What have we learned from recent guidelines on the treatment of venous thromboembolism?
Jeanne SHELDON
University of Calgary,
Calgary, Canada
ABSTRACT
Overwhelming evidence indicates that the quality of randomized, controlled trials reporting has been suboptimal. Accordingly, the extended CONSORT statementprovides recommendations that will profoundly impact the design, conduct, and reporting of new drug trials. Additionally, registration of all trials in a public repository ensures that every trial becomes part of the public record, allowing clinicians to explore the full range of clinical evidence. Finally, QUORUM addresses standards for improving the quality of reporting meta-analyses of randomized, controlled, clinical trials. These improvements in the reporting of trials will strengthen evidence-based medicine guidelines. Evidence-based medicine guidelines have resulted in accepted standards of care for treating venous thromboembolism. Low-molecular-weight heparin is the initial treatment of choice for in-hospital and out-of-hospital therapy of deep vein thrombosis and, more recently, for submassive pulmonary embolism. A key uncertainty is the optimal duration of long-term treatment after a first episode or recurrent episodes of venous thrombosis.
BACKGROUND
Evidence-based medicine guidelines are critically dependent upon the quality of the evidence providing the basis for them. To improve the conduct and reporting of randomized clinical trials, standards have been set, which must be met for publication in high-impact journals.
These accepted standards focus on three areas:
1) the conduct and reporting of rigorous randomized trials, namely the Consolidated Standards of Reporting Trials (CONSORT) statements1,2
2) avoiding a reporting bias in randomized trials by the requirement of mandatory clinical trial registration,3 and
3) the need to improve the quality of reporting of meta-analyses of randomized clinical trials, namely the QUORUM requirements.4 Many high-impact journals require mandatory compliance with these standards for reporting individual trial results or performing meta-analyses.
In more detail:
1) The CONSORT statements identify, “in response to overwhelming evidence and the consequences of poor quality reporting of randomized, controlled trials,” the need for clear standards in the conduct and reporting of trials.2 “Many medical journals and editorial groups have now endorsed the CONSORT statement, a 22-item checklist and flow diagram” (Table I and Figure 1).2 Recently, an extension of the CONSORT statement, the Better Reporting of Harms in Randomized Trials statement (Table II), provided upgraded guidelines that set the standard for the conduct and reporting of randomized clinical trials, including better reporting of benefits and harm.2 Adherence to the CONSORT statement requirements is now widely accepted and will further strengthen the scientific validity of evidence-based guidelines for treatment of venous thromboembolism.
2) Avoiding a reporting bias in the publication of data: the mandatory use of clinical trial registries Many high-impact journals now require mandatory prior registration of randomized clinical trials as a prerequisite for publication. The rationale behind move this was articulated clearly in a recent article setting this standard:3 “Altruism and trust lie at the heart of research on human subjects.” Selective reporting of trials distorts the body of evidence available for clinical decision-making. Mandatory registration of clinical trials reveals the existence of all clinical research, an important step in avoiding “selective reporting” of clinical trials.3 Public registration of all clinical trials at inception ensures that “every trial’s existence is part of the public record,” and enables clinicians to explore the aggregate of clinical evidence.3
3) Improving the quality of reporting of meta-analyses (QUORUM) The QUORUM4 conference was convened to address standards for improving the quality of reporting of meta-analyses of randomized, controlled, clinical trials. The QUORUM checklist and flow diagram are available on The Lancet’s website (www.thelancet.com) (Table III and Figure 2). The authors state, “we hope that this document will generate further interest in the field of meta-analysis and that, like the CONSORT initiative, the QUORUM statement will become available in different languages and locations as it is disseminated.”4 Adherence to QUORUM recommendations is widely accepted.
EVIDENCE-BASED MEDICINE GUIDELINES
The ongoing use of the CONSORT statements, clinical trials registration, and the QUORUM requirements for meta-analyses as standards will substantially improve the quality of published evidence and provide clearer practical clinical recommendations. Two evidence-based medicine guidelines on the treatment of venous thromboembolism are widely accepted. These are Prevention and Treatment of Venous Thromboembolism: International Consensus Statement5,6 (Nicolaides et al) and the Seventh American College of Chest Physicians (ACCP) Conference on Antithrombotic and Thrombolytic Therapy.7-9 Both international guidelines use an evidence-based approach.10,11 The use of evidence-based guidelines “reflects the emphasis on an evidence-based approach to making recommendations.” The development of evidence-based guidelines requires a clear and explicit definition of each question,12 a definition that specifies eligibility criteria, including the relevant population, alternative management strategies, and the outcomes.7 Both consensus reports5,9 provide grades of recommendation based upon the methodological quality of the evidence underlying the recommendation.
The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy authors used the following grades of recommendation based on the methodologic quality of the evidence:8
– consistent results from randomized clinical trials generate Grade A recommendations;
– inconsistent results from randomized clinical trials generate Grade B recommendations;
– observational studies generate Grade C recommendations, or secure generalizations from randomized clinical trials.
In addition, the Seventh ACCP Conference uses the following grades of recommendation:
Grade 1) Experts are very certain that benefits do, or do not, outweigh risks, burdens, and costs, ie, a strong recommendation;
Grade 2) Experts are less certain of the magnitude of the benefits, risks, burdens, and costs, and thus of their relative impact, ie, a weaker recommendation.
It is evident that these expert opinions concerning Grade 1 and 2 recommendations may be affected by both national and regional differences in viewpoint. In addition, the use of costs to provide an expert grade of recommendation may confound the recommendations due to differing costs among health care systems. The use of costs would be better restricted to the national or local health care level in establishing grades of recommendation. Finally, the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy consensus report8,13 has included values and preferences as part of their criteria for assigning grades of recommendation. The choice guiding the use of a particular therapeutic regimen may well be influenced by local values and preferences that are not shared internationally. Values and preferences, therefore, may be better used at the local rather than international level. An example of such a dichotomy is the widespread use of vitamin-Kantagonist prophylaxis in major orthopedic surgery in the United States and Canada based on a strong grade of recommendation, whereas low-molecular-weight heparin is the prophylaxis of choice in Western Europe.
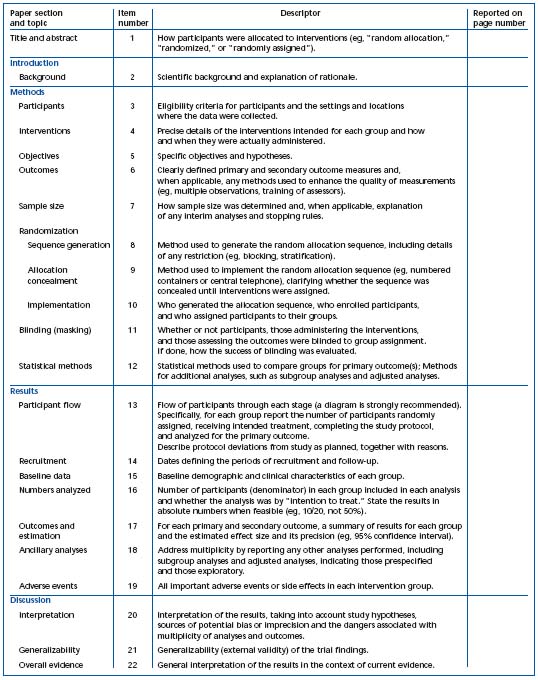
Table I. Checklist of items to include when reporting a randomized trial.2 Reprinted with permission from Annals of Internal Medicine.
Evidence-based grades based on the quality of the methodologic evidence are likely to be more consistent, unlike the grades of opinion. The authors of the Prevention and Treatment of Venous Thromboembolism: International Consensus Statement5,6 used similar grades of methodological evidence. Due to the potential variability amongst expert opinions based upon regional differences, the Grade 1 and Grade 2 criteria and values and preferences were not used.
Their levels of evidence include the following:
Grade A) level 1 evidence from multiple randomized trials with consistent results (eg, in systematic reviews), which are directly applicable to the target population;
Grade B) level 1 evidence from randomized trials with less consistent results, limited power, or other methodological problems;
Grade C) based on level 2 evidence from well-conducted observational studies with consistent results, directly applicable to the target population.
These two widely acknowledged consensus reports provide guidelines for the treatment of venous thromboembolism that are largely in harmony concerning the methodologic grades.5,8
OVERVIEW OF THE TREATMENT OF VENOUS THROMBOEMBOLISM
Venous thromboembolism occurs not only as a complication in patients who are sick and hospitalized, but also in otherwise healthy ambulant individuals. Anticoagulant drugs—unfractionated heparin, lowmolecular- weight heparin, and vitamin K antagonists— are the mainstay of the management of venous thromboembolism. Low-molecular-weight heparin has replaced unfractionated heparin for many therapeutic indications. Unfractionated heparin, given by continuous intravenous infusion with laboratory monitoring using the activated partial thromboplastin time, in conjunction with warfarin starting on day 1 or day 2 and continued for three months or more, has historically been the standard treatment for established venous thromboembolism (deep vein thrombosis and pulmonary embolism). If unfractionated heparin is used initially, the therapeutic range must be reached within 24 hours.14 Over the past decade, low-molecular-weight heparin has supplanted unfractionated heparin and constitutes one of the most frequently used therapeutic regimens in the treatment of venous thromboembolism. Low-molecular-weight heparins have been evaluated against different treatments, including unfractionated heparin, for the prevention and treatment of venous thromboembolism. In many countries, the lowmolecular- weight heparins have replaced unfractionated heparin for both the prevention and treatment of venous thromboembolism. Lowmolecular- weight heparin therapy allows outpatient treatment of uncomplicated patients with deep vein thrombosis. For vitamin-K-antagonist therapy, the importance of maintaining a therapeutic international normalized ratio (INR) (2.0-3.0) is well documented; this requires frequent INR monitoring. The optimal duration of oral anticoagulant therapy, after initial or recurrent episodes of venous thromboembolism, is becoming better understood. For patients with cancer and venous thromboembolism, long-term treatment with lowmolecular- weight heparin is preferred.15,16
Initial antithrombotic therapies
Low-molecular-weight heparin:
For treating established venous thromboembolism, lowmolecular- weight heparin, given by subcutaneous injection, has distinct advantages over continuous intravenous unfractionated heparin: once-daily (or twice-daily) subcutaneous administration and the antithrombotic response to low-molecular-weight heparin is highly correlated with body weight, permitting administration of a fixed-dose without laboratory monitoring. The use of low-molecular-weight heparin allows outpatient therapy in many patients with uncomplicated deep vein thrombosis.9 As lowmolecular- weight heparins have become widely available for treatment, they have replaced intravenous unfractionated heparin in the initial management of most patients with venous thromboembolism.
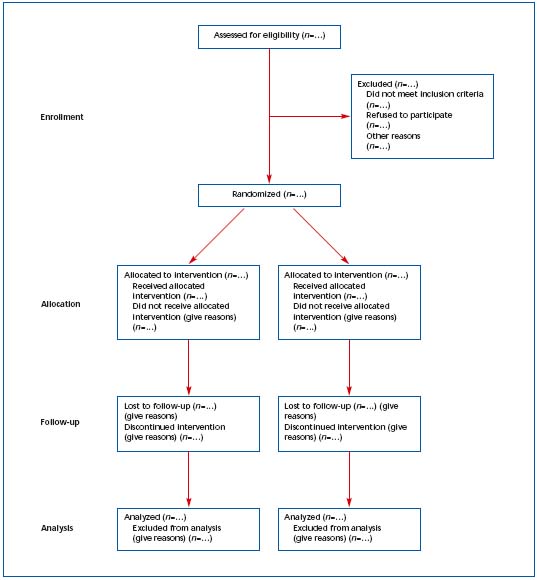
Figure 1. Revised template of the CONSORT (Consolidated Standards of Reporting Trials) diagram showing the flow of participants
through each stage of a randomized trial.1 Reprinted with permission from Annals of Internal Medicine.
Evidence is accumulating that complications such as bleeding, osteoporosis, and heparin-induced thrombocytopenia are indeed less serious and less frequent with the use of low-molecular-weight heparin when compared with unfractionated heparin.9,17 The findings of a recent meta-analysis17 suggest that the frequency of heparin-induced thrombocytopenia with low-molecularweight heparin is 0.2% whereas the risk is 2.6% with unfractionated heparin. The low-molecular-weight heparins all cross-react with unfractionated heparin and, therefore, cannot be used as an alternative therapy in patients who develop heparin-induced thrombocytopenia. Upon diagnosis of heparin-induced thrombocytopenia, low-molecular-weight heparin must be stopped immediately.18 In patients requiring ongoing anticoagulation, alternate therapy is required, for example argatroban.
Unfractionated heparin:
Classic anticoagulant therapy for venous thromboembolism involves a combination of continuous intravenous heparin using a heparin protocol19 and an oral vitamin K antagonist. Initial intravenous heparin therapy is administered for 5 days, or until the INR remains within the therapeutic range (2.0 to 3.0) for 2 consecutive days.9 Simultaneous use of initial heparin and warfarin has become clinical practice for all patients with venous thromboembolism who are medically stable.9
Clinical trials have established the need for initial heparin (or low-molecular-weight heparin) treatment in patients with venous thromboembolism.8 Randomized clinical trials have shown that achieving the lower limit of the therapeutic range within 24 hours is required to adequately prevent recurrent venous thromboembolism in patients receiving intravenous heparin.14,20 Anticoagulant monitoring of unfractionated heparin therapy is described elsewhere.19 In most patients with deep vein thrombosis and many patients with submassive pulmonary embolism, low-molecularweight heparin has appropriately supplanted the use of unfractionated heparin, avoiding entirely the problems associated with anticoagulant monitoring.
The main adverse effects of heparin therapy include bleeding, thrombocytopenia, and osteoporosis.18,19,21 Patients at particular risk are those who have had recent surgery or trauma, or who have other clinical factors that predispose to bleeding when on heparin, such as peptic ulcer, occult malignancy, liver disease, hemostatic defects, weight, age >65 years, and female gender. The development of thrombocytopenia may be accompanied by arterial or venous thrombosis, which may lead to serious consequences such as death or limb amputation. Upon diagnosis of heparin-induced thrombocytopenia, heparin must be stopped immediately.18 In patients requiring ongoing anticoagulation, alternate therapy is required, for example argatroban.Osteoporosis has been reported in patients receiving unfractionated heparin for more than 6 months. Demineralization can progress to the fracture of vertebral bodies or long bones, and this defect may not be entirely reversible.19
Fondaparinux:
The synthetic pentasaccharide, fondaparinux, is effective at treating deep vein thrombosis and submassive pulmonary embolism.9,22,23 This new agent will become visible in the therapeutic arena, supported by future evidence-based medicine guidelines, once approval by the regulatory affairs agencies has been completed.
Thrombolytic therapy:
It is widely accepted that patients with acute massive pulmonary embolism may benefit from this adjunctive therapy.9 However, thrombolytic therapy remains controversial, particularly due to the risk of bleeding, and it is not indicated for the routine treatment of venous thromboembolism.
Long-term antithrombotic therapies
Vitamin-K-antagonist therapy:
The anticoagulant effect of vitamin-K-antagonist therapy is delayed until after the normal clotting factors have been cleared from the circulation, and the peak effect does not occur until 36 to 72 hours after drug administration.24 The use of initial daily doses of 5 to 10 mg is the preferred approach for initiating vitamin- K-antagonist treatment; many clinicians advocate starting with 5 mg. The dose-response relationship to vitamin-K-antagonist therapy varies widely between individuals, therefore frequent monitoring of the INR is required, particularly initially, to establish therapeutic anticoagulation.9,24 A number of factors influence the anticoagulant response of vitamin-K-antagonist therapy in individual patients, including dietary changes and drugs that interfere with the metabolism of vitamin K antagonist.24 Heparin or low-molecular-weight heparin therapy is discontinued on the fifth day following initiation of vitamin-K-antagonist therapy, provided the INR remains in the recommended therapeutic range (INR 2.0 to 3.0) for at least two consecutive days.
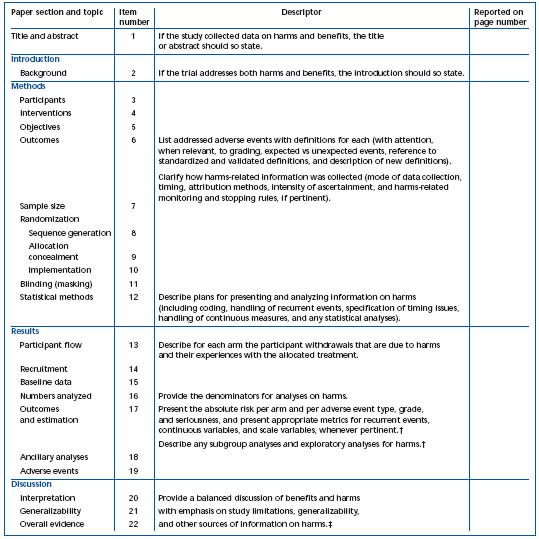
* This proposed extension for harms includes 10 recommendations that correspond to the original CONSORT checklist.
† Descriptors refer to items 17, 18, and 19.
‡ Descriptors refer to items 20, 21, and 22.
Table II. Checklist of items to include when reporting harms in randomized trials.2* Reprinted with permission from Annals of Internal Medicine.
Once the anticoagulant effect and the patient’s warfarin dose requirements are stable, the INR should be monitored every 1 to 3 weeks throughout the course of warfarin therapy. However, if there are factors that may produce an unpredictable response to warfarin (eg, concomitant drug therapy), the INR should be monitored more frequently to minimize the risk of complications due to poor anticoagulant control. To promote standardization of the prothrombin time for monitoring oral anticoagulant therapy, the World Health Organization (WHO) developed an international reference thromboplastin from human brain tissue and recommended that the prothrombin time ratio be expressed as the INR. The INR is the prothrombin time ratio obtained by testing a given sample using the WHO reference thromboplastin. For practical clinical purposes, the INR24 for a given plasma sample is equivalent to the prothrombin time ratio obtained using a standardized human brain thromboplastin known as the Manchester Comparative Reagent, which has been widely used in the United Kingdom. In recent years, thromboplastins with a high sensitivity have been commonly used. In fact, many centers have been using the recombinant tissue factor, which has an ISI value between 0.9 and 1.0 giving an INR equivalent to the prothrombin time ratio.24
The duration of anticoagulant therapy is influenced by the knowledge of multiple parameters: first episode versus recurrent episode of venous thromboembolism; transient, continuing, or unknown predisposing risk factors; and the risk of bleeding, to name some. There is increasing awareness that venous thromboembolism should be considered a chronic disease with a potential continued risk of venous thromboembolism often associated with minor provocation.25
In many patients, there will be considerable uncertainty as to the duration of long-term anticoagulant therapy (see evidence-based guidelines for the long-term treatment of patients with venous thromboembolism). For this reason, it is important to include patient preferences in the decision-making process concerning duration of anticoagulant therapy (Figure 3). In deep vein thrombosis or pulmonary embolism patients who receive indefinite anticoagulant treatment, the riskbenefit of continuing such treatment should be reassessed in the individual patient at periodic intervals. Where appropriate, the patient should be involved in the decision process.
Long-term low-molecular-weight heparin therapy:
Long-term low-molecular-weight heparin has been compared in randomized clinical trials15,16,26,27 against warfarin therapy in patients presenting with venous thromboembolism. Long-term low-molecular-weight heparin is a useful alternative to vitamin-K-antagonist therapy, and is the preferred therapy for up to 6 months or so in cancer patients with venous thromboembolism. 15,16
Adjunctive therapy
Inferior vena caval interruption:
To understand the role of the inferior vena cava filter in patients with venous thromboembolism, it is important to consider the natural history of venous thromboembolism. Patients with untreated proximal venous thrombosis, with or without pulmonary embolism, have a poor prognosis off therapy: intervention is required. What line of defence can be offered to the patient with proximal venous thrombosis or pulmonary embolism for whom immediate anticoagulant therapy is contraindicated due to hemorrhagic complications, or who have an unacceptable risk of bleeding? Since the early 1970s, the answer has been insertion of an inferior vena cava filter, the use of which is less harmful to the patient than inferior vena cava ligation. The clinical use of the inferior vena cava filter has markedly increased over the past two decades; indeed by the late 1990s at least 30 000 – 40 000 filters were inserted in patients annually in the United States.28,29 Retrievable filters represent a new generation of inferior vena cava filters with the potential for considerably less harm.30 Retrieval of the filter may result in less thrombotic complications in the long term, which is a particular problem with permanent filters.31-33 Due to the paucity of rigorous clinical trial data including randomized trials, it remains difficult to definitively assess the benefit-to-harm relationship, not only of permanent inferior vena cava filters, but also of their potential successor, the retrievable vena cava filter.
Catheter interventions:
Catheter-tip devices for the extraction or the fragmentation of pulmonary embolism have the potential for producing immediate relief from massive pulmonary embolism. Catheter-tip interventions may have a role in patients in whom there is a contraindication for thrombolytic therapy.
Thrombectomy and embolectomy:
The routine use of venous thrombectomy and embolectomy is not recommended.
EVIDENCE-BASED GUIDELINES FOR TREATMENT OF VENOUS THROMBOEMBOLISM: GRADES OF RECOMMENDATION
The recommendations used in this document are consistent with and adapted from those reported in the Seventh ACCP Conference on Antithrombotic Therapy and Thrombolytic Therapy, 9 and from those recently reported that are entirely consistent with the evidence. The international consensus guidelines are in press.5 With few exceptions, patients with deep vein thrombosis or pulmonary embolism are treated similarly.
Initial treatment of patients with venous thromboembolism
Initial regimen:
For patients with objectively confirmed deep vein thrombosis or pulmonary embolism, short-term treatment with subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin is recommended (Grade A). Subcutaneous unfractionated heparin may be used in deep vein thrombosis patients (Grade A). For patients with a high clinical suspicion of deep vein thrombosis or pulmonary embolism, treatment with anticoagulants while awaiting the outcome of diagnostic tests is suggested (Grade C). In patients with deep vein thrombosis or acute nonmassive pulmonary embolism, low-molecular-weight heparin, instead of unfractionated heparin, is recommended (Grade A). Uncomplicated deep vein thrombosis patients may be treated as outpatients (Grade C). In patients with acute deep vein thrombosis or nonmassive pulmonary embolism treated with low-molecular-weight heparin, routine monitoring with anti-factor Xa levels is not recommended (Grade A). In patients with severe renal failure, intravenous unfractionated heparin rather than low-molecular-weight heparin is suggested (Grade C).
Duration of initial treatment:
In acute deep vein thrombosis or pulmonary embolism, initial treatment with low-molecular-weight heparin or unfractionated heparin for at least 5 days is suggested (Grade C).
Commencing vitamin-K-antagonist therapy:
Initiation of vitamin K antagonist together with lowmolecular- weight heparin or unfractionated heparin on the first treatment day and discontinuation of heparin when the INR is stable and >2.0 is recommended (Grade A).
Adjunctive initial therapy
Thrombolytic therapy:
In patients with deep vein thrombosis or pulmonary embolism, the routine use of systemic thrombolytic treatment is not recommended (Grade A). In selected deep vein thrombosis patients, such as those with massive ileofemoral deep vein thrombosis at risk of limb gangrene secondary to venous occlusion, intravenous thrombolysis is suggested (Grade C). In selected patients with pulmonary embolism, systemic administration of thrombolytic therapy is suggested (Grade B). For pulmonary embolism patients who are hemodynamically unstable, use of thrombolytic therapy is suggested (Grade B). For patients with pulmonary embolism who receive thrombolytic regimens, use of thrombolytic regimens with a short infusion time over those with prolonged infusion times is suggested (Grade C). In pulmonary embolism patients, it is suggested that local administration of thrombolytic therapy via a catheter should not be used (Grade C). In patients with deep vein thrombosis, the routine use of catheterdirected thrombolysis is not suggested (Grade C). In deep vein thrombosis patients, confining catheter-directed thrombolysis to selected patients such as those requiring limb salvage is suggested (Grade C).
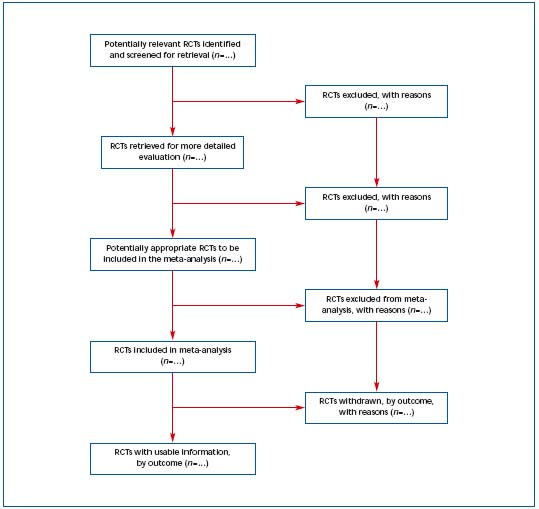
Figure 2. Progress through the stages of a meta-analysis for RCTs.4 Reprinted with permission from The Lancet.
Nonsteroidal anti-inflammatory agents:
For the initial treatment of deep vein thrombosis, the use of nonsteroidal anti-inflammatory agents is not recommended (Grade B).
Ambulation:
For deep vein thrombosis patients, it is recommended that these patients be permitted ambulation as tolerated (Grade B).
Long-term treatment of patients with venous thromboembolism
Intensity of long-term vitamin-K-antagonist therapy:
In patients with deep vein thrombosis or pulmonary embolism, adjusting the dose of vitamin K antagonist to maintain a target INR of 2.5 (range, 2.0 and 3.0) for all treatment durations is recommended (Grade A). Highintensity vitamin-K-antagonist therapy (INR range, 3.1 to 4.0) is not recommended (Grade A). Low-intensity therapy (INR range, 1.5 to 1.9) compared with an INR range of 2.0 to 3.0 is not recommended (Grade A).
Long-term low-molecular-weight heparin treatment:
For most patients with deep vein thrombosis or pulmonary embolism and concurrent cancer, treatment with low-molecular-weight heparin for at least the first 3 to 6 months of long-term treatment is recommended (Grade A). For these patients, anticoagulant therapy, indefinitely or until the cancer is resolved, is suggested (Grade C).
Duration of long-term vitamin-K-antagonist therapy
Transient (reversible) risk factors:
For patients with a first episode of deep vein thrombosis or pulmonary embolism secondary to a transient (reversible) risk factor, long-term treatment with a vitamin K antagonist for at least 3 months over treatment for shorter periods is recommended (Grade A).
Idiopathic:
For patients with a first episode of idiopathic deep vein thrombosis or pulmonary embolism, treatment with a vitamin K antagonist for at least 6 to 12 months is recommended (Grade A). Considering patients with first-episode idiopathic deep vein thrombosis or pulmonary embolism for indefinite anticoagulant therapy is suggested (Grade A).
Presence of a thrombophilia:
For patients with a first episode of deep vein thrombosis or pulmonary embolism who have documented antiphospholipid antibodies or who have two or more thrombophilic conditions (eg, combined factor V Leiden and prothrombin 20210 gene mutations), treatment for 12 months is recommended (Grade C). Indefinite anticoagulant therapy in these patients is suggested (Grade C). For patients with a first episode of deep vein thrombosis or pulmonary embolism who have documented deficiency of antithrombin, deficiency of protein C or protein S, or the factor V Leiden or prothrombin 20210 gene mutation, homocysteinemia, or high factor VIII levels (>90th percentile of normal), treatment for 6 to 12 months is recommended (Grade A). Indefinite therapy as for patients with idiopathic thrombosis is suggested (Grade C).
Recurrent venous thromboembolism:
For patients with two or more episodes of objectively documented deep vein thrombosis or pulmonary embolism, indefinite treatment is recommended (Grade A).
Indefinite anticoagulant treatment:
In deep vein thrombosis or pulmonary embolism patients who receive indefinite anticoagulant treatment, the risk-benefit of continuing such treatment should be reassessed in the individual patient at periodic intervals (Grade C).
Prognostic testing
In patients with deep vein thrombosis or pulmonary embolism, repeat testing with compression ultrasonography for the presence or absence of residual thrombosis or measurement of plasma d-dimer is suggested (Grade C).
Vena caval filter
For most patients with deep vein thrombosis, the routine use of a vena cava filter in addition to anticoagulants is not recommended (Grade A). In deep vein thrombosis or pulmonary embolism patients, the placement of an inferior vena caval filter in patients with a contraindication for, or a complication of, anticoagulant treatment is suggested (Grade C), as well as in those with recurrent thromboembolism despite adequate anticoagulation (Grade C).
Catheter interventions
For most patients with pulmonary embolism, use of mechanical approaches is not recommended (Grade C). In selected highly compromised patients who are unable to receive thrombolytic therapy or whose critical status does not allow sufficient time to infuse thrombolytic therapy, use of mechanical approaches is suggested (Grade C).
Thrombectomy and embolectomy
In patients with deep vein thrombosis, the routine use of venous thrombectomy is not recommended (Grade C). In selected patients such as patients with massive ileofemoral deep vein thrombosis at risk of limb gangrene secondary to venous occlusion, venous thrombectomy is suggested (Grade C). For most patients with pulmonary embolism, pulmonary embolectomy is not recommended (Grade C). In selected highly compromised patients who are unable to receive thrombolytic therapy or whose critical status does not allow sufficient time to infuse thrombolytic therapy, pulmonary embolectomy is suggested (Grade C).
Post-thrombotic syndrome (PTS)
The use of an elastic compression stocking with a pressure of 30 to 40 mm Hg at the ankle for a duration of 2 years after an episode of deep vein thrombosis is recommended (Grade A). A course of intermittent pneumatic compression for patients with severe edema of the leg due to PTS is suggested (Grade B).
The use of elastic compression stockings for patients with mild edema of the leg due to PTS is suggested (Grade C). In patients with mild edema due to PTS, administration of rutosides is suggested (Grade B). In individual deep vein thrombosis or pulmonary embolism patients who, for example, receive indefinite anticoagulant treatment, the risk-benefit of continuing such treatment should be reassessed in the individual patient at periodic intervals. Where appropriate the patient should be involved in the decision process. The patient provides guidance in the assessment process by completing the Patient Planner prior to his or her review in our clinic (Figure 3).

Figure 3. Patient contracting. Reprinted with permission from Vascular Surgery.

REFERENCES
2 Ioannidis JPA, Evans SJW, Gøtzsche PC, et al, for the CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781-788.
3 De Angelis C, Drazen JM, Frizell FA, et al. Clinical trial registration: a statement from the international committee of journal editors. Ann Intern Med. 2004;141:477-478.
4 Moher D, Cook DJ, Eastwood S, et al, for the QUORUM Group. Improving the quality of reports of meta-analyses of randomized controlled trials: QUORUM. Lancet. 1999;354:1896-900.
5 Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism: international consensus statement (guidelines according to scientific evidence). Int Angiol. 2006;25:101-161.
6 Nicolaides AN, Breddin HK, Fareed J, et al. Cardiovascular Disease and Educational and Research Trust and the International Union of Angiology. Int Angiol. 2001;20:1- 37.
7 Hirsh J, Guyatt G, Albers GW, Schunemann HJ. Evidence-based guidelines. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:172S-173S.
8 Guyatt G, Schunemann HJ, Cook D, et al. Applying the grades of recommendation for antithrombotic and thrombolytic therapy. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:179S-187S.
9 Buller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S- 428S.
10 Sackett DL, Straus SE, Richardson WS, et al. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Edinburgh, Scotland: Harcourt Brace & Co. Ltd; 2005.
11 Straus SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based medicine: how to practice and teach EBM. 3rd ed. Edinburgh, Scotland: Harcourt Brace & Co. Ltd; 2000.
12 McKibbon A, Hunt D, Richardson SW, et al. Finding the evidence. In Guyatt G, Rennie D, eds. Users’ guides to the medical literature: a manual for evidence-based clinical practice. Chicago, Il: American Medical Association Press; 2002:16.
13 Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S-400S.
14 Writing Committee for the Galilei Investigators. Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose lowmolecular- weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med. 2004;164:1077-1083.
15 Hull RD, Pineo GF, Brant RF, et al. Longterm LMWH versus usual-care in proximalvein thrombosis patients with cancer. Am J Med. In press.
16 Lee AYY, Levine MN, Baker RI, et al, for the CLOT Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153.
17 Martel N, Lee J, Wells PS. Risk for heparininduced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a metaanalysis. Blood. 2005;106:2710-2715.
18 Warkentin TE, Greinacher A. Heparininduced thrombocytopenia: recognition, treatment, and prevention. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126: 311S-337S.
19 Hirsh J, Raschke R. Heparin and lowmolecular- weight heparin. The seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126: 188S-203S.
20 Hull RD, Raskob GE, Brant RF, et al. The relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157: 2562-2568.
21 Levine MN, Raskob G, Beyth RJ, et al. Hemorrhagic complications of antithrombotic treatment. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:287S- 310S.
22 The Matisse Investigators. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349:1695-1702.
23 Buller HR, Davidson BL, Decousus H, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140:867-873.
24 Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonist. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:204S- 233S.
25 Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med. 2001;345:165-169.
26 Hull RD, Pineo GF, Brant RF, et al. Selfmanaged long-term LMWH therapy: the balance of benefits and harms. Am J Med. In press.
27 Van der Heijden JF, Hutten BA, Buller HR, Prins MH. Vitamin K antagonist or lowmolecular- weight heparin for the longterm treatment of symptomatic venous thromboembolism. The Cochrane Database of Systematic Reviews 2002: The Cochrane Library. 2002;(1):CD002001. Available at: http//www.cochrane.org.
28 Stein PD, Kayali F, Olson RE. Twenty-oneyear trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164:1541- 1545.
29 Magnant JG, Walsh DB, Juravsky LI, Cronenwett JL. Current use of inferior vena cava filters. J Vasc Surg. 1992;16:701-706.
30 Hull RD. Changes in the technology of inferior vena cava filters promise improved benefits to the patient with less harm, but a paucity of evidence exists. J Thromb Haemost. 2005;3:1368-1369.
31 Ferris EJ, McCowan TC, Carver DK, McFarland DR. Percutaneous inferior vena cava filters: follow-up of seven designs in 320 patients. Radiology. 1993;188:851-856.
32 Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena cava filters in the prevention of pulmonary embolism in patients with proximal deep vein thrombosis. N Engl J Med. 1998;338:409-415.
33 Athanasoulis CA, Kaufman JA, Halpern EF, et al. Inferior vena cava filters: review of a 26-year single center clinical experience. Radiology. 2000;216:54-66.
