Management of combined venous and lymphatic malformations
and lymphatic malformations
Director of the Department of Vascular Surgery
and Center for Vascular Malformations,
“Stefan Belov,” Clinical Institute Humanitas
“Mater Domini,” Castellanza (Varese) Italy
Abstract
Vascular malformations are congenital vessel malformations that include one or more venous, lymphatic, or arteriovenous defects. Klippel-Trenaunay syndrome occurs when there is a combination of venous and lymphatic malformations in the limbs; however, the definition is still controversial. The 2013 international venous malformation consensus established that Klippel-Trenaunay syndrome is a combination of venous malformations that involve the whole limb and lymphatic malformations. Although, if two venous malformations are present (eg, extratruncular and truncular), then a lymphatic malformation is not necessary to meet the definition for Klippel-Trenaunay syndrome. The classic triade of signs, ie, limb overgrowth, nevus and dilated superficial veins was not present in all cases of a patient series we analyzed (n=46). The diagnostic goal should be to recognize vascular malformations in individual patients. Investigations should involve the following (in the order presented): (i) a clinical examination; (ii) duplex scan to rules out arteriovenous malformations, study the morphology and flow in the veins, and establish flow in dysplastic peripheral vascular masses; (iii) MRI to confirm morphology of deep veins and determine the site of infiltrating malformations; and (iv) lymphoscintigraphy to identify the main deep and superficial lymphatic channels. Three treatment techniques–surgery, alcohol sclerotherapy of dysplastic vessels, and an interstitial or a superficial laser procedure–are available for Klippel-Trenaunay syndrome, which may be performed in stages and it may involve a combination of techniques. Significant improvement is possible if there is a complete diagnosis and correct treatment planning.
Introduction
Congenital vascular malformations arise due to an error in vessel development in the embryo. According to the type of vessel involved–artery, vein, or lymphatic duct–arterial, venous, lymphatic, and arteriovenous malformations can occur.1 The anomalies are divided into defects of the main vessel, which are called truncular defects by the Hamburg classification,2 or defects of the major named vessels according to the International Society for the Study of Vascular Anomalies (ISSVA)3 and areas of dysplastic vessels in tissues, which are called extratruncular or simple according to the Hamburg classification or the ISSVA, respectively.
A combination of malformations may occur in the same patient, which often results in a more complex disease that can be difficult to understand and treat. In this paper, we will discuss combinations of venous and lymphatic malformations.
Venous malformations are the most common type of congenital vascular malformation with an incidence >50%, lymphatic malformations are less common, and combinations of venous and lymphatic malformations have a lower incidence.4,5 Table I shows the distribution of congenital vascular malformations from a recent patient series. Venous malformations may be combined with lymphatic malformations in different truncular or extratruncular forms, resulting in the following possible combinations:
• Truncular venous malformations (aplasia, hypoplasia, or dilatation of the main venous trunks) with truncular lymphatic malformations (aplasia, hypoplasia, or dilatation of the main lymphatic ducts).
• Truncular venous and extratruncular lymphatic malformations (ie, mass of dysplastic lymphatics situated in the tissues).
• Extratruncular venous (ie, mass of dysplastic veins situated in the tissues) and truncular lymphatic malformations.
• Extratruncular venous and lymphatic malformations.6
• Truncular and extratruncular venous and lymphatic malformations may coexist in the same patient.
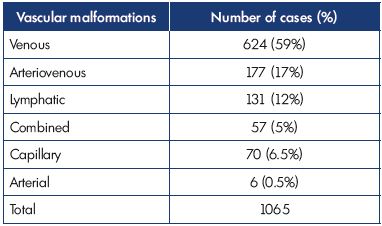
Table I. Distribution of congenital vascular malformations during
4 years of observation (2011-2015) in our Vascular Malformation
Center of Castellanza (Italy).
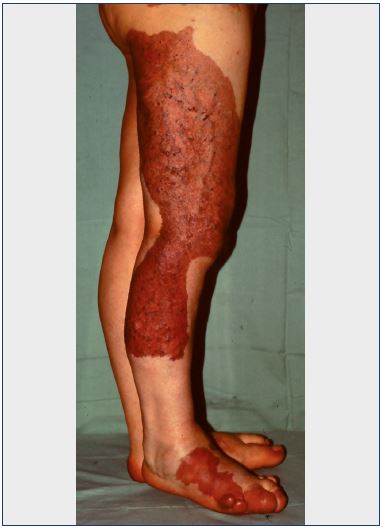
Venous malformations may also have capillary defects (ie, the so-called port-wine stains) that vary from extensive cutaneous involvement to an almost complete absence of capillary skin defects (Figure 1).
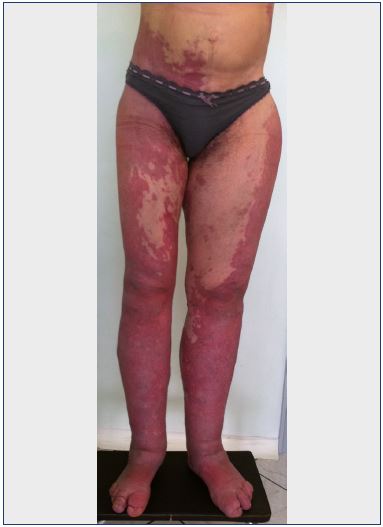
The combination of venous and lymphatic malformations in the limbs has been defined as the Klippel-Trenaunay syndrome, which comes from the original description by the French authors Maurice Klippel and Paul Trenaunay in 1900. They described cases with a triad of clinical signs on the lower limbs that included dilated superficial veins, nevus, and limb hypertrophy (Figure 2).7 At that time, no diagnostic instruments were available to recognize the vascular malformations existing in those patients. Some years later, the German dermatologist Frederick Parkes Weber described similar cases that presented with the triad of signs, but also clear signs of arteriovenous malformations.8,9 With the introduction of angiography, it was possible to recognize that patients with Klippel-Trenaunay syndrome had venous malformations without arteriovenous fistulae, while the cases described by Parkes Weber (also known as Parkes-Weber syndrome) did, which helped distinguish between the two syndromes.
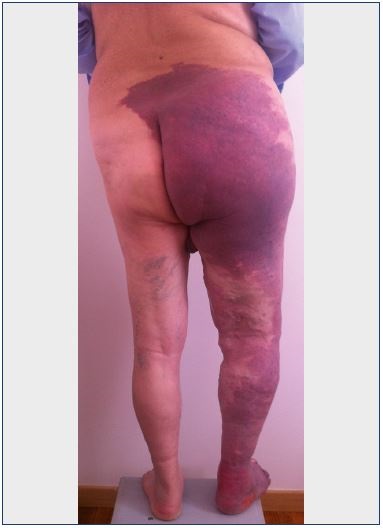
Figure 2. A case where the triad of signs for Klippel-Trenaunay
syndrome is present–nevus, limb overgrowth, and dilated
superficial veins.
The concept of Klippel-Trenaunay syndrome is still not clearly defined in the literature. Some authors have also used the term Klippel-Trenaunay-Weber syndrome, indicating cases with or without arteriovenous malformations, which increases the confusion between Klippel-Trenaunay syndrome and Parkes-Weber syndrome. Associated lymphatic malformations have been considered, but without a clear definition of the type of lymphatic malformations (truncular or extratruncular). Moreover, vascular malformations located in other parts of the body, such as the head or pelvis, have also been classified as Klippel-Trenaunay syndrome.
An attempt to clarify the concept of Klippel-Trenaunay syndrome has been done with the international consensus about venous malformations, where Klippel-Trenaunay syndrome was defined as a diffuse venous malformation that involved the whole limb and where a combination of two malformations was present (ie, truncular or extratruncular venous or lymphatic malformations), without arteriovenous malformations. Malformations involving only a part of the limb (thigh, calf, or foot) or locations only outside the limbs should not be defined as Klippel-Trenaunay syndrome. Diffuse arteriovenous malformations of a limb should be classified as Parkes-Weber syndrome.10
Clinical signs
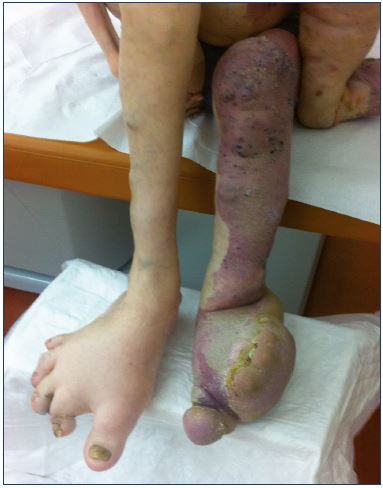
Klippel-Trenaunay syndrome may manifest in the lower limbs with the clinical triad–dilated abnormal superficial veins, nevus, and limb–length discrepancy due to overgrowth or shortening of the affected limb. However, the nevus may be absent and limb–length discrepancies may not be constant (Table II). Bilateral involvement (Figure 3) and deformity by overgrowth of the foot are possible (Figure 4). An abnormal, lateral vein, called a marginal vein, is often present. This vein is valves and may create stasis, pain, and sometimes, a pulmonary embolism.11 Patients often complain of heaviness, swelling, and pain, which may be localized to specific areas of the limb. Pelvic involvement is possible, including the genitals or the rectum with bleeding.
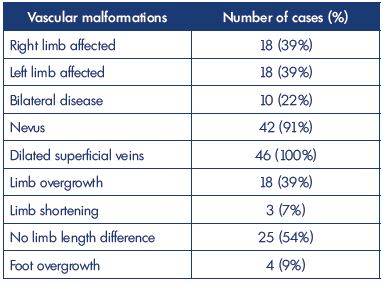
Table II. Clinical signs observed in 46 cases of Klippel-Trenaunay
syndrome in our Vascular Malformation Center of Castellanza
(Italy) from 2011 to 2015.
Diagnostic
As Klippel-Trenaunay syndrome often appears as a complex of congenital vascular malformations, diagnosis may be difficult. Often unnecessary tests, such as angiography, were performed, while, in other cases, no examinations were done and the diagnosis was based on a simple clinical evaluation. To correctly diagnose the syndrome, a step-by-step procedure is recommended, beginning with the least invasive procedure, as follows:
• Clinical evaluation
• Comparative radiography of the limbs
• Duplex scan
• MRI with and without contrast
• Lymphoscintigraphy
• Other tests, if necessary
The clinical examination should focus on evaluating the extension of the nevus, recognizing and/or excluding differences in limb length, noticing the presence and extension of dilated superficial veins, and checking for signs of arteriovenous malformations, such as abnormal vascular pulsations (ie, thrills). The clinical signs of Klippel- Trenaunay syndrome vary and may include the classic triad of signs, but these may manifest with different frequencies, and some signs may not be constant. Table II shows the clinical signs that we identified in 46 cases of Klippel- Trenaunay syndrome.
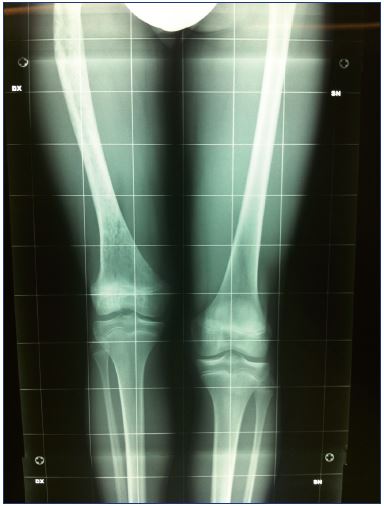
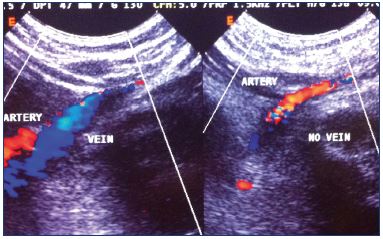
Comparative radiography of the limbs is useful to recognize overgrowth or shortening of the affected limb, presence of phlebolythes (a typical sign of venous malformations), and bone structure anomalies (Figure 5). Duplex scanning provides hemodynamic and morphologic data on the congenital vascular malformations. Analyzing the deep and superficial venous systems with duplex scanning may demonstrate anomalies of the deep and superficial veins (Figure 6). Vascular masses situated in tissues should be analyzed to determine the type of flow: low flow indicates venous dysplasia; high flow is typical of arteriovenous malformations; and areas with liquid cysts with no flow (ie, no flow areas) indicate lymphatic extratruncular malformations. Combinations of low flow and no flow vascular areas may coexist (Figure 7). MRI is an excellent diagnostic tool to identify the location and extent of the extratruncular venous and lymphatic malformations, which are often located inside the muscles. Truncular venous malformations have also been well documented (Figure 8). Experience and knowledge of congenital vascular malformations is a requirement for the radiologist in order to acquire high-quality images that are specific for vascular malformations.12
Lymphoscintigraphy is necessary to study the lymphatic drainage system because anomalies are common in Klippel- Trenaunay syndrome and these cannot be determined using other examinations. A separate study for deep and superficial lymphatic drainage systems is necessary to identify the location and extent of the malformations. Anomalies of the deep lymphatic trunks, such as aplasia or hypoplasia in segments or even the whole vessel, are the most common lymphatic malformations recognized in Klippel-Trenaunay syndrome (Figure 9). However, nuclear medicine laboratories usually do not perform this type of study because they are normally requested to analyze total lymph drainage of the limb in patients with lymphedema.13
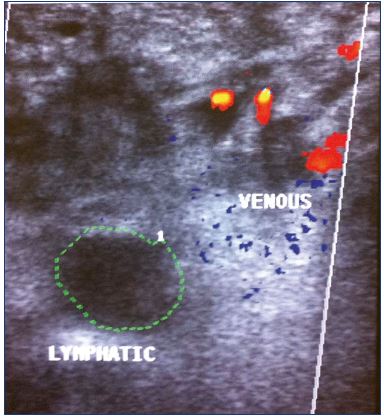
Figure 7. Duplex scan showing the presence of dysplastic
intramuscular veins (low flow) and a large lymphatic area (no
flow).
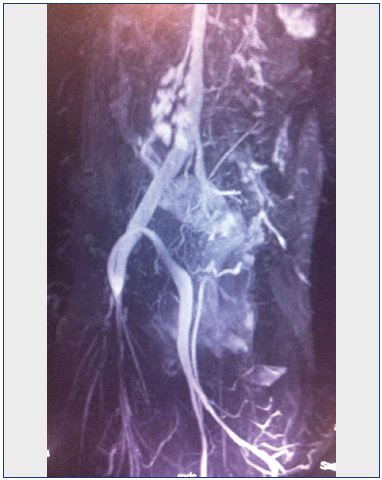
Figure 8. Magnetic resonance angiography demonstrating
aplasia of the left iliac vein and spontaneous suprapubic leftright
bypass.
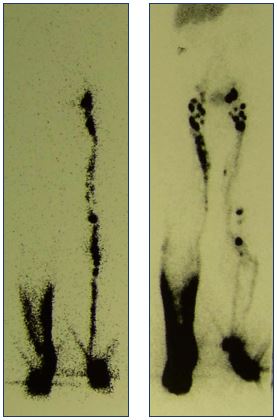
Figure 9. Lymphoscintigraphy of the deep and superficial
lymphatic drainage system.
Panel A shows an absence of draining lymphatic vessels (right)
and dermal backflow in the deep system. Panel B shows a slow
drainage in the superficial system (right).
The diagnostic process concludes when a precise definition of the vascular anomalies, according to classification, is possible. The incidences of different types of vascular anomalies discovered in our cases by diagnostic procedures are shown in Table III. This table shows that cases can be very different and that a complete diagnosis using the tests described is essential for complete recognition of the anomalies in a single case.
Treatment
Treatment should be planned according to some priorities that include pain; clinical evolution of malformations, such as progression of limb elongation or shortening; risk of complications, such as a pulmonary embolism (ie, in the marginal vein); and esthetic discomfort (the last point to consider). Pain mainly occurs due to repeated thrombosis in venous extratruncular masses where blood stasis often occurs. Venous aneurysms in the femoral or popliteal vein may also cause pain due to blood stasis. Progression of limb elongation is often due to the marginal vein, which creates stasis, and due to a slight arteriovenous malformation located in the dysplastic tissues. Limb shortening is due to extensive venous dysplastic masses pressing on bones, which inhibits their growth. Pulmonary embolisms may originate from both large marginal veins and venous aneurysms.
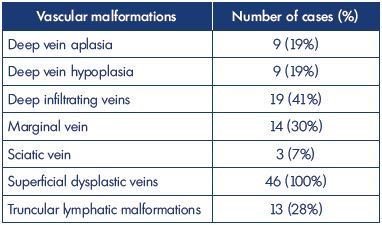
Table III. Vascular defects observed in 46 cases of Klippel-
Trenaunay syndrome in our Vascular Malformation Center of
Castellanza (Italy) from 2011 to 2015.
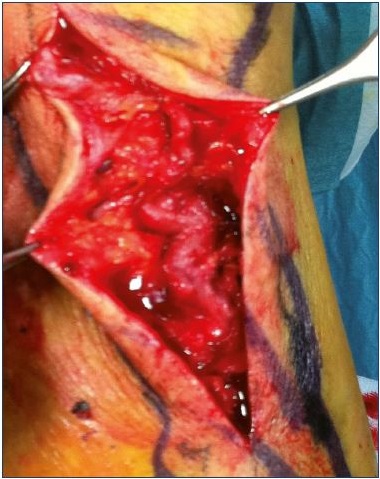
Available treatment techniques include surgery, sclerotherapy, and laser treatment. Surgery is often the best technique; however, it should be well planned based on a complete recognition of the malformation and the causes of discomfort. Surgical removal of extratruncular masses that cause pain or affect limb growth can considerably improve a patient’s condition (Figure 10). In our experience, the best results are obtained with a step-by-step procedure, which avoids extensive single operations that may have complications, such as infection, difficult wound healing, and thrombosis. Marginal veins should be removed surgically in an open procedure; closed stripping should be avoided due to bleeding complications that can arise from the rupture of larger perforators, if present.11 This procedure is not indicated for deep vein aplasia because, in this case, the marginal vein is the main draining vessel. For deep hypoplasia, the marginal vein can be resected, as deep veins are able to dilate spontaneously to an almost normal size after resection. Endovascular treatment of marginal veins using laser treatment has been reported.14 Venous aneurysms can be treated by tangential resection and vein reconstruction using a Satinsky clamp, which is our preferred technique, or by resection and substitution with an autologous venous graft.
Sclerotherapy of dysplastic veins is an excellent and less invasive technique. However, classic sclerotherapy with sclerosants for varicose veins (eg, sodium tetradecyl sodium, polidocanol, etc) is less effective for venous malformations than for varicose veins and there is a high incidence of early recurrence. The presence of slight arteriovenous malformations in the dysplastic veins may explain the difference. The introduction of alcohol for sclerotherapy has dramatically improved the results because ethanol is the strongest sclerosant that can almost completely occlude the treated vessels. Ethanol is considered the reference sclerosant for venous malformations.10 Alcohol is best used for treatment of extratruncular dysplastic venous malformations, whereas truncular malformations are treated better with surgery (Figure 11).
For extratruncular vascular masses, laser treatment using an interstitial technique that positions the laser fiber in the mass can be used to occlude dysplastic vessels. Radial fibers may be useful to increase the effect of treatment. Leaking extratruncular lymphatic malformations with repeated inflammation can be treated successfully using laser treatment. Superficial and deep occlusion of leaky points is effective to treat inflammation, which occurs due to an infection that enters through the leaky points. Superficial laser treatment of the nevus may have an esthetic goal, but this option should only be used after other, more severe, disturbances have been treated. Simple superficial laser treatments have no effect on deep malformations that result in severe symptoms. Treatment is often performed in stages by combining the three treatment modalities (Figure 12).
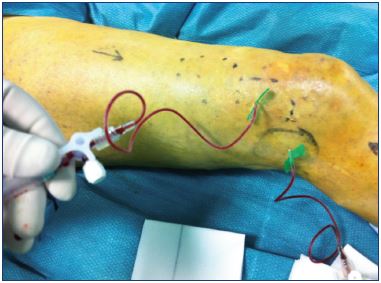
Figure 11. Alcohol is injected directly into the malformation
and outflow is controlled using a contrast injection, which is
administered before the alcohol.
Orthopedic techniques are effective if limb–length discrepancies develop.15 During childhood, epiphysiodesis is effective to temporarily block limb growth. The expected growth phase should be accurately predicted to determine when to implant the elongation device. In adults and after growth has stopped, limb elongation of the contralateral extremity is possible using the Ilizarov technique. Osteotomy to shorten the affected limb is performed less frequently.
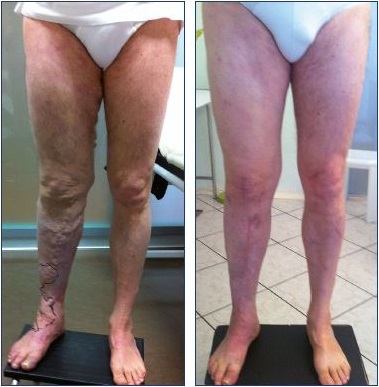
Figure 13. Results of surgical and alcohol treatment for Klippel- renaunay syndrome.
Diagnosis demonstrates abnormal, diffuse, superficial veins;
hypoplasia of the superficial femoral vein; and deep lymphatic
dysplasia. Panel A. Before treatment. Panel B. After treatment.
REFERENCES
1. Belov S. Classification of congenital vascular defects. Int Angiol. 1990;9(3):141-146.
2. Lee BB, Laredo J, Lee TS, Huh S, Neville R. Terminology and classification of congenital vascular malformations. Phlebology. 2007;22(6):249-252.
3. Wassef M, Blei F, Adams D, et al; ISSVA Board and Scientific Committee. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015;136(1):203- 214.
4. Lee BB, Laredo J, Neville RF, et al. Epidemiology of vascular malformations. In: Mattassi R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations: an Atlas of Diagnosis and Treatment. Milan, Italy: Springer; 2015: 165-169.
5. Mattassi R. Die Endovaskuläre Therapie venöser Malformationen (Endovascular treatment of venous malformations). In: Netzer F, ed. Endoluminale Varizentherapie. Berlin, Germany: Walter De Gruyter GmbH; 2015: 178.
6. Lee BB, Laredo J, Neville R, Mattass R. Primary lymphedema and Klippel- Trenaunay syndrome. In: Lee BB, Bergan J, Rockson SG, eds. Lymphedema. London, UK: Springer; 2011: 427-436.
7. Klippel M, Trenaunay P. Du naevus variqueux et osteohypertrophique. Arch Gen Med. 1900;3:641-672.
8. Weber FP. Haemangiectasic hypertrophies of the foot and lower extremity. Congenital or acquired. Med Press (London). 1908;136:261-266.
9. Weber FP. Haemagiectasis hypertrophy of limbs. Congenital phlebarteriectasias and so called congenital ‘varicose veins.’ Br J Child Dis. 1918;15:13-17.
10. Lee BB, Baumgartner I, Berlien P, et al. Guideline: diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP): updated-2013. Int Angiol. 2014 Jun 10. Epub ahead of print.
11. Mattassi R, Vaghi M. Management of marginal vein: current issues. Phlebology. 2007;22(6):283-286.
12. Dubois J, Alison M. Vascular anomalies: what a radiologist needs to know. Pediatr Radiol. 2010;40:895-905.
13. Dentici R, Mattassi R. Nuclear medicine diagnostics. In: Mattassi R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations: an Atlas of Diagnosis and Treatment. Milan, Italy: Springer 2nd Ed. 2015:223-236.
14. King K, Landrigan-Ossar M, Clements R, Chaudry G, Alomari A. The use of endovenous laser treatment in toddlers. J Vasc Interv Radiol. 2013;24(6):855-858.
15. Hauert J, Loose DA. Orthopedic problems. In: Mattassi R, Loose DA, Vaghi M, eds. Hemangiomas and Vascular Malformations: an Atlas of Diagnosis and Treatment. Milan, Italy: Springer; 2015: 369-378.
16. Maslow A. Psychology of Science. Anna Maria, FL, USA: Maurice Bassett: 1966.