Pharmacological treatment of chronic venous disorders
and George GEROULAKOS2
2. Consultant Vascular Surgeon,
Ealing and Charing Cross Hospital,
Senior Lecturer, Department of Vascular
Surgery, Imperial College of Science
Technology and Medicine, London, UK
The term chronic venous disorder (CVD) is used to denote all abnormal clinical changes that result from venous disease of the lower extremities, and that have a chronic course.1 According to this definition, CVD includes patients who present with so-called symptoms and/or signs of venous disease that characterize each class of CVD in the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification,2 from class C0s to class C6.
A review of the literature3 shows that CVD is most commonly manifested by the following symptoms: heaviness in the legs, pain, a sensation of swelling, restless legs, paresthesia, nighttime cramps, tiredness, and itching. It should be stated that none of these symptoms is specific to CVD, and even less so pathognomonic. Since it is not possible to confirm whether such symptoms are related to venous disease, it is important to characterize them using the following secondary criteria:
• Variability with position or physical activity: Symptoms generally occur after prolonged standing, at the end of the day, and do not exist, or are diminished, in the morning, in the supine position, or with the legs elevated;
• Variability with temperature: Symptoms are exacerbated by warmth, summer temperatures season, hot baths, hot waxing to remove body hair, floor-based heating systems, and regress; symptoms are diminished in winter and on exposure to low temperatures;
• Variability with levels of circulating sex hormones: Symptoms fluctuate with the menstrual cycle; they can occur with hormonal therapy (estrogens or estrogen-progestin), and disappear with discontinuation of such treatment.
The existence of at least two secondary criteria is necessary to confirm that symptoms are related to CVD, but the absence of such criteria does not rule out the possible venous-related origin of the symptoms. Whatever the therapy used, the effect of treatment on symptoms is difficult to quantify because these symptoms are subjective. Therapeutic efficacy can be assessed more readily based on a certain number of signs, such as edema or venous ulcers.
CLASSIFICATION OF PHLEBOTROPIC DRUGS
Phlebotropic drugs belong to several different chemical families. The majority of them are plant-derived compounds. Some have been produced by chemical synthesis. The main phlebotropic drugs4 are summarized in Table I.

Table I. Classification of the main phlebotropic drugs.
PATHOPHYSIOLOGICAL TARGETS OF PHLEBOTROPIC DRUGS
It is important to specifically identify the mode of action of phlebotropic drugs depending on the pathophysiological mechanisms that they aim to treat. Among such mechanisms, we can differentiate the following:
• those that are identified before microcirculatory disorders occur, consisting mainly of alterations in the venous wall; and
• those consisting of microcirculatory disorders.
Pharmacological targets before the occurrence of abnormal changes in the microcirculation
Two major mechanisms may be responsible for pain in the absence of trophic changes:
• first, venous wall tension, which results from dilation of the vein in a normal subject in the erect position, and the valvular incompetence during dynamic movement in the erect position in a subject with valvular insufficiency; and
• second, hypoxia of the tunica media of the venous wall due to alteration of the vasa vasorum. Pain seems more related to hypoxia. In the early stages of CVD, superficial venous distensibility is slight, while pain is more severe than in the advanced stages of CVD where venous pressure is elevated, and venous wall pressure is therefore high.5 However, the venous remodeling phase that precedes the development of varicose veins, which is accompanied by the process of venous distention, hemorrheological disturbances, and conditions of hypoxia can be painful.
Pain and heaviness in the legs
Phlebotropic drugs are intended to decrease sensation of heaviness in the legs, pain, and ankle edema at the end of the day. The first target of such therapy is increased venous wall pressure. Distensibility is increased by 10% to 50% in patients,6 and this is due to a decrease in venous tone. The second target is hypoxia of the tunica media which is related to disease of the vasa vasorum.5
Restless legs, nighttime cramps
These symptoms most often occur during the latter half of the night, but can also occur during prolonged sitting.
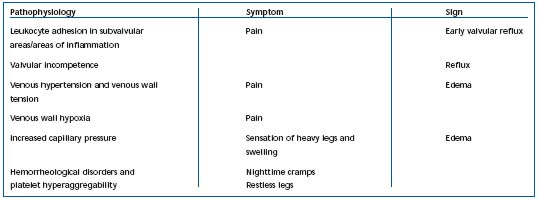
Table II. Possible links between pathophysiological variables, symptoms, and signs of chronic venous disorders.
They can also be related to hypoxia of the tunica media, but more specifically may be associated with hemorheological disorders.5 In fact, red blood cell hyperviscosity and hyperaggregation are constant findings in venous disease.7,8 It appears likely that hemorheological disorders worsen the circulation in the vasa vasorum. Hypoxia in the tunica media, in turn, induces deterioration of the venous wall. Hypoxia has a potent effect in inducing metabolic disorders: triggering of enzymatic activities, such as those of matrix metalloproteinases (MMP), and dedifferentiation, and migration of smooth muscle cells, which secrete growth factors. Fibrosis of the venous wall governs the development of the varicose vein. The diseased venous wall generates several metabolic disorders, including hypofibrinolysis due to elevated levels of plasminogen activating inhibitor (PAI-1). The links between pathophysiology, symptoms, and clinical signs of CVD are summarized in Table II.
Pharmacological targets related to microcirculatory disorders
The process of edema is manifestly due to increased capillary permeability related to permanent venous hypertension, 5 whose mechanisms vary: reflux or obstruction. Two stages should be distinguished in the progression of capillary disorders as venous disease becomes progressively worse: a functional disorder, followed by development of a lesional disorder, which characterizes chronic venous insufficiency.
Capillary functional disorder
At a relatively early stage, capillary permeability is observed in patients, as assessed by ankle plethysmography, following proximal venous hypertension, with fluorescein capillary angioscopy, and the Gibbon-Landis radioisotope test. A second aspect is capillary fragility demonstrated by the suction cup test. Traditionally, such disorders are taken as targets when studying the effects of phlebotropic drugs (Table III). However, it is not known whether they play a part in the development of lesional disorders. One argument in favor of their involvement is that they are accompanied by microedema and hemorrheological disorders, which occur at an early stage of venous disease. And yet, it is known that red blood cell hyperaggregation promotes microcirculatory disorders.
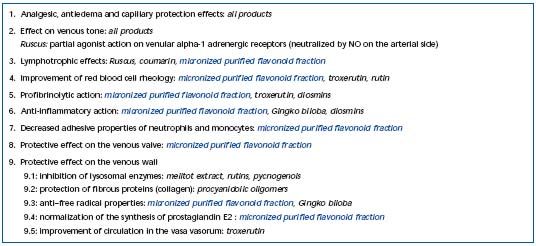
Table III. Pharmacological targets of phlebotropic drugs.
Microvascular lesional disease Alteration of the cutaneous microcirculation in the lower extremities is the long-term result of permanent venous hypertension, as the distal venous valves gradually become incompetent. The result is trophic changes whose incidence is proportional to the increase in ambulatory venous pressure. Over the last few years, advances have been made in understanding the pathophysiology of events, allowing better identification of the structures targeted by phlebotropic drugs:
• Doppler-laser investigation has shown an increase in cutaneous blood flow at rest, related to increased con- centrations of circulating red blood cells. This involves impairment of blood distribution affecting the most superficial areas where hypoxia develops, while intraand subcutaneous PO2 concentrations are normal.9
• Hypofibrinolysis increases concomitantly with increasing severity of trophic changes, usually with very high levels of PAI-1.10
• The hemorheological disorder, in particular, red blood cell hyperaggregation, is exacerbated and correlated with the clinical severity of the disease.
• Hypoxia, and excess delivery of oxygenated free radicals in the most superficial capillaries promote endothelial and leukocyte activation.11
Therefore, leukocyte accumulation and activation in dilated capillary loop worsen the condition, which is extensively involved in the pathogenesis of venous ulcer.11 In summary, microcirculatory disorders progressively worsen with hemodynamic alterations. During this process, leukocyte adhesion to the vascular endothelium produces endothelial activation with release of proteolytic enzymes and free radicals in the tissues.12 In addition, in vitro studies have demonstrated the release of prostaglandin (especially PGF2) and basic fibroblast growth factor (bFGF), which may be directly involved in venous wall remodeling.13 These mediators are found in abnormal quantity in varicose veins.14 During this process, venous valves may be the first to be damaged.15 The interaction between leukocyte and endothelium may be the key component in the pathogenesis of CVD and its complications, and may be an essential entity targeted by phlebotropic drugs. Pharmacological action of phlebotropic drugs on these different targets Remacle’s team has demonstrated the ability of phlebotropic drugs to inhibit the release of mediators of inflammation in endothelial cells placed under conditions of hypoxia (Figure 1).16,17
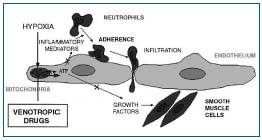
Figure 1. Possible protection of mechanism of endothelial cell by
phlebotropic drugs.
The interaction between leukocyte and endothelium may be the key component in the pathogenesis of CVD and its complications, and may be an essential entity targeted by phlebotropic drugs.
Pharmacological action of phlebotropic drugs on these different targets
Remacle’s team has demonstrated the ability of phlebotropic drugs to inhibit the release of mediators of inflammation in endothelial cells placed under conditions of hypoxia (Figure 1).16,17
A recent pharmacological study has demonstrated the ability of the micronized purified flavonoid fraction (MPFF) to protect venous valves from destruction caused by venous hypertension.18 It is by inhibiting the expression of adhesion molecules on the surface of leucokytes and the endothelium that this phlebotropic drug limits leukocyte adhesion, and subsequently leukocyte inflitration into the valvular subendothelium, thereby limiting inflammatory events.18 This effect had been demonstrated previously with the same preparation in the microcirculation. 19
CLINICAL TRIALS OF PHLEBOTROPIC DRUGS
Action on symptoms
Pain and heaviness in the legs are the two symptoms most commonly identified in studies on symptoms of venous disease.3 Symptoms and, in particular, pain, can be assessed with self-evaluation rating scales. Three types of scales have been validated:20 the simple verbal scale, the numerical scale, and the visual analog scale. All these scales evaluate pain intensity but do not provide information on the nature of the pain. They can be used to compare intraindividual variations between groups of patients in evaluation studies, or longitudinal observational surveys. Symptoms have an effect on the quality of life of patients with CVD. Overall assessment of quality of life allows quantification of the impact of symptoms on functional ability. Several quality-of-life questionnaires have been specifically adapted to CVD.21-23
Evaluation of symptoms associated with venous disease, and of the expected benefit of therapy with phlebotropic drugs, is not easy because many intercurrent factors exist. However, many double-blind, placebo-controlled studies with a washout period have been conducted using measurable criteria for evaluation of pain and heaviness in the legs. We will briefly summarize the studies conducted on calcium dobesilate,24-28 Horse chestnut extract,29,30 hydroxyrutin, 31-33 and micronized purified flavonoid fraction,34-37 and to which a few meta-analyses and reviews may be added.38-47 All these studies have confirmed the reduction in symptoms with all the different therapeutic agents. Quality of life was assessed during treatment with a phlebotropic drug. It markedly improved after 6 months of treatment, in particular in symptomatic patients, and was greater in patients in whom reflux had not been identified.48
Action on edema
Several methods have been used to measure edema and study the efficacy of phlebotropic drugs on this sign. The simplest method is measurement of ankle circumference, as done most often with a Leg-O-Meter®. This instrument, which has been validated,49 takes into account the height at which the measurement is made. However, changes observed in ankle circumference are not always correlated with changes in volume of the lower limb. This is why methods to measure differences in leg volume are preferable. The most well-known is the volumetric method of fluid displacement,50,51 which has been validated.
Volumetric measurement has been used to show that the most painful legs are those affected by edema. Furthermore, the volumetric method has demonstrated that the standing position, or even prolonged sitting with no activity of the calf muscle pump system, produces an increase in leg volume. Moreover, such edema is correlated with the degree of venous insufficiency.52 Thus, this accounts for leg edema during long-distance airline travel. Other methods have been used to assess edema in CVD: the optoelectronic method,53 the tomographic method,54 high-resolution magnetic resonance imaging, and X-ray absorptiometry.54
In the literature, randomized, controlled studies have demonstrated the efficacy of phlebotropic drugs on edema: Jaeger et al25 and Casley–Smith26 on calcium dobesilate, Vayssairat’s study on naftazone,55 Diehm’s study on horse chestnut extract,56 that of Blume on micronized purified flavonoid fraction,57 and a study by Cesarone et al on the effect of hydroxyrutin58,59 on edema associated with long-distance airline travel. These studies have demonstrated a significant decrease in edema.
Action on chronic venous insufficiency: classes C4–C6
Few phlebotropic drugs have been studied in the treatment of chronic venous insufficiency. The phlebotropic drug most widely studied by far in venous ulcer and its complications is micronized purified flavonoid fraction.60-63 A recent meta-analysis of five clinical trials with this drug revealed its beneficial action on reduction of time needed for healing of venous ulcer.64 Among phlebotropic drugs, horse chestnut seed extract,55,65 and hydroxyrutosides66 reduce both edema and symptoms of chronic venous insufficiency, but are not desmontrably better than compression in advanced chronic venous insufficiency,65 or in preventing venous ulcer recurrence.66 This may be because reduction in edema alone is insufficient to treat leg ulceration. Additional factors must be influenced in order to speed ulcer healing, which the micronized purified flavonoid fraction might be able to address. Recently, much attention has been focused on the involvement of growth factors, 67 and leukocytes in the development of venous ulceration.19 This has opened up new areas of investigation.
By reducing the likelihood of leukocyte adhesion, micronized purified flavonoid fraction presumably acts through an anti-inflammatory mechanism.19 Thus, among the many mechanisms at work in the pathogenesis of venous ulceration, the mechanism involving leukocyte activation and interaction with the endothelium seems at present to be the most responsive to pharmacological treatment.
ROLE OF PHLEBOTROPIC DRUGS IN THE TREATMENT OF CHRONIC VENOUS DISORDERS
Phlebotropic drugs have a well-established effect on edema. They also effectively decrease the so-called symptoms of venous disease, such as heaviness of the legs, pain, sensation of swelling, and nighttime cramps.
In both patients classified as having stage C0s disease, and in those classified as C1s and C2s for whom invasive therapy (sclerotherapy, surgery) does not appear warranted, phlebotropic drugs appear to be good first-line treatment of chronic venous disorder, possibly in conjunction with compression therapy.
At more advanced disease stages, phlebotropic drugs have no demonstrable additional benefit over compression on improvement of skin changes, or in ulcer healing, except for micronized purified flavonoid fraction, which may be used in conjunction with sclerotherapy, surgery, and/or compression therapy, or as an alternative treatment when surgery is not indicated or is not feasible.46


REFERENCES
2. Porter JM, Moneta GL. International Consensus Committee on chronic venous disease. Reporting standards in venous disease: an update. J Vasc Surg. 1995;21: 635–645.
3. Garde C, Perrin M, Chleir F, Henriet JP, et al. First meeting of review and consensus on venoactive agents on the symptoms of chronic venous disease (in French). Phlébologie. 2003;56:103–109.
4. Ramelet AA, Kern P, Perrin M, eds. Varicose veins and telangiectasias. Paris: Masson, 2003. Chapter 13 (Drug therapy) pp. 166–168.
5. Boisseau MR. Pharmacologie des médicaments veinotoniques: données actuelles sur leur mode d’action et les cibles thérapeutiques (in French). Angéiologie. 2000;52:71–77.
6. Clarke GH, Vasdekis SN, Hobbs JT, Nicolaides AN. Venous wall function in the pathogenesis of varicose veins. Surgery. 1992;111:402–408.
7. Boisseau M, Freyburger G, Busquet M, et al. Hemorheological disturbances in venous insufficiency after induced stasis. Clin Hemorheol. 1989;9:161–163.
8. Le Devehat C, Boisseau MR, Vimeux M, et al. Hemorheological factors in the pathophysiology of venous disease. Clin Hemorheol. 1989;9:861–870.
9. Taccoen A, Belcaro G, Lebard C, Zuccarelli F. Etiologies et mécanismes des varices: réalités et perspectives. STV. 1997;9: 354–363.
10. Boisseau MR, Taccoen A, Garreau C, et al. Fibrinolysis and hemorheology in chronic venous insufficiency: a double blind study of troxerutin efficiency. J Cardiovasc Surg. 1995;36:369–374.
11. Saharay M, Shields DA, Georgiannos SN, et al. Endothelial activation in patients with chronic venous disease. Eur J Vasc Endovasc Surg. 1998;15:342–349.
12. Michiels C, Arnould T, Thibaut- Vercruyssen R, et al. Perfused human saphenous veins for the study of the origin of varicose veins: role of the endothelium and of hypoxia. Int Angiol. 1997;16:135–141.
13. Michiels C, De Leener F, Arnould T, Dieu M, Remacle J. Hypoxia stimulates human endothelial cells to release smooth muscle cell mitogens: role of prostaglandins and bFGF. Exp Cell Res. 1994;213:43–54.
14. Badier-Commander C, Couvelard A, Henin D, et al. Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol. 2001;193:398–407.
15. Takase S, Pascarella L, Bergan J, Schmid- Schönbein GW. Hypertension-induced valve remodeling. J Vasc Surg. 2004;39:1329–1334.
16. Janssens D, Delaive E, Houbion A, et al. Effect of venotropic drugs on the respiratory activity of isolated mitochondria and in endothelial cells. Br J Pharmacol. 2000;130:1513–1524.
17. Michiels C, Bouaziz N, Remacle J. Role of the endothelium and blood stasis in the appearance of varicose veins. Int Angiol. 2002;21:1–8.
18. Takase S, Pascarella L, Lerond L, Bergan J, Schmid-Schönbein GW. Venous hypertension. Inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28:484–493.
19. Shoab SS, Porter JB, Coleridge-Smith PD. Effect of oral micronised flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg. 2000;31:456–461.
20. Agence Nationale d’Accréditation et d’Evaluation de la Santé (in French). Evaluation et suivi de la douleur chronique chez l’adulte en médecine ambulatoire (in French). www.anaes.fr.
21. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lower limb venous insufficiency (CIVIQ). Qual Life Res. 1996;5:539–550.
22. Lamping DL, Abenhaim L, Kurz X, et al. Measuring quality of life and symptoms in chronic venous disorders of the leg: development and psychometric evaluation of the VEINES-Qol/SYM questionnaire. Qual Life Res. 1998;7:621–622.
23. Klyscz T, Junder M, Schanz S, et al. Quality of Life in chronic venous insufficiency (CVI). Results of a study with the newly developed Tubingen questionnaire for measuring quality of life with CVI. Hautartz. 1998;49:372–381.
24. Hachen HJ, Lorenz P. Double-blind clinical and plethysmographic study of calcium dobesilate in patients with peripheral microvascular disorders. Angiology. 1982;33:480–487.
25. Labs KH, Degischer S, Gamba G, Jaeger KA. Effectiveness and safety of calcium dobesilate in treating chronic venous insufficiency: randomized, double-blind, placebo-controlled trial. Phlebology. 2004;19:123–130.
26. Casley-Smith JR. A double blind trial of calcium dobesilate in chronic venous insufficiency. Angiology. 1988;39:853–857.
27. Pecchi S, De Franco V, Damiani P, et al. Calcium dobesilate in the treatment of primary venous insufficiency of the lower limbs. A controlled clinical study. Clin Ter. 1990;32:409–417.
28. Widmer L, Biland L, Barras JP. Doxium 500 in chronic venous insufficiency: a double-blind placebo-controlled multicentre study. Int Angiol. 1990;9:105–110.
29. Le Devehat C, Lemoine A, Roux E, et al. Aspects clinique et hemodynamique de Cyclo 3 dans l’insuffisance veineuse (in French). Angeiologie. 1984;36:119–122. 30. Sentou Y, Bernard-Fernier MF, Demarez JP, et al. Symptomatologie et pléthysmographie: parallélisme des résultats obtenus lors d’un traitment par Cyclo 3 de patients porteuses d’une insuffisance veineuse chronique (étude en double insu contre placébo). Gaz Med. 1985;92:73–77.
31. Petruzzellis V, Troccoli T, Candiani C, et al. Oxerutins (Venoruton): efficacy in chronic venous insufficiency–a double-blind, randomized, controlled study. Angiology. 2002;53:257–263.
32. Balmer A, Limoni C. A double blind placebo controlled clinical trial of Venoruton on the symptoms and signs of chronic venous insufficiency. The importance of patient selection. VASA. 1980;9:1–7.
33. MacLennan WJ, Wilson J, Rattenhuber V, et al. Hydroxyethylrutosides in elderly patients with chronic venous insufficiency: its efficacy and tolerability. Gerontology. 1994;40:45–52.
34. Chassignolle J-F, Amiel M, Lanfranchi G, et al. Activité thérapeutique de MPFF at a dose of 500 mg dans l’insuffisance veineuse fonctionnelle (in French). J Int Med. 1987;99:32–37.
35. Gilly R, Pillion G, Frileux C. Evaluation of a new venoactive micronized flavonoid fraction (S 5682) in symptomatic disturbances of the veinolymphatic circulation of the lower limb: a double-blind, placebocontrolled study. Phlebology. 1994;9:67–70.
36. Cospite M, Dominici A. Double blind study of the pharmacodynamic and clinical activities of 5682 SE in venous insufficiency. Advantages of the new micronized form. Int Angiol. 1989;8:61–65.
37. Tsouderos Y. Are the phlebotonic properties shown in clinical pharmacology predictive of a therapeutic benefit in chronic venous insufficiency? Our experience with MPFF at a dose of 500 mg. Int Angiol. 1989;8:53–59.
38. Ciapponi A, Laffaire E, Roque M. Calcium dobesilate for chronic venous insufficiency: a systermatic review. Angiology. 2004;55:147–154.
39. Arceo A, Berber A, Trevino C. Clinical evaluation of the efficacy and safety of calcium dobesilate in patients with chronic venous insufficiency of the lower limbs. Angiology. 2002;53:539–544.
40. Poynard T, Valterio C. Meta-analysis of hydroxyethylrutosides in the treatment of chronic venous insufficiency. VASA. 1994;23:244–250.
41. Wadworth AV, Faulds D. Hydroxyethylrutosides: a review of its pharmacology, and therapeutic efficacy in venous insufficiency and related disorders. Drugs. 1992;44:1013–1032.
42. Boyle P, Diehm C, Robertson C. Metaanalysis of clinical trials of Cyclo 3 Fort in the treatment of chronic venous insufficiency. Int Angiol. 2003;22:250–262.
43. Siebert U, Brach M, Sroczynski G, et al. Efficacy, routine effectiveness, and safety of horsechestnut seed extract in the treatment of chronic venous insufficiency. A meta-analysis of randomized controlled trials and large observational studies. Int Angiol. 2002;21:305–315.
44. Pittler MH, Ernst E. Horse-chestnut seed extract for chronic venous insufficiency. A criteria-based systematic review. Arch Dermatol. 1998;134:1356–1360.
45. Geroulakos G, Nicolaides AN. Controlled studies of MPFF at a dose of 500 mg in chronic venous insufficiency. Angiology. 1994;45:549–553.
46. Lyseng-Williamson KA, Perry CM. Micronised Purified Flavonoid Fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71–100.
47. Boada JN. Therapeutic effect of venotonics in chronic venous insufficiency. Clin Drug Invest. 1999;18:413–432.
48. Jantet G, and the RELIEF Study Group. Chronic venous insufficiency: worldwide results of the RELIEF study. Reflux assEssment and quaLity of life improvEment with micronized Flavonoids. Angiology. 2002;53:245–256.
49. Bérard A, Kurz X, Zuccarelli F, et al. Reliability study of Leg-O-Meter, an improved tape measure device in patients with chronic venous insufficiency of the leg. Angiology. 1998;49:169–173.
50. Brijker F, Heijdra YF, Van den Elshout FJJ, et al. Volumetric measurements of peripheral oedema in clinical conditions. Clin Physio. 2000;20:56–61.
51. Vayssairat M, Maurel A, Gouny, et al. La volumétrie: une méthode précise de quantification en phlébologie (in French). J Mal Vasc. 1994;19:108–110.
52. Garde C, Nicolaides A, Guex JJ, Grondin L, et al. Consensus meeting on the action of phlebotropic drugs on chronic venous disease-related edema (in French). Phlébologie. 2004;57:7–13.
53. Stanton AWB, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (Perometer®). Lymphology. 1997;30:77–97.
54. Perrin M, Guex JJ. Edema and leg volume: methods of assessment. Angiology. 2000;51:9–12.
55. Vayssairat M. and the French Venous Naftazone Trial Group. Placebo-controlled trial of naftazone in women with primary uncomplicated symptomatic varicose veins. Phlebology. 1997;12:17–20.
56. Diehm C, Trampish HJ, Lange S, Schmidt C. Comparison of leg compression stocking and oral horse-chestnut extract therapy in patients with chronic venous insufficiency. Lancet. 1996;347:292–294.
57. Blume J, Langenbahn H, de Champvallins M. Quantification of edema using the volometer technique;therapeutic application of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology. 1992;7:S37–S40.
58. Cesarone MR, Belcaro G, Brandolini R, et al. The LONFLIT4-Venoruton Study: a randomized trial–prophylaxis of flightedema in venous patients. Angiology. 2003;54:137–142.
59. Cesarone MR, Belcaro G, Geroulakos G, et al. Flight microangiopathy on long-haul flights: prevention of edema and microcirculation alterations with Venoruton. Clin Appl Thromb Hemost. 2003;9:109–114.
60. Guilhou J-J, Dereure O, Marzin L, et al. Efficacy of MPFF at a dose of 500 mg in venous leg ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology. 1997;48:77–85.
61. Glinski W, Chodynicka B, Roszkiewicz J, et al. The beneficial augmentative effect of micronised purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open, multicentre, controlled, randomised study. Phlebology. 1999;14:151–157.
62. Roztocil K, Stvrtinova V, Strejcek J. Efficacy of a 6-month treatment with MPFF at a dose of 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol. 2003;22:24–31.
63. Ramelet AA. Clinical benefits of MPFF at a dose of 500 mg in the most severe stages of chronic venous insufficiency. Angiology. 2001;52:S49–S56.
64. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198–208
65. Ottillinger B, Greeske K. Rational therapy of chronic venous insufficiency—chances and limits of the therapeutic use of horsechestnut seeds extract. BMC Cardiovasc Disord. 2001;1:5.
66. Wright DD, Franks PJ, Blair SD, et al. Oxerutins in the prevention of recurrence in chronic venous ulceration: randomized controlled trial. Br J Surg. 1991;78: 1269–1270.
67. Jull A, Waters J, Arroll B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet. 2002;359:1550.
