Investigations for identifying and treating iliac venous stenosis
The RANE Center for Venous & Lymphatic
Diseases, Jackson, Mississippi, USA
Abstract
Iliac vein stenosis is a commonly present lesion in the general population that remains silent in the majority of individuals. It is, however, a permissive lesion that becomes symptomatic if homeostasis is upset by secondary insults such as trauma, infection, thrombosis, or onset of additional pathology. Duplex ultrasound is useful to rule out acute deep venous thrombosis (DVT), identify associated reflux, and exclude other relatively rare nonstenotic venous pathology such as tumor compression, arteriovenous (A-V) fistula, etc. Iliac vein stenosis is best graded on absolute residual area rather than relative stenosis compared with an adjacent reference segment. Intravascular ultrasound (IVUS) is the current reference standard in iliac vein caliber metrics. Duplex ultrasound is severely handicapped by an unacceptably high false positive rate for identifying stenosis. Duplex ultrasound assessment of common iliac vein caliber is a quarter smaller than measured by IVUS and a third smaller in case of external iliac vein. Assessments of caliber via magnetic resonance venography differs from IVUS assessment by nearly the same degree. Computed tomography (CT) imaging with contrast remains the most reliable diagnostic tool at present with only 2.5% mean variance from IVUS caliber measures for common iliac vein and 7.3% for the external iliac vein. The “two-segment” method of assessing the common iliac and external iliac vein calibers individually improves diagnostic accuracy. IVUS is also the favored procedural tool for iliac vein stent placement. There is no role for diagnostic investigations or prophylactic correction of silent iliac vein stenosis.
Introduction
Chronic iliac venous stenosis seldom poses a threat to life or limb. Most symptomatic lesions should be treated by conservative measures. Patients are often relieved to learn of the essentially benign nature of the lesion. Reassurance is an essential part of treatment. Interventional correction may be offered when conservative treatment fails to resolve symptoms within a reasonable time frame, when symptoms progress, or complications ensue. Initial investigation is influenced by the severity of clinical presentation. Duplex ultrasound is routinely used initially but has several limitations, including a very high false positive rate for identifying stenosis.
Silent lesions
Iliac vein stenosis is a common incidental finding during autopsy in the general population. The frequent presence of the lesion in silent form (around 30% of autopsies) was soon recognized after the lesion was initially described by pathologists in the last century.1,2 Some degree of iliac vein stenosis may be found as an incidental finding in as many as 70% of imaging studies carried out for other purposes.3 Yet severe iliac vein stenosis is the causative lesion in some patients presenting with specific severe symptoms.4 This paradox is a feature of permissive pathologies that are a common cause of human disease.5 A classic example is patent foramen ovale, which is present in about 20% of the general asymptomatic population. However, it is a source of embolic complications in a significant fraction of stroke victims. Innocent ureteral reflux is a common finding and requires no specific treatment. However, when urinary infection is superimposed, specific correction is indicated. Previously silent iliac vein stenoses often become symptomatic when homeostasis is perturbed by injury, infection, thrombosis, or onset of new reflux.6 Iatrogenic trauma of joint surgery or onset of saphenous reflux with age are common examples in referral practice. Innumerable other examples of permissive lesions exist in virtually every organ/system disease: carotid stenosis and transient ischemic attack (TIA), obesity, and diabetes are other common examples. Symptoms may resolve if the secondary insult is reversible and corrected; when irreversible, correction of the permissive pathology itself is recommended to prevent recurrent or worsening symptoms. Silent iliac vein stenosis often coexists with other commonly occurring largely benign venous pathology, for example, varicose veins. There has been increasing concern that this may result in unethical and unwarranted iliac vein interventions. There is no role for prophylactic correction of silent iliac vein stenosis regardless of its severity. There are numerous reports in the literature of deep venous thrombosis (DVT) associated with iliac vein stenosis. But there are no epidemiological or longitudinal studies at present to support preventative treatment of silent iliac stenosis, including institution of anticoagulation, with significant morbidity of its own.
Routine investigation of patients for iliac vein stenosis in the absence of relevant symptoms is not recommended.
Absolute and relative stenosis
Relative stenosis is a feature of estimating the severity of arterial stenosis. For example, major regional arteries such as the renal or the femoral do not exhibit flow reduction until the stenosis exceeds a 60% to 70% threshold (Figure 1).7 This is due to the presence of autoregulation in arterial inflow. As arterial stenosis progressively increases, there is compensatory distal vasodilatation, which results in an increased pressure gradient to augment flow in compensatory fashion. After a certain point, the mechanism tops out and is no longer able to maintain normal distal perfusion; further increase in the stenosis results in a rather abrupt drop in distal perfusion and pressure. The phenomenon underlies the papaverine test to estimate adequacy of “distal runoff.” Correction of arterial stenosis is not usually considered until the stenosis exceeds the characteristic threshold (60%-70% for large regional arteries).
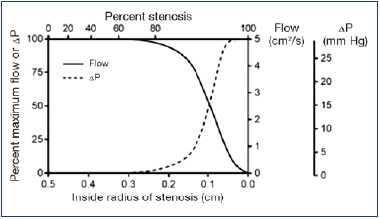
Figure 1. Relationship between flow and pressure drop and percentage stenosis. Flow and pressure remain fairly constant until stenosis reaches about 70%. Further increase in stenosis results in a precipitous drop in flow pressure.
After reference 7: Kassab and Raju. J Vasc Surg Venous Lymphat Disord. 2019;7(2):151-152. © 2018, Society for Vascular Surgery. Published by Elsevier, Inc. Reprinted by permission of Elsevier.
Such autoregulation is weak or absent in venous stenosis. A continuous rise in peripheral venous pressure with increasing stenosis devoid of any lag can be shown to occur in experimental simulations (Figure 2).8 Nevertheless, many interventionalists apply a 50% stenosis threshold before correction of iliac vein stenosis. There is no published basis for the 50% threshold. It appears to be an ad hoc modification derived from arterial practice. In a recent analysis of 480 consecutive limbs, treated at our facility, the 50% stenosis threshold assessed by intravascular ultrasound (IVUS) had no correlation with severity of initial presentation, CEAP clinical class (clinical, etiology, anatomy, pathology classification system), peripheral venous pressure, or outcome.9
An absolute stenosis value in the iliac veins based on an optimal normal caliber area can be argued on the basis of the governing Poiseuille flow equation. Since the outflow in the iliac veins and the pressure gradient vary within a narrow range in the population, a caliber of 200 mm2 and 150 mm2 respectively for the common and external iliac veins can be calculated from the equation to maintain “normal” peripheral venous pressures (<11 mm Hg).10 The effect of any reduction in these caliber values on peripheral venous pressure and related parameters is likely to be nonlinear (tube law).11 There is recent interest on the effect of aspect ratio changes on flow resistance.12 Whereas an adverse flow effect is demonstrable in experimental simulations, few lesions in clinical practice evince a change in aspect ratio alone without a reduction in caliber area.13,14
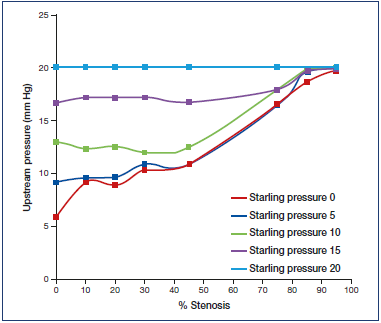
Figure 2. Relationship between outflow stenosis and upstream pressure for a variety of external (Starling) pressures surrounding a Penrose conduit. Note the initial sharp increase in upstream pressure without any lag as outflow stenosis increases from 0% to 10%. There is a further progressive increase in upstream pressure with increasing outflow stenosis. There is some flattening of the pressure curve as outflow pressure approaches external (Starling) pressures.
After reference 8: Raju et al. J Vasc Surg Venous Lymphat Disord. 2014;2(1):52-59. © 2014, Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved. Reprinted by permission of Elsevier.
Investigative tests to assess severity of iliac vein stenosis should preferably provide accurate caliber metrics. Relative stenosis percentage based on an adjacent “normal” segment used in evaluating arterial stenosis is unreliable in assessment of venous stenosis; the adjacent segment is often variably involved in a long diffuse stenosis (“Rokitanski” stenosis) that is common in iliac veins (Figure 315).16 If used as a reference, the net result will be an underestimation of the degree of stenosis.
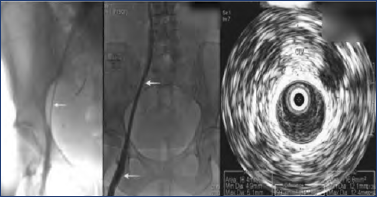
Figure 3. Extreme examples of Rokitansky stenosis are easily recognized (left). Milder examples are less obvious (middle). A subtle sign is the smaller caliber of the iliac vein compared with the common femoral vein (CFV; arrows). On intravascular ultrasound (IVUS) examination, the common iliac vein (CIV) measured 116 mm2, a 42% area of stenosis (right).
After reference 15: Montminy et al. J Vasc Surg Venous Lymphat Disord. 2019;7(6):801-807. © 2019, The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
Investigative tests
Duplex ultrasound assessment
Duplex ultrasound is widely used as the initial screening test for iliac vein stenosis in symptomatic patients. It is readily available and inexpensive. The major limitation of the test is the dimensional disparity present between Duplex ultrasound and IVUS (Figure 4).17 Duplex calibers are a third to one fourth less than corresponding IVUS measurements in the iliac veins, ie, duplex ultrasound tends to overestimate caliber stenosis. Because of this disparity, frequent false positives mixed in with true positives are inherent with this technique. It is mainly used to detect acute venous thrombosis, chronic occlusions, associated reflux, and other infrequent pathology that may present with similar leg symptoms. These include tumors, retroperitoneal fibrosis, pre- or poststenotic dilatations, retroperitoneal fibrosis, or arteriovenous (A-V) fistula. In some patients, the findings may be intermediate with indirect signs for a stenosis, such as thickened venous walls, collaterals, and high or low iliac venous velocities. A more invasive investigation for definitive diagnosis is usually required to confirm such duplex ultrasound findings.
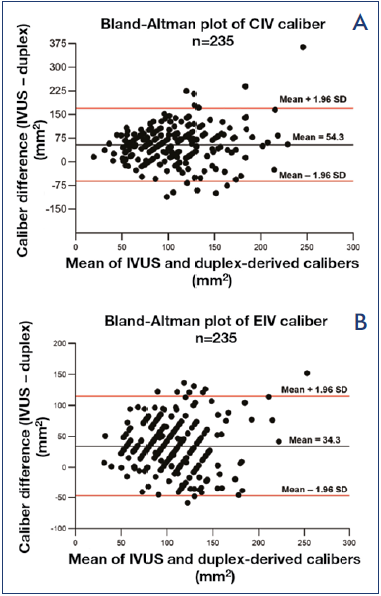
Figure 4. Bland-Altman plots of the difference in duplexderived calibers compared with intravascular ultrasound (IVUS) measurements for A) common iliac vein (CIV); and B) external iliac vein (EIV). Duplex calibers were smaller by 54 mm2 and 34 mm2 respectively compared with IVUS.
After reference 17: Raju et al. Vasc Med. 2021;26(5):549- 555. © 2021, The Author(s). Reprinted by permission of SAGE Publications.
The absence of an iliac stenosis on duplex ultrasound examination in the context of symptoms is an important finding and also requires another invasive test for confirmation; this may be a false- or true-negative duplex ultrasound finding for stenosis. Duplex ultrasound may miss some significant stenotic lesions near the iliac-caval junction underneath the arterial crossover point (false negative). Nonstenotic pathology that may present with stenosis-like limb symptoms (true negatives) include a wide spectrum of diseases that are sourced in other organs and systems; nutritional, metabolic, or immune derangements; and drug interactions, etc. Though this is relatively infrequent compared with stenosis in clinical practice, such patients usually end up undergoing an IVUS examination because compression often fails in these patients. The negative IVUS finding results in an extensive multisystem workup to pinpoint the nonvenous source of leg symptoms. An increasing number of patients present with severe chronic venous disease caused by external compression of the iliac vein due to increased abdominal pressure related to obesity.18 The compression does not result in luminal narrowing but manifests itself by increased femoral venous pressure (peripheral venous hypertension) and decreased velocity in the iliac segment.19-21 Traditional iliac venous stenoses of primary or secondary etiology often coexist with external compression.22,23
Magnetic resonance venography
Magnetic resonance venography (MRV) caliber measurements of the iliac veins were significantly smaller than IVUS area measurements in a blinded comparison of the 2 techniques in 78 limbs at our institution.24 MRV caliber measurements were derived from time of flight (TOF) measurements followed by gadolinium-enhanced images (Table I).24 The differences from IVUS measurements were so large that MRV could not be reliably used for grading stenosis. Others have reported similar deficiency in MRV caliber metrics with and without contrast.5 MRV has additional drawbacks: cost, contrast allergy, renal failure, and intolerance of the technique by patients due to metal implants or claustrophobia. These may preclude use of the technique in 20% to 30% of patients.
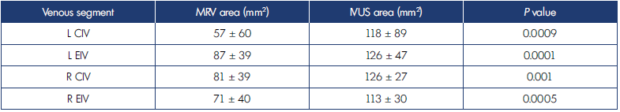
Table I. Comparison of means of minimal areas for external iliac vein and common iliac vein noted on magnetic resonance venography and intravascular ultrasound.
After reference 24: Saleem et al. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1066-1071. 2022, Society for Vascular Surgery.Published by Elsevier Inc. All rights reserved. Reprinted by permission of Elsevier.
CIV, common iliac vein; EIV, external iliac vein; IVUS, intravascular ultrasound; MRV, magnetic resonance imaging. Bold face indicates significant P values.
Computed tomography (CT) venography
Computed tomography venography (CTV) is currently our preferred “go to” definitive technique before IVUS to confirm or rule out iliac venous stenosis. Contrast is administered peripherally through an arm vein. Imaging of abdomen and pelvis is commenced after a standard delay of 120 seconds. The stored images are later scanned in coronal, sagittal, and axial views to identify anatomic variations if any. All measurements in the area of interest are made with calipers from 5-mm interval axial sections.
A “two-segment” caliber metrics is used wherein the narrowest lumen diameter of the common iliac and external iliac veins are considered individually (Table II).26 The narrowest diameter identified at each segment is converted to area for circle (πr2). A stenosis is determined to be present if the caliber at the narrowest point is <200 mm2 and <150 mm2 for the common and external iliac veins respectively. Percentage stenosis is calculated based on these minimum normal thresholds. The increased accuracy of the method over considering only the common iliac vein caliber is based on the fact that the common iliac and external iliac vein are each stenotic in around 80% of limbs; an additional approximately 15% can be picked up by considering the other segment separately because one of the 2 segments is stenotic in individuals when the other is not; ie, common iliac and/or external iliac is found to be stenotic in around 95% of limbs. Bland-Altman plots show only 2.5% and 7.3% variance for common iliac and external iliac veins respectively between CTV and IVUS (Figure 5).26
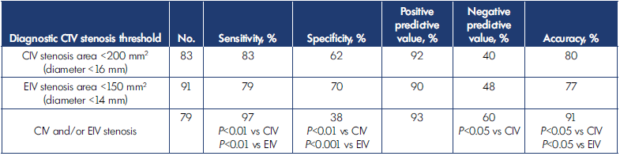
Table II. Diagnostic accuracy detail of computed tomography venography (CTV) assessment for iliac vein stenosis.
After reference 26: Raju et al. J Vasc Surg Venous Lymphat Disord. 2020;8(6):970-977. © 2019, The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
CIV, common iliac vein; EIV, external iliac vein.
Two-segment diagnostic comparison was significantly superior to single-segment analysis.
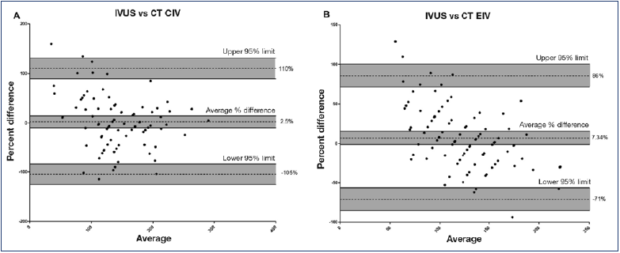
Figure 5. Bland-Altman plot of mean caliber difference between computed tomography (CTV) and intravascular ultrasound (IVUS).The difference was A) only 2.5% for common iliac vein (CIV); and B) 7.3% for external iliac vein (EIV).
After reference 26: Raju et al. J Vasc Surg Venous Lymphat Disord. 2020;8(6):970-977. © 2019, The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
Procedural tests to guide stent implantation
Transfemoral venography
The use of traditional contrast venography has sharply declined in recent years. Legacy equipment used for the technique lacked the vital internal scale integrated in modern imaging equipment. Nevertheless, the traditional technique provides a panoramic venous map useful in complex postthrombotic cases. Many interventionalists with a radiology background continue to use traditional venography as a procedural guide during stent placement.
Key minimum requirements of a procedural tool in venous stenting are as follows: identification and grading of the stenosis and localizing optimal proximal and distal landing zones. In a blinded comparison of IVUS and venography in 155 stented limbs, venography was inferior in all respects15: venography failed to identify IVUS-positive lesions in 19%, and the median maximal area stenosis was significantly less (P<0.001). A typical example percentage stenosis estimation of the same lesion by contrast venography and IVUS are shown in Figure 6.15 Venography missed the location of maximal stenosis in over two-thirds of limbs. The iliac-caval confluence location was lower with venography than IVUS by as much as the height of 1 vertebral body. Agreement between venography and IVUS on location of distal landing zone free of disease was only 26%. Mislocating optimal proximal and distal landing zones is likely to result in recurrence of stenosis near these sites. The misestimation of confluence level is due to merging of contrast from the 2 sides resulting in obscuration of the boundary at the junction. The error is magnified when only the contrast-injected side opacifies and the estimation of confluence location is made on the basis of caliber or course change of contrast flow stream (Figure 7).15
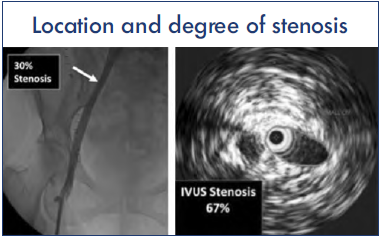
Figure 6. Disparity between venography and intravascular ultrasound (IVUS) in estimating maximal stenosis. In the example shown, the common iliac vein (CIV) was identified as the site of maximal stenosis with an estimated 30% diameter stenosis (53% area stenosis) as shown (left). IVUS estimation of area stenosis at the same location was higher at 67% (right). See text.
After reference 15: Montminy et al. J Vasc Surg Venous Lymphat Disord. 2019;7(6):801-807. © 2019, The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
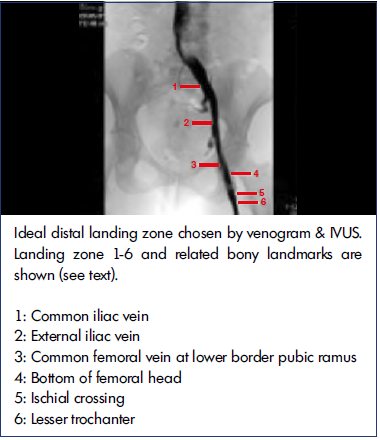
Figure 7. Where is the confluence? Lack of contralateral iliac opacification makes venographic localization difficult. Intravascular ultrasound (IVUS)-identified confluence was a vertebral body higher than estimated by venography (n=128). There were wide discrepancies between venogram and IVUS in choosing the distal landing site as well; see text.
After reference 15: Montminy et al. J Vasc Surg Venous Lymphat Disord. 2019;7(6):801-807. © 2019, The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
IVUS is frequently referred to as the “gold standard” for imaging iliac vein stenosis. Yet, it has deficiencies of its own that results in suboptimal imaging in as many as 15% to 20% of cases.15 This arises because the IVUS catheter is not coaxial and exhibits a luminal bias toward one side or the other in the complex 3-dimensional (3D) spiral of the iliac vein anatomy. This bias is particularly prominent at tributary junctions at the confluence of the internal/external iliac veins and the common iliac/inferior vena cava (IVC) junction. This results in incomplete visualization of the stenotic lesion where part of the circumference appears to be missing (Figure 8); incomplete caliber metrics is the result. It is generally possible to determine if the lesion is high or low grade by “balloon sizing” of the lesion and noting the degree of waisting at the site. A CTV if obtained before IVUS is able to provide caliber metrics in all such lesions.26
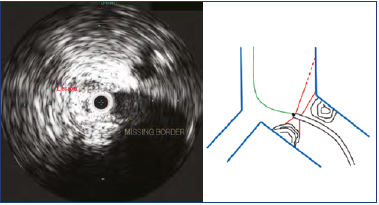
Figure 8. “Missing border” of a high-grade stenosis at the iliac confluence (left). This artefact results from tilting of the intravascular ultrasound (IVUS) catheter tip due to lack of a centering mechanism (right). Only part of the lesion is imaged with the sound beam missing the rest of the lesion. High-grade lesions will demonstrate waisting on “balloon sizing.” More precise caliber measurement can be obtained from computed tomography venography (CTV) imaging if available. See text.
Notwithstanding this deficiency, IVUS is superior to venography as a procedural guide during stenting. It can be used as frequently as necessary without incurring undue radiation hazard or contrast-related problems. It can identify and measure lesions that may be missed by venography because too much or too little contrast was present for proper visualization. Similar comments apply to preference for IVUS to identify post-stent defects such as stent shelving, incomplete overlap, inadequate expansion, etc. The lack of internal scale is a distinct disadvantage in detecting late stent compression on contrast venography (Figure 916).
Iliac vein stenting was carried out with IVUS and fluoroscopy alone in 31 limbs where contrast venography was prohibited due to renal failure or contrast allergy. The diagnostic yield of IVUS in highly symptomatic patients with features of chronic obstruction is very high. About 20% of procedures are currently carried out in our practice with IVUS guidance alone under fluoroscopy without use of contrast.
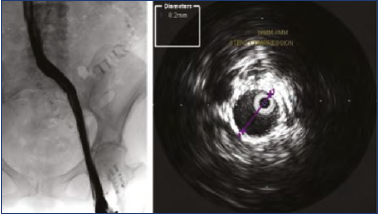
Figure 9. Post-stent venogram showed a smooth contrast profile without any apparent stenosis. Intravascular ultrasound (IVUS) showed severe stent stenosis due to compression. An 18-mm stent had been compressed to 8 mm with a caliber reduction of >75%.
After reference 16: Raju and Davis. J Vasc Surg Venous Lymphat Disord. 2014;2(3):260-267. © 2014, Society for Vascular Surgery. Published by Elsevier Inc. All Rights Reserved. Reprinted by permission of Elsevier.
Multiplanar venography
Modern equipment has substantially improved the use ability of contrast venography for iliac vein assessment. In the multicenter VIDIO trial (Venogram versus Intravascular ultrasound for Diagnosing and treating Iliofemoral vein Obstruction), multiplanar venography was compared with IVUS as procedural guide.27 IVUS identified lesions in 26% of limbs missed by multiplanar venography. The treatment plan was significantly revised in 57% as a result of IVUS findings not evident on venography. Twenty-three percent required a longer stent stack than indicated by venography. Stenting was avoided in 3% because IVUS did not detect a lesion falsely identified on multiplanar venography. The authors used the 50% relative stenosis threshold in this study.
Saleem evaluated IVUS versus all contrast-enhanced multiplanar techniques (multiplanar venography, CTV, MRV) in a recent systematic review.28 CTV appeared to have the highest sensitivity among the techniques using IVUS as the reference standard. However, all techniques had low sensitivities not useable for clinical decision-making in symptomatic patients.
Emerging techniques
Most radiologists use 5-mm slices from CTV images for identification and measurement of stenotic lesions. Even so, some lesions particularly near the iliac-IVC confluence can be missed. Even smaller slice cuts (0.6 mm) and higher image resolution are possible with newer equipment. 3D image generation with automated measurement algorithms holds the promise of increased diagnostic accuracy (Figure 10).29 3D software specific for iliac venous stenosis is currently under development. Since iliac venous stenting in chronic disease is usually an elective procedure, the technique holds promise of broad adoption. Customized manufacture of stents to suit individual anatomy is a future possibility.
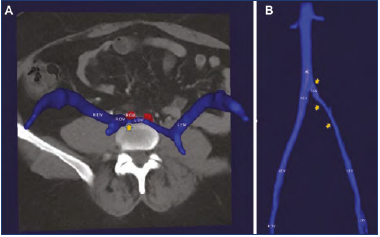
Figure 10. Three-dimensional (3D) computed tomography venogram (CTV) reconstructions in the A) axial and B) coronal views demonstrating severe left iliofemoral venous obstruction. Orange arrows point to areas of obstruction.
Abbreviations: CFV, common femoral vein; CIA common iliac artery; CIV, common iliac vein; EIV, external iliac vein; IVC, inferior vena cava; L, left; R, right.
After reference 29: Jayaraj and Raju. J Vasc Surg Venous Lymphat Disord. 2021;9(1):73-80.e1. © 2020, Published by Elsevier Inc. on behalf of the Society for Vascular Surgery. Reprinted by permission of Elsevier.
A clinical algorithm
The level of venous testing required in individual cases depends on the intensity of clinical presentation. Mild leg swelling and pain are common symptoms of diverse origin. Most of recent onset do not persist long term. It is appropriate to start compression therapy in such patients after ruling out acute DVT and other unexpected pathology by duplex ultrasound examination. Additional expensive or complex investigations looking for confirmation of stenosis is not necessary at this stage. If symptoms do not improve within a few weeks, additional testing may be desirable. If initial presentation is more severe (eg, CEAP class 3 or higher) a CTV may be ordered. Many patients and referring physicians desire a confirmatory test for iliac venous stenosis short of IVUS. CTV fits this role. This may be unnecessary in geriatric or frail patients as contrast imaging is a potential source of renal or allergic complications. It is often possible to perform single-stage diagnostic IVUS and stenting at the same sitting with informed consent.
Conclusions
Chronic iliac vein stenosis is a commonly occurring lesion in the general population. Most remain silent lifelong. Investigation or treatment of asymptomatic patients is not warranted. There is a role for interventional correction of iliac vein stenosis in patients in whom compression fails or when symptoms progress to tissue damage or complications set in. An initial duplex ultrasound examination is useful to rule out acute thrombosis and other uncommon pathology that requires a different treatment approach. IVUS is the current reference standard for grading iliac stenosis severity. Relative stenosis grading is popular, but an absolute stenosis grading based on residual caliber is probably more appropriate for central veins. Several imaging techniques including duplex ultrasound, traditional venography, and MRV do not have dimensional parity with IVUS. Contrast-enhanced routine CT imaging of the iliac veins displays excellent dimensional parity (<10% variance) with IVUS for measuring iliac vein caliber. The common iliac and external iliac veins should be individually assessed for residual caliber. Considering both segments individually can increase sensitivity and reduce false positives. IVUS procedural guidance is superior to contrast venography including multiplanar venography for stent placement.

REFERENCES
1. Ehrich WEKEB. A frequent obstructive anomaly of the mouth of the left common iliac vein. Am Heart J. 1943(26):18-31.
2. Negus D, Fletcher EW, Cockett FB, Thomas ML. Compression and band formation at the mouth of the left common iliac vein. Br J Surg. 1968;55(5):369-374.
3. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937- 943.
4. Cockett FB, Thomas ML. The iliac compression syndrome. Br J Surg. 1965;52(10):816-821.
5. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143;discussion 44.
6. Raju S, Oglesbee M, Neglen P. Iliac vein stenting in postmenopausal leg swelling. J Vasc Surg. 2011;53(1):123-130.
7. Kassab G, Raju S. Grading venous stenosis is different from arterial lesions. J Vasc Surg Venous Lymphat Disord. 2019;7(2):151-152.
8. Raju S, Kirk O, Davis M, Olivier J. Hemodynamics of “critical” venous stenosis and stent treatment. J Vasc Surg Venous Lymphat Disord. 2014;2(1):52-59.
9. Jayaraj A, Powell T, Raju S. Utility of the 50% stenosis criterion for patients undergoing stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1408-1415.
10. Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology. 2018;33(7):451-457.
11. Shapiro AH. Steady flow in collapsible tubes. J Biomech Eng. 1977;99:126-147.
12. Lichtenberg M. Lumen shape: a new measurement to consider in the treatment of iliofemoral venous outflow obstruction. Endovasc Today Eur. 2018;6:9-11.
13. Brecher GA. Venous Return. Grune & Stratton; 1956.
14. Raju S, Kuykendall R. Experimental analysis of aspect ratio in iliac vein stenosis. J Vasc Surg Venous Lymphat Disord. 2021;9(4):1041-1050e1.
15. Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord. 2019;7(6):801-807.
16. Raju S, Davis M. Anomalous features of iliac vein stenosis that affect diagnosis and treatment. J Vasc Surg Venous Lymphat Disord. 2014;2(3):260-267.
17. Raju S, Walker W, Noel C, Kuykendall R, Powell T, Jayaraj A. Dimensional disparity between duplex and intravascular ultrasound in the assessment of iliac vein stenosis. Vasc Med. 2021;26(5):549-555.
18. Jayaraj A, Powell T, Raju S. Effect of body mass index on initial presentation and outcomes after stenting for quality of life-impairing chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2022;10(2):325-333e1.
19. Guyton AC, Adkins LH. Quantitative aspects of the collapse factor in relation to venous return. Am J Physiol. 1954;177(3):523-527.
20. Raju S, Varney E, Flowers W, Cruse G. Effect of external positive and negative pressure on venous flow in an experimental model. Eur J Vasc Endovasc Surg. 2016;51(2):275- 284.
21. Thurin A, Goushegir P, Ask P, Thulesius O. Venous flow in an in vitro model: effect of extravascular pressure. J Vasc Invest. 1997;3:124-129.
22. Padberg F Jr, Cerveira JJ, Lal BK, Pappas PJ, Varma S, Hobson RW 2nd. Does severe venous insufficiency have a different etiology in the morbidly obese? Is it venous? J Vasc Surg. 2003;37(1):79-85.
23. Raju S, Darcey R, Neglen P. Iliac-caval stenting in the obese. J Vasc Surg. 2009;50(5):1114-1120.
24. Saleem T, Lucas M, Raju S. Comparison of intravascular ultrasound and magnetic resonance venography in the diagnosis of chronic iliac venous disease. J Vasc Surg Venous Lymphat Disord. 2022;10(5):1066- 1071.
25. Kusiak A, Budzynski J. Usefulness of noncontrast- enhanced magnetic resonance imaging prior to venous interventions. Postepy Kardiol Interwencyjnej. 2019;15(3):338-344.
26. Raju S, Walker W, Noel C, Kuykendall R, Jayaraj A. The two-segment caliber method of diagnosing iliac vein stenosis on routine computed tomography with contrast enhancement. J Vasc Surg Venous Lymphat Disord. 2020;8(6):970-977.
27. Gagne PJ, Tahara RW, Fastabend CP, et al. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5(5):678-687.
28. Saleem T, Raju S. Comparison of intravascular ultrasound and multidimensional contrast imaging modalities for characterization of chronic occlusive iliofemoral venous disease: a systematic review. J Vasc Surg Venous Lymphat Disord. 2021;9(6):1545-1556.
29. Jayaraj A, Raju S. Three-dimensional computed tomography venogram enables accurate diagnosis and treatment of patients presenting with symptomatic chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9(1):73-80.e1.
