior consultation with an anesthesiologist, which is essential, this test is generally performed under local anesthesia, with/without placement of a urinary catheter. It confirms the diagnosis and provides all information necessary for treatment: anatomy, degree of valvulation, and of dilation of the four major venous trunks involved, direction of blood flow. It is also useful to detect communicating vessels.
Two approaches are possible: brachial or femoral.
After inserting a 4/5 French Desilet, the patency and abnormalities of the major collecting vessels, the iliac vein, vena cava and left renal vein, are checked. Then, the ovarian veins opposite and the internal iliac major veins are partly opacified during a Valsalva maneuver. The diameter of the veins, the size of varicoceles, the degree of stasis of the contrast medium, and points of leakage are assessed, which will allow treatment to be adapted.
Catheters and guidewires used are as follows:
• By the femoral approach: a Cobra 1.2 for the left uteroovarian vein and the internal iliac veins and a Simmons 1.2 for the right ovarian vein;
• By the brachial approach: a multipurpose catheter, a Picard catheter of appropriate length (125 cm);
• Hydrophilic guidewires: 0.35 from 135 to 230 cm;
• The author’s personal variation: opacification with an occlusion balloon catheter to better assess the extent of the varicoceles and enhance demonstration of points of leakage.
ENDOVASCULAR TREATMENT: EMBOLIZATION
Embolization is performed subsequent to phlebography but is not always complete in a single phase. This procedure starts by treating the incontinent ovarian veins and then the points of leakage.
Endovenous navigation runs into some difficulties, in particular, in relation to the hypogastric veins, with tortuous varicosities, valves, collateral branches, venous compliance that varies with position, and respiratory time. This is to be taken into account in adjustment of coil diameter, which should be overvalued by 1-2 mm.
Materials for embolization: To inject the veins, the same catheters are used as for phlebography, sometimes a 3F coaxial catheter for the right ovarian vein.
Materials differ depending on the areas to be embolized:
• Sclerotherapy agents (Aetoxysclerol) for venous networks and taking the collateral vessels into account, with a 30% dextrose/water solution.
• Coils for occlusion of the large venous vessels, most often these two materials are used in combination.
• More rarely, a polymerizing agent (Histoacryl) mixed with ultrafluid Lipiodol is used.
Embolization starts with the left utero-ovarian vein (Figures 10 and 11).
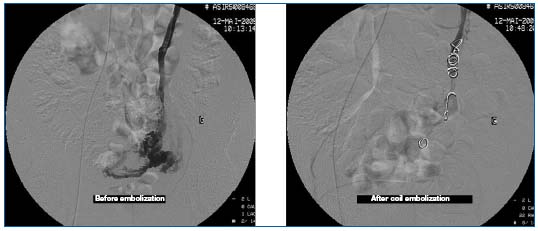
Figures 10 and 11. Phlebography prior to embolization: incontinence of the ovarian vein and left para-uterine varicosities. Repeat phlebography after embolization.
Four to five coils 8 to 15 mm in diameter over 4 to 20 cm in length are released; either into the main collateral veins, or at the origin of the vein (promontory). Some operators in addition use sclerotherapy agents between the coils.2 The collaterals identified along the main venous axis are occluded by the smallest coils (3-5 mm). The right ovarian vein is systematically embolized by some authors,14 while this is done by other clinicians only if this vein is incontinent.4 In the hypogastric veins, some clinicians treat only incontinent collaterals, with coils and sclerotherapy agents,2 while others perform sclerosis of the hypogastric veins en masse (Figures 12 and 13).14
Author’s personal variation: the sclerotherapy agent (4- 8 cc 3% Aetoxysclerol foam) is injected under protection of a balloon catheter kept inflated for 5 minutes, then is supplemented by release of coils before deflation.
Post-procedure therapy: Antibiotics are not systematically prescribed, analgesics, and anti-inflammatory agents in principle are given for 5 days.
Serious treatment-related complications are rare: migration of the embolization material. In the posttherapy period, the following manifestations may be observed: pelvic heaviness and pain, left low back pain, fever. These symptoms can be controlled perfectly with treatment and rapidly regress.
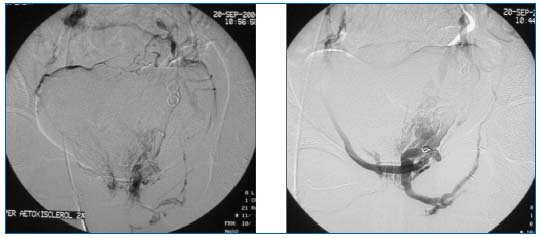
Figures 12 and 13. Phlebography of the left hypogastric vein: demonstration of an extra-pelvic point of leakage. Repeat phlebography after sclerotherapy and coil embolization.
Immediate technical results: A 95% to 100% success rate for embolization of the ovarian veins in the majority of studies, but lower success rates for leakage points dependent on the hypogastric veins: 85%.2
Clinical results: It is difficult to classify results according to baseline clinical criteria: PCS and/or concomitant perineal varices and/or varicosities of the lower limbs. The majority of publications in particular involve PCS. They only amount to a few hundred cases and results do not necessarily appear better when the two ovarian veins are systematically occluded.4,14-16 The results reported in this context in particular are expressed in terms of symptom improvement and are around 70%, in a follow-up period ranging from 6 to 22 months. Clinical improvement occurs only 3 to 4 weeks after the start of therapy. In the setting of veins that flow back to the lower limbs, the more complete the treatment, the better the results. Only one (large) study reported an 90% improvement in symptoms.2
CONCLUSION
Doppler ultrasound is the basic method of investigation of PVI and its consequences in the lower limbs. It is an essential procedure prior to phlebography, which should only be performed with intent to treat. Embolization has been shown to be an effective, low-risk procedure to rule out involvement of the ovarian veins. In the hypogastric veins and their pathological branches, the procedures for embolization and the materials used can be improved. The clinical results of this new therapeutic procedure remain to be evaluated by multicenter studies.
This article is a translation of the original article published in the French medical journal “Angéiologie: Insuffisance veineuse pelvienne périnéale et varices des members inférieurs”. Angéiologie. 2006;68(3):44-52. Published here with the kind permission of Dr Michèle Cazaubon.

REFERENCES
2. Monedero JL. Pelvic venous pathology. Embolising treatment. Phlébologie. 1999;52:299-310.
3. Allaert FA, Barthelemy P, Jamin C, Leteuff G, Cazzala. Angéiologie, livre des résumés. 2004;56:33-34.
4. D’Archambeau O, Maes M, De Schepper AM. The pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatment. JBR-BTR. 2004;87:1-8.
5. Kamina P. Les voies de suppléance de la vascularisation veineuse pelvienne chez la femme. Rev Fr Gynécol Obstet. 1987;77:393- 402.
6. Franceschi C, Bahnini A. Points de fuite pelviens viscéraux et varices des membres inférieurs. Phlébologie. 2004;57:37-42.
7. Coakley FV, Varghese SL, Hricak H. CT and MRI of pelvic varices in women. J Comput Assist Tomogr. 1999;23:429-434.
8. Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES Jr. Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. Am J Roentgenol. 2001;176:119-122.
9. Agence nationale d’accréditation et d’évaluation en santé. Indications du traitement chirurgical des varices essentielles des membres inférieurs. Paris: Anaes; 2004.
10. Labropoulos N, Kang SS, Mansour MA, et al. Primary superficial vein reflux with competent saphenous trunk. Eur J Vasc Endovasc Surg. 1999;18:201-206.
11. Labropoulos N, Tiongson J, Pryor L, et al. Nonsaphenous superficial vein reflux. J Vasc Surg. 2001;34:872-877.
12. Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. Am J Roentgenol. 2004;182:683-688. 13. Haag T, Manhès H. Veines et algies pelviennes chroniques. J Mal Vasc. 1999;24:267-274.
14. Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Inter Radiol. 2002;13:171-178.
15. Capasso P. Endovascular treatment of varicoceles and utero-ovarian varices. J Radiol. 2000;81:1125-1126.
16. Maleux G, Stockx L, Wilms G, et al. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11:859-864.
