Management of chronic deep venous obstructive disease
Kush Rajesh Desai, MD, FSIR
Northwestern University,
Feinberg School of Medicine
Division of Interventional
Radiology, Chicago, IL, USA
ABSTRACT
Chronic deep venous obstructive disease occurs in both the lower and upper extremities. Although they share similar features, particularly with respect to pathophysiologic changes associated with postthrombotic obstruction, they are clinically and epidemiologically distinct processes. As a result, there are significant differences in disease course, clinical approach, and management. While conservative measures, including elastic compression, remain the mainstay for symptom management, endovascular treatment of obstruction has become a vital approach for persistent, debilitating symptoms in both entities. Permanent stent placement remains far more common in lower-extremity obstruction due to iliofemoral/iliocaval outflow obstruction. By contrast, upper extremity obstruction frequently requires adjunctive open surgical approaches, most commonly in the setting of venous thoracic outlet syndrome, whereas open surgery is less common in lower-extremity disease and can include endophlebectomy of the common femoral vein and venovenous bypass for iliac/caval obstruction. In this chapter, the epidemiology, clinical course, and endovascular management of upper- and lower-extremity venous obstructive disease will be reviewed.
Introduction
Chronic lower-extremity deep venous disease
Lower-extremity deep venous occlusive disease is most commonly separated into postthrombotic and nonthrombotic etiologies. Postthrombotic syndrome (PTS) occurs with varying severity in approximately 50% of all patients after lower-extremity acute deep venous thrombosis (DVT).1 Involvement of the iliofemoral (common femoral, external iliac, and common iliac) venous segments and inferior vena cava (IVC) are frequently associated with more severe PTS symptoms, which can include pain/fatigue, severe edema, dermatitis, and soft tissue ulceration.2,3 The pathophysiology of PTS consists of a combination of luminal obstruction from organization of thrombus into type 3 and type 1 collagen, as well as inflammation that damages venous valves leading to reflux; together, they clinically result in ambulatory venous hypertension.4 Treatment of PTS is via both medical and interventional means. Elastic compression stockings (ECS) have not been shown to prevent development of PTS5; however, it is accepted as an important adjunct in symptom management and improvement in quality of life.6 Supervised exercise programs and venoactive medications, when tolerated, also play a role. Postthrombotic obstruction can be treated via the endovascular placement of self-expanding venous stents, which can reduce pain, edema, and promote ulcer healing.7 Treatment of superficial reflux is also an important component in the management of PTS.8
Nonthrombotic venous obstruction is most commonly caused by external compression of deep veins by arteries. Left iliac vein compression syndrome (frequently referred to as “May-Thurner syndrome”) is the most commonly encountered type, where there is compression of the left common iliac vein between the right common iliac artery and vertebral body; various other compression phenomena can occur, including obstruction of the external iliac vein by the ipsilateral external iliac artery. Although compression syndromes can result in acute iliofemoral DVT, they can also cause venous stasis symptoms in the absence of thrombus, including pain/fatigue, edema, ulceration, and in females, symptoms of female pelvic venous disease (commonly known as pelvic congestion syndrome); such lesions are commonly referred to as nonthrombotic iliac vein lesions (NIVLs). However, accurate estimation of the incidence of clinically significant NIVLs is difficult as they can nonpathologically present in a significant portion of the population and are asymptomatic9; thus, a thorough clinical evaluation to exclude other causes is mandatory prior to intervention. Self-expanding venous stents can be placed when treatment is indicated. Finally, extrinsic venous compression associated with adjacent malignancy can occur, and is variably associated with thrombosis of the impacted segment and inflow veins.
Chronic upper-extremity deep venous disease/thoracic central venous obstruction
Unlike lower-extremity venous obstruction, upper-extremity deep venous disease resulting from thoracic central venous obstruction (TCVO) can result from several different etiologies, including indwelling central venous devices, extrinsic compression from musculoskeletal compression or malignancy, and infectious/inflammatory processes. Anatomic compression of the subclavian vein between the first rib/clavicle or anterior scalene muscle/subclavius muscle/first rib can result in chronic occlusion typical of venous thoracic outlet syndrome (vTOS); when there is associated acute axillosubclavian DVT, this is also known as “Paget-Schroetter syndrome.” This entity is most commonly seen with repetitive motions, such as those seen in athletes.
Given the myriad causes and the variety of anatomy that can be affected by TCVO, the epidemiology remains poorly understood; similarly, the clinical course and management can vary significantly.10 Patients can present with severe symptoms such as pain, facial edema, and respiratory distress resulting from superior vena cava (SVC) syndrome or can be relatively asymptomatic. Thus, an individualized approach is necessary to effectively manage patients with TCVO.
Indications for intervention
Postthrombotic lower-extremity deep venous obstruction
Patients with moderate-to-severe PTS have a history of DVT in the index limb and most frequently have a component of iliofemoral and/or iliocaval venous occlusion/ obstruction on noninvasive imaging studies. Patients can have the numerous symptoms/findings to varying degrees; endovascular recanalization therapy is typically considered when symptoms persist despite conservative therapy including elastic compression and venoactive medications. Patients typically have symptoms that classify as C3-C6 disease by the clinical, etiologic, anatomic, pathologic (CEAP) scale; Villalta scores of 10 or greater/ venous clinical severity scores (VCSS) of 8 or greater are most frequently encountered. Patients can experience pain that limits or prevents normal activities of daily living and worsens with short periods of activity or standing or during walking (venous claudication). Edema is frequently present and involves the calf and thigh. Skin damage of the affected limb is common and can include eczema, subcutaneous fibrosis, lipodermatosclerosis, and/or atrophie blanche. The most severe manifestation of PTS is venous stasis ulceration, which is frequently present around the malleoli and pretibial soft tissues.
Nonthrombotic lower-extremity deep venous obstruction
Patients with NIVL may present with symptoms that include asymmetric lower-extremity edema, pain, heaviness, and/or fatigue of the affected extremity with activity or prolonged standing or during walking (venous claudication), and asymmetrically advanced superficial venous disease in the affected limb that may include venous stasis ulceration. Unlike PTS, patients with NIVL may have less than C3 disease, however, in such patients a significant portion of their symptoms must be lifestyle limiting venous claudication. In females, pelvic symptoms may occur, including pain with prolonged periods of standing and with intercourse, and also bladder symptoms. Patients with NIVL, by definition, have no known history of antecedent DVT.
Thoracic central venous obstruction
Symptoms of TCVO are highly variable and depend on the anatomical segment involved, causative factors, and comorbidities. SVC syndrome is most commonly secondary to malignant compression, though can be due to device- or catheter-related occlusion as well as infectious/inflammatory processes (eg, fibrosing mediastinitis). Patients can present with respiratory distress, severe facial/upper extremity edema, and inability to tolerate oral secretions. Note, no objective symptom grading scale for thoracic central venous occlusions is present. Urgent endovascular intervention and/or external radiation therapy is frequently indicated in these settings. However, patients are frequently minimally symptomatic, such as for dialysis patients with catheter induced TCVO.
vTOS is due to extrinsic compression of the subclavian vein by a cervical rib or muscular hypertrophy. When DVT occurs in this setting, this is known as Paget-Schroetter syndrome or “effort-induced” thrombosis and is frequently seen in athletes. Patients may present with sudden onset, unilateral upper-extremity edema.
Contraindications to intervention
The primary absolute contraindication to intervention, particularly for PTS and vTOS, is an absolute contraindication to anticoagulation. Such patients require durable anticoagulation therapy for intervention to be successful. Other absolute contraindications include active systemic infection, uncorrectable coagulopathy, severe contrast reaction refractory to steroid and antihistamine medications, current pregnancy, known severe allergies to stent material (eg, nickel allergy) and non–dialysis-dependent oliguria where contrast nephropathy is a concern; alternatively, carbon dioxide contrast or IVUS can be used based on operator experience.
Relative contraindications to intervention include anemia, likelihood of low benefit for intervention (eg, minimally symptomatic nonambulatory patients) and short life expectancy; palliative treatment of SVC syndrome can be considered in appropriate procedural candidates.
Preprocedure preparation
Postthrombotic lower-extremity deep venous obstruction
As with all venous disease, a thorough history and physical examination should be obtained, with attention to venous thromboembolism history, including prior episodes of acute DVT. Use and compliance with conservative measures, including compression stockings, venous return assist devices, and venoactive medications should be documented. A complete knowledge of the patient’s anticoagulation history is vital; assess for potential risk factors for bleeding from anticoagulation and expected level of patient compliance.
If patients are unlikely to derive benefit from PTS symptom reduction, intervention may not be indicated. For example, selection of patients that are nonambulatory or have a limited life expectancy may not be appropriate candidates for intervention. Thus, it is important to assess for comorbid conditions that may impact the success or safety of an intervention. Furthermore, many PTS patients have phlebolymphedema, which is chronic lymphatic damage that results from chronic venous obstruction and does not typically improve following endovascular intervention; in such cases, counseling and setting appropriate expectations for improvement are key.
Obtain index limb measurements for comparative purposes following intervention. Typically, measure ankle circumference 5 cm above the medial malleolus, calf circumference 5 cm below the tibial tubercle, and thigh measurement above the patella. If a prior or current venous stasis ulcer is present, assess length of time it was or has been present. If an active ulcer is present, obtain measurements of the ulcer and assess for infection. If infected, prescribe appropriate antibiotic therapy. If involving muscular, tendinous, or osseous structures, obtain wound care/surgical consultation for further management.
Evaluate patients according to venous disease scoring systems to guide decision-making. This includes the CEAP score, VCSS, and Villalta score.
Review noninvasive imaging studies. Evaluate abdominopelvic venous structures with computed tomographic or magnetic resonance venography (CTV or MRV, respectively), or IVC/ iliac duplex ultrasonography. The choice of imaging study will be dependent on local practice/physician preference and expertise. Assess for presence of >50% luminal stenosis or occlusion, length of stenosis, and predisposing factors that resulted in occlusion (eg, left iliac vein compression, or in an iliocaval occlusion, an obstructed IVC filter). Inflow assessment is vital to promote stent patency; assess venous inflow at the level of common femoral vein (CFV) with venous duplex ultrasound. If the CFV is occluded or severely stenotic, assess the femoral vein and profunda femoris vein (PFV) for flow and stenosis. The PFV in particular is critical in maintenance of stent patency in the absence of a normal CFV. Evaluate superficial veins for reflux as they may require treatment after successful deep venous recanalization. In patients with stasis ulceration, foam sclerotherapy of the ulcer bed is frequently necessary.
In patients undergoing recanalization, initiate anticoagulation prior to the procedure to ensure tolerance and compliance.
In the periprocedural and immediate postprocedural period, low molecular weight heparin (LMWH) is preferred by many due to consistent antithrombotic activity and theoretical anti-inflammatory properties. Anticoagulation is generally continued through the procedure.
Nonthrombotic lower-extremity deep venous obstruction
Obtain a thorough history and physical examination, focusing on venous disease–specific symptoms. Specifically, assess whether superficial venous disease workup and treatment has occurred, as well as trials of conservative therapy (eg, ECS). Given that asymptomatic patients frequently have compressions, it is important to assess for alternative explanations for symptoms. For example, lower-extremity edema can be caused by numerous disorders, including lymphedema, heart failure, hypoalbuminemia, and various medications including calcium channel blockers.
Similar to postthrombotic occlusions, imaging for venous compression is important in preprocedural planning. CTV/MRV and/or IVC/iliac duplex ultrasound are useful to assess for venous compression syndromes. On duplex ultrasound, the presence of ipsilateral internal iliac vein flow reversal may increase confidence that the compression is hemodynamically significant and therefore the cause of the patient’s symptoms. Similarly, a duplex ultrasound insufficiency on examination to assess for the presence of deep venous reflux (typically, >1 second) or nonphasic flow adds to diagnostic confidence.
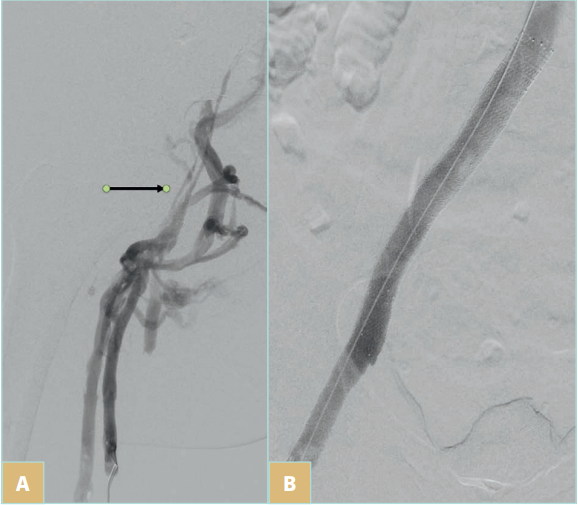
Figure 1. Postthrombotic left iliofemoral venous obstruction. A) Digital subtraction left common femoral venography of a patient in the prone position demonstrates postthrombotic obstruction of the left common femoral and left iliac vein. The arrow denotes the true lumen of the occluded iliac vein; other veins represent collateral drainage. B) Venography after stent placement demonstrates recanalized left common femoral and left iliac vein; collaterals are no longer visualized.
Thoracic central venous obstruction
While obtaining a history and physical examination, specifically evaluate for the cause and risk factors for the occlusion, as well as the time course and severity of symptoms, which will dictate urgency of intervention.
As TCVO can be caused by numerous factors and can have varying extents of disease, it is important to evaluate imaging to determine the feasibility and durability of a potential intervention. For example, in patients with SVC syndrome, it is important to assess the extent of SVC occlusion and cause (tumor, catheter/device). Focal SVC lesions tend to have better outcomes as inflow is preserved; involvement of inflow veins (eg, innominates/subclavian) can have a significant negative impact on long-term patency and treatment options. In vTOS, imaging can identify the compression lesion as well as the presence of associated acute thrombus, which would require concomitant thrombectomy/thrombolysis. In catheter/ device-related occlusions, imaging assists in evaluating the ongoing need for devices (eg, dialysis catheters, cardiac leads), as well as potentially involves the necessary subspecialists to assist in decision-making regarding necessity of intervention and/or device placement following recanalization.
Procedure
There are significant similarities in basic procedural techniques in deep venous recanalization, which are summarized in a stepwise fashion below. Where there are differences or variations, they are specifically noted.
Postthrombotic lower-extremity deep venous obstruction
1. Select a primary venous access site. The primary consideration is to ensure that inflow into a stent is preserved.
a. If the CFV is uninvolved, either CFV or great saphenous vein adjacent to the saphenofemoral junction may be used.
b. If the CFV is involved, either popliteal, small saphenous, posterior tibial, mid-thigh femoral, or internal jugular vein access may be used per operator preference. CFV access would compromise inflow and would place the stent at risk for occlusion.
c. For iliocaval or other complex occlusions, multiple accesses are necessary to visualize normal/pathologic anatomy and facilitate traversal of the occlusion.
2. Using a linear high-frequency ultrasound transducer for guidance, administer local anesthetic to the soft tissues and obtain ultrasound-guided access.
3. Place a sheath at the access site; typical sizes needed for chronic occlusions are 9 Fr or 10 Fr.
4. Perform venography to assess the extent of the occlusion.
5. Traverse the occlusion with a wire and catheter; most commonly, hydrophilic wires are used. Attempt to identify the occluded vein, which may be obscured by collaterals, by performing multiplanar venography (Figure 1). Note, a crossing/support catheter may be necessary to provide sufficient support for crossing the occlusion. The use of sharp/radiofrequency techniques is common but carries increased procedural risk and should only be performed by experienced operators.
6. Perform venographic confirmation of traversal into normal venous anatomy; pay specific attention to ensure that collateral vessels/azygos system are not mistaken for the IVC.
7. Perform intravascular ultrasound (IVUS) to assess the length of the occlusion (using both markers on the catheters and imaging findings), the inflow vessels (ie, PFV) in the event of CFV compromise, and cranial/caudal stent landing zones. Note, IVUS is of limited utility in selecting stent diameter in PTS given that there frequently is not a suitable “normal” reference vessel to measure.
8. Administer systemic anticoagulation prior to predilation and stent placement; typically 70-100 units/kg unfractionated heparin or bivalirudin/argatroban in patients with heparin-induced thrombocytopenia. It may be helpful to monitor activated clotting time through the remainder of the procedure at approximately 30-minute intervals, with a target of 200-300 seconds.
9. Pre-dilate the occluded venous segments to the target stent diameter via balloon angioplasty; typically, 14-16 mm in iliac veins and 12-14 mm in the CFV. The diameter chosen will depend upon the stent model that is selected. In the event of an iliocaval occlusion, pre-dilate to the target diameter of the selected stent; note that as of present, there are no on-label IVC stents, and there are several approaches to IVC stent placement, including stents designed for tracheobronchial applications, larger diameter iliofemoral approved stents, and placement of iliofemoral stents in a “double-barrel” configuration. Consider balloon angioplasty of the inflow vessels as needed to optimize inflow.
a. If an IVC filter was the cause for iliocaval occlusion, consider retrieval based on local filter retrieval expertise.11
10. Deploy on-label self-expanding venous stents in the occluded segments. Stent placement below the inflow of the PFV (roughly at the level of the lesser trochanter) is rarely performed due to low patency rates.
a. Sizing of stents depends on whether nitinol stents (which deploy true to size) or Elgiloy stents (a woven stent which has variable diameter based on its deployed length) are chosen. 11. Perform post-dilation balloon angioplasty of each stent to its rated diameter.
12. Perform venography and IVUS to assess luminal restoration and determine if there is adequate flow. Ensure that there is adequate coverage of the lesion by the stents.
13. Remove the sheath and achieve hemostasis with direct compression.
Variations in the procedure for NIVL
1. Nonthrombotic lesions can typically always be treated from groin (CFV, great saphenous) access.
2. IVUS has a significantly different role in NIVLs, as in most cases there is a “normal” reference segment. Classic teaching has been to select patients with at least 50% area stenosis at the lesion site relative to a normal vein segment; newer data suggests that patients that improve with stent placement typically have a >61% minimum diameter stenosis relative to a normal vein segment (Figure 2).12 Ensure that you do not compare with a prestenotically dilated common iliac vein; typically, the best segment for comparison is the external iliac vein. Similarly, use the external iliac vein as the reference segment when selecting stent size for placement; follow the instructions for use for sizing (there is some oversizing that is typical). Analysis of the published literature suggests that stents shorter than 60 mm in length and smaller than 14 mm in diameter had a greater likelihood of migration.13
3. Baseline therapeutic anticoagulation may not be necessary; these patients do not have thrombotic disease. Intraprocedural anticoagulation may be sufficient.
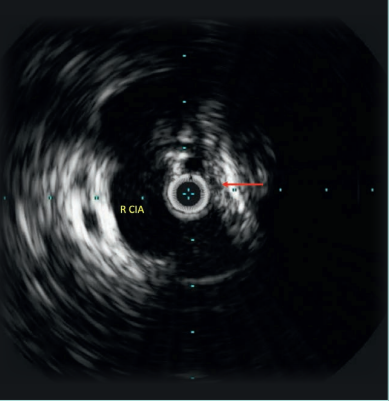
Figure 2. 10-MHz Intravascular ultrasound of the left common iliac vein (red arrow) demonstrates compression by the right common iliac artery (R CIA). Note that the vein wall is thickened and echogenic.
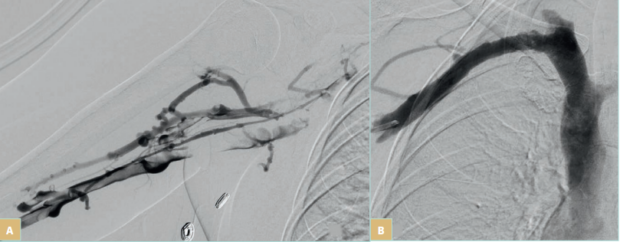
Figure 3. Acute right subclavian vein thrombosis. A) Digital subtraction right basilic venography demonstrates acute thrombotic occlusion through the axillosubclavian veins on the basis of venous thoracic outlet compression. B) Right subclavian venography following thrombectomy and angioplasty of the compression demonstrates subtotal luminal restoration. This patient went on to have transaxillary first rib resection to remove the cause of extrinsic compression.
Variations in the procedure for TCVO
1. Typical access sites are basilic, brachial, or cephalic veins. If additional sites are needed for complex occlusions, groin venous access may be of value.
2. Many of the tools for traversal of chronic lower-extremity postthrombotic occlusions apply here as well. Again, the use of sharp/radiofrequency techniques is common but carries increased procedural risk and should only be performed by experienced operators. Risks include mediastinal, lung, or cardiac injury, including tamponade. If using such techniques in close proximity to the heart, adequate surgical backup and preparation for emergent chest or pericardial drainage is mandatory.
3. For vTOS/Paget-Schroetter syndrome with acute thrombus, perform thrombectomy with a device of the operator’s choice (Figure 3). Notably, stent placement in these patients is not advised due to the high risk for stent fracture at the mechanical compression site, typically between the clavicle and first rib. Stent placement in this location should be reserved for highly selected scenarios with limited options.
4. No stent is specifically approved for used in TCVO, thus all placements are off-label. Stent size selection is variable with minimal data to provide guidance. Typically, 12- to 14-mm stents in the innominate veins are sufficient. For focal SVC stenosis, consider using IVUS to measure the uninvolved portion of the SVC for guidance on stent selection.
5. For extensive reconstructions involving subclavian and innominate veins and SVC, ensure that the patient’s symptoms are severe enough to dictate that an intervention is likely to be helpful, and that the inflow into the occluded segments appears sufficient to support stent patency.
Results
Postthrombotic recanalization results: A meta-analysis demonstrated a 1-year primary patency of 79% and 5-year projected patency of 60%.14 However, this data was with many stents being placed off-label and before techniques for treatment of PTS were refined to the level used today. Furthermore, several new on-label venous stents are available, which will lead to the generation of new data.
NIVL treatment results: Meta-analysis data suggests high primary patency rates at 1 year, approximately 96%, and projected 5-year patency rates of approximately 90%.14
These high patency rates are corroborated in numerous investigational device exemption trials.
TCVO recanalization results: Outcomes are difficult to assess, given the variety of anatomy that may be involved, the different contributing comorbidities, the lack of systematic approaches to treatment, and the lack of follow-up. Limited data are available on the treatment of Paget-Schroetter syndrome, with a meta-analysis suggesting clinical improvement in approximately 90% following thrombectomy and surgical decompression.15
Postprocedure management
For patients with postthrombotic occlusions or extensive TCVO, including vTOS/Paget-Schroetter syndrome, anticoagulation should be administered post procedure. LMWH is preferable early in the postprocedural course per expert consensus, due to its pleiotropic anticoagulant and anti-inflammatory effects. At 4 to 6 weeks, it likely can be transitioned to an oral agent. The duration of anticoagulation will depend on the nature and severity of disease, as well as the patient’s prior thrombosis history. Consider comanagement with a hematologist.
The use of antiplatelet agents in the setting of stents is controversial with little supporting data. Consider a short period of clopidogrel (3 months) followed by low-dose aspirin (81 mg) indefinitely. As stated above, NIVL patients may not require anticoagulation or antiplatelet therapy. Compression therapy for lower-extremity disease should be encouraged for symptom reduction. Starting at lower intensity, such as 20- to 30-mm-Hg compression, is reasonable if compliance is a concern; for severe postthrombotic disease, consider 30 to 40 mm Hg or higher, as tolerated. For patients with a component of phlebolymphedema, consider lymphedema therapy, including manual lymphatic drainage and pneumatic compression.
Imaging follow-up will be dictated by local preference and expertise and can include duplex ultrasound or CTV. MRV will be of limited utility for assessing stent patency due to metal related susceptibility artifact. Perform imaging and clinical follow-up at 1 month. Ongoing long-term follow-up should be considered for patients with extensive postthrombotic disease or complex TCVO, as reintervention may be needed to assist patency and address symptom recurrence.
Complications
Common procedural complications can occur, including bleeding from the access or intervention site, or infection related to an invasive procedure, including site infection or stent infection. Serious complications of this nature are rare with proper technique.
Pulmonary embolism is a very rare complication in this type of procedure. Stent occlusion is a complication that is not fully understood. The short-term consequences of loss patency and recurrence is known; however, the effect of a permanently implanted malfunctioning device is not.
Patients with chronic venous disease often require long-term anticoagulation, which carries a risk of bleeding diatheses. Ongoing assessment of the need for anticoagulation should occur in patients on long-term therapy.
Conclusion
The endovascular management of chronic venous obstructive disease has witnessed significant growth, mirrored by the rapid pace of device innovation and ongoing robust clinical trials. As more data emerges, proper patient selection, technical expertise, and diligent follow-up are necessary to ensure optimal outcomes.
CORRESPONDING AUTHOR
Kush Rajesh Desai, MD, FSIR
Chicago, IL, United States
email: kdesai007@northwestern.edu
References
1. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252.
2. Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139(9):1162-1173.
3. Strandness DE Jr, Langlois Y, Cramer M, Randlett A, Thiele BL. Long-term sequelae of acute venous thrombosis. JAMA. 1983;250(10):1289-1292.
4. Shull KC, Nicolaides AN, Fernandes é Fernandes J, et al. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg. 1979;114(11):1304-1306.
5. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383(9920):880-888.
6. Lurie F, Lal BK, Antignani PL, et al. Compression therapy after invasive treatment of superficial veins of the lower extremities: clinical practice guidelines of the American Venous Forum, Society for Vascular Surgery, American College of Phlebology, Society for Vascular Medicine, and International Union of Phlebology. J Vasc Surg Venous Lymphat Disord. 2019;7(1):17-28.
7. Neglen P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979-990.
8. Nayak L, Hildebolt CF, Vedantham S. Postthrombotic syndrome: feasibility of a strategy of imaging-guided endovascular intervention. J Vasc Interv Radiol. 2012;23(9):1165-1173.
9. Kibbe MR, Ujiki M, Goodwin AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937- 943.
10. Dolmatch BL, Gurley JC, Baskin KM, et al. Society of Interventional Radiology Reporting Standards for Thoracic Central Vein Obstruction: Endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI). J Vasc Interv Radiol. 2018;29(4):454-460e3.
11. Desai KR, Xiao N, Karp J, et al. Single session inferior vena cava filter removal, recanalization, and endovenous reconstruction for chronic iliocaval thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7(2):176-183.
12. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6(1):48- 56e1.
13. Sayed MH, Salem M, Desai KR, O’Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord. 2022;10(2):482-490.
14. Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10):e002772.
15. Peek J, Vos CG, Unlu C, van de Pavoordt H, van den Akker PJ, de Vries JPM. Outcome of surgical treatment for thoracic outlet syndrome: systematic review and meta analysis. Ann Vasc Surg. 2017;40:303- 326.
