Management of chronic venous disease: the example of MPFF at a dose of 500 mg
ABSTRACT
Chronic venous disease (CVD) is a common condition and represents a spectrum of disorders. The CEAP classification has been adopted worldwide to facilitate meaningful communication about the disease and to serve as a basis for more scientific analysis of treatment options. In addition to improved methods of defining CVD, there is also now increased understanding of the pathological processes involved in its development. Leukocyte-endothelium interactions are one of the earliest pathophysiological mechanisms at work and appear to be important in many aspects of the disease. The sequence of leukocyte adhesion, endothelial interaction, activation, and migration, and its association with valvular damage has focused attention on available molecules with activity known to modify this chain of events. The micronized purified flavonoid fraction (MPFF, MPFF at a dose of 500 mg), consisting of 90% diosmin and 10% other flavonoids expressed as hesperidin, diosmetin, linarin, and isorhoifolin, currently possesses the most appropriate profile. It has been shown to reduce leukocyte interaction with the endothelium in acute venous hypertension and inflammation and is used clinically to treat CVD.
INTRODUCTION
Chronic venous disease (CVD) is a common presenting condition in industrialized countries, although prevalence estimates vary depending on the design of the particular study, the population sampled, and the disease definition used.1 This is illustrated by data from the most representative epidemiological studies of the last 10 years, which have observed prevalence rates ranging from 40% to 90%. General population-based surveys in Germany,2 Italy,3 Scotland,4 and the USA5 have reported CVD prevalence rates of 90%, 77%, 85%, and 71%, respectively. These surveys recruited adults on a spontaneous and voluntary basis, by selecting them from registers2,4 or by print or television advertising.3,5 The high prevalence of disease in these surveys might indicate that respondents were more likely to have a history of diagnosed venous disease than nonrespondents.4
Although CVD is often mild in presentation, it is potentially a progressive condition.
Despite this it is often deemed a minor problem and frequently ignored by patients and physicians alike. In the past, one of the reasons for this may have been the lack of a standard definition for a disease, which covers a broad spectrum of venous complaints, from telangiectasias to varicose veins and ultimately to chronic venous ulcers, all of which are grouped in the same category of diseases. However, over the past decade the diagnosis and treatment of CVD has developed rapidly. An important aspect of this has been the development of a universal classification system that allows physicians to clearly delineate between the different types of venous complaints, thus facilitating comparisons between studies. The CEAP classification was introduced in 19946 and characterized each of the seven clinical classes (C0-C6) by a subscript for the presence of symptoms (S, symptomatic) or absence of symptoms (A, asymp – tomatic) (see Table I).
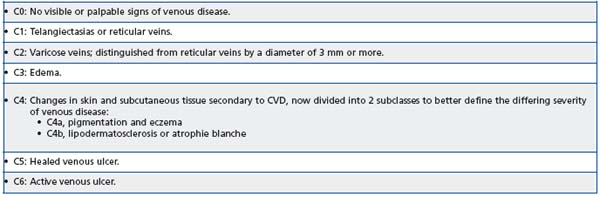
Table I. Clinical definitions of the CEAP classification.7
In 2004, a revision of the classification further refined the definitions of CVD, important amendments being the introduction of subclasses in skin changes (C4a and C4b) and the addition of a new descriptor, n, for E, A, and P items when no venous abnormality is identified.7 This made it possible to classify the often encountered C0s, En, An, Pn subject, which describes a patient with socalled venous symptoms but without any visible or detectable sign of CVD (usually termed “C0s patient”).
Most recently, the VEIN TERM consensus document has been developed to provide universal agreement on the definition of many widely used clinical venous terms.8 In this document, the term chronic venous disorder encompasses the full spectrum of venous abnormalities, while CVD is reserved for the major subset of individuals with venous complaints and/or manifestations requiring investigation or care or both.8 The term chronic venous insufficiency is reserved for those with advanced signs. The document also provides recommendations for venous clinical terminology based on the type of symptoms as well as aggravating factors. Thus, venous symptoms are defined as tingling, aching, burning, pain, muscle cramps, swelling, sensations of throbbing or heaviness, itching skin, restless legs, leg tiredness and/or fatigue, which may be exacerbated during the course of the day or by heat, but is relieved with leg rest or elevation or both. The document defines venous signs as visible manifestations of venous disorders, which include dilated veins (telangiectasias, reticular veins, varicose veins), leg edema, skin changes, and ulcers, as described in the CEAP classification.7
CVD may be associated with a wide range of lower limb symptoms, which may be present from the outset even before any visible signs of CVD have been identified. The recent clarifications of the definitions of CVD should help physicians identify patients who may be amenable to effective medical therapy. A number of venoactive drugs of plant or synthetic origin are available for the treatment of the symptoms of CVD, as illustrated in Table II.9,10 Using the example of micronized purified flavonoid fraction (MPFF, MPFF at a dose of 500 mg, Servier, France), this review will provide an update on the pathological processes involved in CVD and illustrate the benefits of treating the underlying disease process.
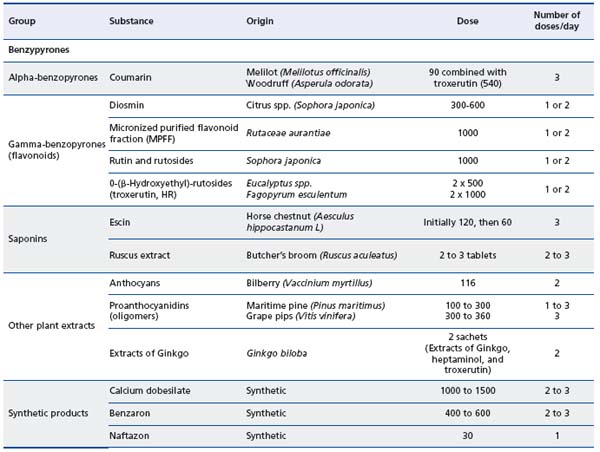
Table II. Classification of the main venoactive drugs.9,10
RECENT ADVANCES IN OUR UNDERSTANDING
OF THE PATHOPHYSIOLOGY OF CHRONIC
VENOUS DISEASE
The venous inflammatory theory
Despite the diversity of signs and symptoms associated with CVD, venous hypertension appears to underlie most or all signs of the condition and in most cases, is caused by reflux through incompetent venous valves.11 While the valves and vein walls can withstand raised venous pressure for limited periods, when it is prolonged adverse effects occur. A number of theories have been put forward to explain how venous hypertension may lead to venous reflux through failed valves, but a prominent role for an inflammatory reaction in the process of venous valve destruction emerged when it was observed that the valve leaflets and venous wall from vein specimens of patients with CVD were infiltrated with monocytes and macrophages, whereas control specimens were not.12 Over the past decade evidence for the inflammatory theory has accumulated and it is now widely recognized to play a key role in the pathogenesis of CVD.11
The trigger for the inflammatory process is thought to be abnormal venous flow in the form of altered shear stress. While shear stress is important in maintaining healthy vasculature, low shear stress, such as that associated with venous hypertension, promotes the expression of inflammatory gene products by the endothelium11 and results in enhanced leukocyte activation, adhesion, and migration through the endothelium of venous valves and the vein wall.12,13 The acute effects of increased venous pressure cannot be readily studied in man, but a number of animal models are available and have provided information on the mechanisms involved in valve destruction. In a rat model of venous occlusion, the effects of venous hypertension could be observed by comparing regions on either side of the occlusion.14
Venous pressure was only elevated on the upstream side of the occlusion and was associated with increased rolling, adherent, and migrating leukocytes as well as with increased numbers of apoptotic cells in the parenchyma, which are markers of the inflammatory cascade and tissue injury.14
Elevation of femoral venous hypertension by a femoral arterial-venous fistula for a period of 3 weeks in a rat saphenous vein model has revealed that valve failure occurs as a result of dilation of the venous wall and shortening of valve leaflets eventually leading to incomplete valve closure and subsequent reflux.15 Examination of the valves revealed infiltration with leukocytes including granulocytes, monocytes, and T-lymphocytes. In addition, the expression of the endothelial cell membrane adhesion molecules, Pselectin and ICAM-1, on the endothelial cells of the saphenous vein wall was increased. Activated leukocytes on the venous endothelium migrate into the venous wall and produce toxic metabolites and free oxygen radicals that participate in valve destruction and venous wall weakening. Enzymatic degradation of the valve leaflets and venous wall by metalloproteinases is thought to play a key role in this process through their degradation of elastin and collagen.16 The leukocyte-endothelium interaction resulting from altered shear stress contributes to the inflammation and subsequent remodeling of the venous wall and valves. Incompetent valves allow reflux to occur, reinforcing venous hypertension and perpetuating disease progression (Figure 1).
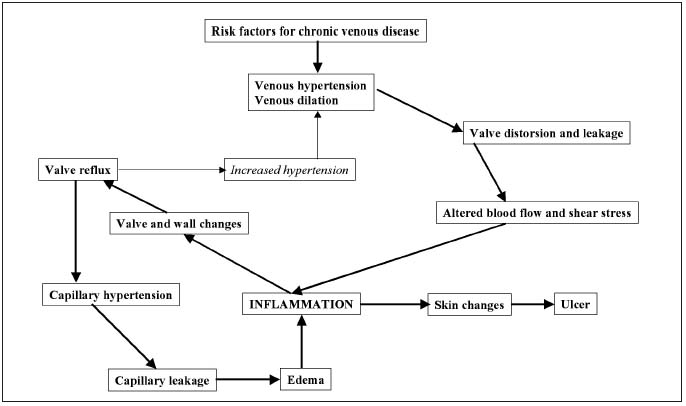
Figure 1. The vicious circle of venous hypertension/venous inflammation. Adapted from Bergan et al, 2006.11
Hypotheses on venous pain and the C fiber theory
Venous pain is a common complaint in patients with CVD and is associated with all stages of the disease. It is now thought that the pain related to CVD may mirror endothelial activation and the cascade of inflammatory mediators released early in the disease process.17 Pain of venous origin can be induced by both mechanical and chemical stimuli and it is suggested that the release of proinflammatory mediators as part of the inflammatory cascade may activate nociceptors located in the venous wall and in the connective tissue, as well as causing capillary damage and leakage resulting in swelling and pressure on nerve endings. In later stages of the disease, remodeling and stretching of the venous wall due to the inflammatory process may also stimulate nociceptors in the venous wall. With such a theory, it would be expected that the levels of some inflammatory markers would be correlated with the intensity of pain in CVD. However, no such correlation has been found, and there is also no association between pain and the severity of the disease. For example, pain is often more severe in the early stages of CVD when superficial venous distensibility is slight than in more advanced stages when pressure on the venous wall is high. This has led researchers to suggest that the cascade of inflammatory reactions that activate venous nociceptors can occur before any significant remodeling of large venous vessels arises. This may account for the occurrence of pain from the earliest stages of CVD. The fact that venous pain is not closely correlated with vein remodeling or incompetent valves suggests that the primary activation site of venous nociceptors may not be in the large venous vessels, but in the microcirculation, where contact between nerve endings, the arteriole, the vein, and the capillary is much closer.17 The involvement of an inflammatory process in the pain related to CVD is important as it suggests that pharmacological treatments acting on venous inflammation may provide relief from this symptom.
THE MECHANISM OF ACTION OF VENOACTIVE
DRUGS AND MPFF (MPFF at a dose of 500 mg)
Most venoactive drugs prolong the vasoconstrictor effect of noradrenaline on the vein wall, increasing venous tone, and thereby reducing venous capacitance, distensibility, and stasis. This increases the venous return and reduces venous pressure in patients suffering from CVD. Studies of the mechanism of action of MPFF have shown that it prevents or delays the occurrence of CVD by: (i) increasing venous tone (this results in restoration of normal blood flow, dispersion of red cell aggregates, and better oxygenation); (ii) improving capillary hyperpermeability and lymph flow thus protecting the microcirculation and decreasing the risk of edema; and (iii) inhibiting leukocyte adhesion to endothelial cells and the transmigration of leukocytes into the venous wall (an effect seen only with MPFF).
Venous tone
The beneficial effects of MPFF on venous tone have been studied in three double-blind, placebo-controlled trials in patient populations with varying degrees of CVD including women with venous insufficiency related to a postthrombotic syndrome,18 venous insufficiency related to pregnancy,19 and women without any venous pathology.20 MPFF at a dose of two tablets per day had an acute effect on increasing venous tone beginning 1 hour after administration in all three groups of women. In the trial of women without any venous pathology,20 MPFF significantly improved venous distensibility for 4 hours after administration compared with placebo (P<0.05). When treatment was continued for 1 week, the significant effect on venous distensibility compared with placebo was maintained for 24 hours (P<0.05).
In a study aimed at determining the effect of MPFF in 25 female volunteers aged 18-35 years with abnormal venous elasticity, but without varicose veins,21 twelve women received a single dose of two tablets of MPFF for 4 weeks and 13 women in the control group received no treatment. Venous tone was significantly improved compared with baseline in patients receiving MPFF (P<0.02). In contrast, venous elasticity did not change significantly from baseline in patients in the control group.
Capillary hyperpermeability
When subjected to prolonged venous hypertension, capillaries become elongated and dilated and develop abnormal permeability. The increased permeability causes interstitial edema. The beneficial effects of MPFF on capillary hyperpermeability have been demonstrated in two trials.22,23 In a 6-week, placebo-controlled trial in 30 patients with idiopathic cyclic edema, MPFF significantly improved capillary hyperpermeability (as measured by labeled albumin retention) compared with placebo (P<0.05).22 This was accompanied by a mean weight loss of at least 1.5 kg and a decreased sensation of swelling indicating a concomitant decrease in edema. In a 4-week study in patients with venous hypertension, MPFF given either two or three times daily significantly decreased the capillary filtration rate from baseline values in a dose-dependent manner (P<0.05).23
Lymphatics
In patients with advanced CVD, there is an increase in intralymphatic pressure and diameter, and in permeability of lymphatic capillaries leading to the transendothelial diffusion of fluids.24 MPFF is thought to improve lymph flow by increasing both the frequency and amplitude of contraction of lymphatic capillaries, as well as increasing the number of functional capillaries. This reduces edema by facilitating the drainage of interstitial fluid into the lymphatic system. In a 4-week study in 24 patients with severe CVD, but no active ulceration, MPFF significantly decreased the diameter of lymphatic capillaries and the intralymphatic pressure from baseline (P<0.001).25 In addition, the number of functional lymphatic capillaries was also significantly increased (P<0.001).
Leukocyte-endothelial interactions
The well-established role of leukocytes in the pathophysiology of CVD has focused attention on drugs able to block leukocyte adhesion to the venous valves and wall and thereby stop venous inflammation very early in the disease process. MPFF is up to now the only available venoactive drug with documented evidence of its ability to attenuate the effects of various mediators of the inflammatory cascade, particularly leukocyteendothelial interactions, which are important in many aspects of the disease.
For more than 10 years it has been accepted that CVD is related to a primary failure of venous valves that are affected by inflammation.15,16 As a result, guidelines now mention that early pharmacological treatment directed towards preventing or inhibiting the inflammatory response at all stages of the disease may play a significant role in preventing or slowing the development and recurrence of the signs and symptoms of CVD.26 Key findings were those provided by the rat fistula model of venous hypertension with MPFF.15 In this model, venous hypertension caused by a femoral arterial-venous fistula resulted in the development of venous reflux and an inflammatory reaction in venous valves. In animals treated with MPFF there was a significant, dosedependent reduction in the reflux rate. MPFF also inhibited the expression of the endothelial cell adhesion molecules P-selectin and ICAM-1, reduced leukocyte infiltration, and decreased the level of apoptosis in the valves in a dose-dependent manner. These data suggest that in the rat model of venous hypertension, MPFF delays the development of reflux and suppresses damage to the valve structures by decreasing the interaction between leukocytes and endothelial cells.
The above observations were confirmed in a study using the same animal model.16 The administration of MPFF reduced the edema and the fistula blood flow produced by the acute arterial-venous fistula. MPFF also reduced granulocyte and macrophage infiltration of valves.
As a consequence of these findings, the most recent guidelines on the management of chronic venous disorders of the lower limbs have expanded the recommendations for the use of venoactive drugs following the realization that the beneficial effects of these agents is not just due to their effects on venous tone.26
INDICATIONS FOR VENOACTIVE DRUGS
CVD may be associated with a wide range of lower limb symptoms, and these may be present in patients suffering from any class of the CEAP classification for CVD (C0s–C6s). Leg heaviness, discomfort, itching, cramps, pain, paresthesia, and edema are the most frequent manifestations of CVD and a major reason for medical consultation.
Venoactive drugs may be indicated as a first-line treatment for CVD-related symptoms and edema in patients at any stage of disease.9,26 In the most recent guidelines for the management of chronic venous disorders of the lower limbs, three agents receive a Grade A level of evidence for their effects on venous symptoms: calcium dobesilate, hydroxyethyl-rutosides, and MPFF.9,26
In patients with advanced CVD, venoactive drugs may be used in conjunction with sclerotherapy, surgery, compression therapy or a combination thereof.9,26 MPFF is useful for first-line management of edema as well as associated symptoms of CVD. It continues to be effective at all subsequent stages of the disease and is the only venoactive drug proven to have an additional beneficial effect on leg ulcer healing in a meta-analysis of 5 trials.27 On the basis of this meta-analysis, MPFF was assigned a grade 2B in the treatment of venous leg ulcers in patients with venous thromboembolic disease28 and a grade 1B in the healing of long-standing or large venous ulcers.29 In these guidelines, the authors had reviewed the evidence for therapies added to conventional compression.
CLINICAL EFFICACY OF THE VENOACTIVE DRUGS: THE EXAMPLE OF MPFF
(MPFF at a dose of 500 mg)
Symptoms of CVD
A review of the data for MPFF show that it is effective from the earliest stages of CVD, including in patients with a C0s classification, and that symptom relief is achieved rapidly and sustained. The efficacy of MPFF’s relief of clinical symptoms has been evaluated in two placebo-controlled trials in which the following symptoms were considered: functional discomfort, leg heaviness, pain, fatigue when standing, night cramps, paresthesia, burning sensation, itching, sensation of edema in the evening (summarized in Table III).30,31 In the first trial of 40 patients with CVD, MPFF was associated with a significantly greater improvement in many of the symptoms of CVD compared with placebo with P-values for the global scores of P<0.001.30 In a second placebo-controlled trial of 160 patients with CVD, MPFF was again associated with a significant improvement in symptoms compared with placebo.31 For the symptoms of functional discomfort, sensation of heaviness, nocturnal cramps, and sensation of swelling, these changes were significant after 4 weeks of treatment.
The effects of MPFF on the symptoms of CVD have also been compared with nonmicronized diosmin in a study of 88 patients.32 While statistically significant improv – ements in all subjective symptoms were noted in both treatment groups at the end of 2 months, MPFF was more effective than diosmin for the majority of response measures.
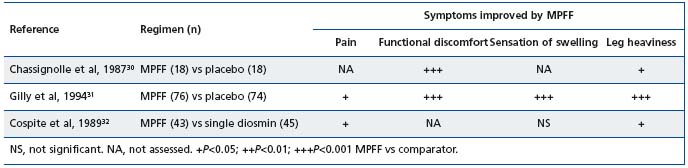
Table III. Clinical efficacy of MPFF in alleviating venous symptoms (adapted from Chassignole et al, 1987;30 Gilly et al, 1994;31
Cospite et al, 198932)
Symptoms after stripping surgery
The benefits of MPFF as part of the pharmacological preoperative care and post-operative recovery for patients with varicose veins who undergo phlebectomy have been evaluated in two trials.33-36 In both studies, MPFF helped to attenuate pain, decrease postoperative hematomas and accelerate their resorption, and to increase exercise tolerance in the early post-operative period. Pre-operative management included prolonged administration of MPFF (4-6 weeks) and compressive therapy in cases of varicose veins with lymphostasis. Post-operatively, MPFF was continued for at least 4 weeks.33,34
Symptoms associated with pelvic congestion syndrome
Venous dysfunction and stasis may be pathophysiologic components of pelvic pain in women with pelvic congestion syndrome. As venous leg disorders have been reported in cases of pelvic congestion syndrome, MPFF treatment has been evaluated in a trial of 20 women with chronic pelvic pain diagnosed with pelvic congestion syndrome by laparoscopy.37 In a cross-over study, 10 women were randomized to receive MPFF 500 mg twice daily for 6 months, and ten a vitamin pill for placebo effect. After 6 months, mean pain scores were significantly lower in the MPFF group compared with placebo (P<0.05).
Leg edema
In the three trials assessing the efficacy of MPFF’s relief of the symptoms of CVD, measures of edema were also taken and all three trials demonstrated a significant correlation between the improvements in the symptom score of sensation of swelling and a decrease in ankle circumference (Table IV).30-32 Three further studies that have used different methods to quantify leg edema have also demonstrated beneficial effects of MPFF. In two placebo-controlled trials of patients with either symptoms or signs of CVD, MPFF resulted in significant reductions in ankle circumference compared with placebo.30,32 In a third study, edema was assessed using a volumeter in 20 patients with varicose veins or postthrombotic syndrome. MPFF was associated with a significant decrease in volume of the more affected lower leg of 263 ml (8%) in all patients and 392 ml (12%) in patients with varicose veins.39 Finally, edema, measured by leg circumference, was also significantly decreased compared with baseline in the Reflux assEssment and quaLity of lIfe improvEment with micronised Flavonoids (RELIEF) study.40
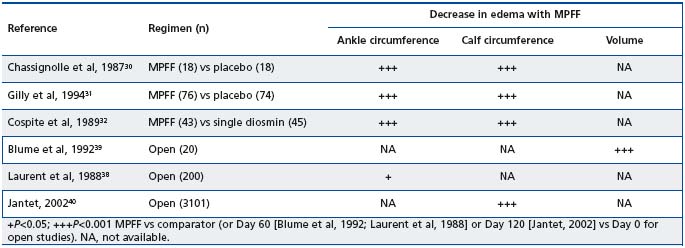
Table IV. Clinical efficacy of MPFF in reducing venous edema (adapted from Chassignole et al, 1987;30 Gilly et al, 1994;31 Cospite et al,
1989;32 Blume et al, 1992;39 Laurent et al, 1988;38 Jantet, 200240).
Leg ulcer healing
MPFF remains of significant benefit for patients at advanced stages of CVD where it may be used with conventional therapy to promote ulcer healing. The efficacy of MPFF in augmenting the healing of venous ulcers has been demonstrated in three randomized, controlled, multicenter trials in which MPFF plus standard venous leg ulcer management was compared with standard venous leg ulcer management (compression therapy plus local treatment) alone41,42 or in addition to placebo43 (Table V).
In all three studies, there was a significantly higher ulcer healing rate in patients treated with MPFF than in the control group. In the Glinski et al study, ulcers with a diameter less than 3 cm were cured in 71% of the MPFF group and 50% of the standard therapy group.41 In the Roztocil et al study, the time to achieve complete healing was significantly shorter in the MPFF group (137 days for MPFF versus 166 days for the control group, P=0.042), and a significantly greater number of patients had complete ulcer healing with MPFF (64.6%) compared with the control group (41.2%, P=0.04).42
A meta-analysis in which 723 patients with venous ulcers treated with MPFF were pooled confirmed that venous ulcer healing is accelerated by adding MPFF to conventional treatments.27 At 6 months, the chance of a healing ulcer was 32% better in patients treated with adjunctive MPFF than in those managed by conventional therapy alone (relative risk reduction: 32%; 95% CI, 3% to 70%; P=0.03). Subgroup analyses suggested that the benefits of MPFF were greatest in ulcers > 5 cm2 and > 6 months in duration.
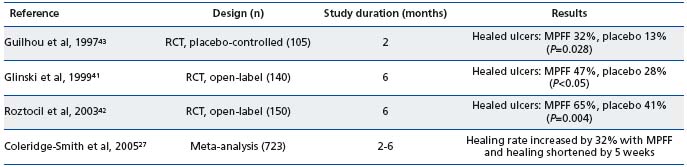
RCT, randomized clinical trial
Table V. Clinical efficacy of MPFF in healing venous ulcers (adapted from Kearon et al, 200828).
CONCLUSION
CVD is a common condition, but epidemiological studies often only focus on one aspect of the disorder, for example varicose veins, and therefore true prevalence rates are difficult to determine. In reality, CVD represents a spectrum of disorders ranging in severity from leg pain, swelling, edema, and skin changes, to venous ulcers. While the CEAP classification has been paramount in providing a uniform basis on which to present diagnostic and treatment results, it is less useful for epidemiological research because of the many subgroups of CVD it distinguishes. In this regard, the recent VEIN-TERM consensus document might prove invaluable in providing uniform recommendations for venous terminology. The use of a common scientific language to allow the global dissemination of knowledge is essential in the rapidly developing field surrounding the investigation and management of CVD.
In order to develop effective treatment regimens for CVD, a clear understanding of the underlying pathological processes is required. Research advances have led to an appreciation of the importance of chronic inflammatory processes throughout the course of the disease and have clarified some of the mechanisms involved in its progression. The wealth of preclinical studies that have been conducted with MPFF have been instrumental in establishing that an interaction between leukocytes and endothelial cells marks the beginning of all pathophysiology in venous disorders and that the resulting inflammation leads to primary failure of venous valves. This discovery has important implications for preventing the development of CVD and, in particular, for treating the C0s class in which patients have symptoms despite the absence of any visible signs of CVD. Patients in this class may be presenting with the earliest form of CVD and treating them may therefore delay further development of the disease. Optimal therapy for CVD would normalize venous physiology, resolving the inflammatory cascade that results in adverse effects on valves and vein walls. While a number of venoactive drugs are available, currently only MPFF has an evidence base demonstrating that it has both acute and long-term anti-inflammatory effects on the venous valves in chronic conditions of venous hypertension. This is translated into clinical benefits with a number of randomized, controlled, double-blind studies demonstrating MPFF’s efficacy in treating the symptoms of CVD, skin changes (C4a-b, C5), and venous leg ulcers (C6). The strength of the evidence is reflected in recent international guidelines, which are strongly evidenced-based and assign MPFF high levels of recommendation for the treatment of patients at all stages of CVD. The management of CVD has therefore reached a point where improved definitions of the disease and understanding of the pathophysiological processes involved have come together to facilitate earlier treatment of CVD with targeted agents. In the long term this may prevent the more serious consequences of the disease, while at the same time improving patient quality of life.

REFERENCES
2. Rabe E, Pannier-Fischer F, Bromen K, et al. Bonner Venenstudie der Deutschen Gesellschaft für Phlebologie. Phlebologie 2003;32:1-14.
3. Chiesa R, Marone EM, Limoni C, Volonte M, Schaefer E, Petrini O. Chronic venous insufficiency in Italy: the 24-cities cohort study. Eur J Vasc Endovasc Surg 2005;30:422-429.
4. Evans CJ, Fowkes FGR, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population. Edinburgh Vein Study. J Epidemiol Community Health 1999;53:149-153.
5. McLafferty RB, Passman MA, Caprini JA, et al. Increasing awareness about venous disease: the American Venous Forum expands the National Screening Program. J Vasc Surg 2008;48:394-399.
6. Porter JM, Moneta GL. International Consensus Committee on Chronic Venous Disease: reporting standards on venous disease: an update. J Vasc Surg 1995;21:635-645.
7. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg 2004;40:1248-1252.
8. Eklöf B, Perrin M, Delis KT, Rutherford RB; the VEIN-Term Transatlantic Interdisciplinary Faculty. Updated terminology of chronic venous disorders: the VEIN-Term Transatlantic Interdisciplinary consensus document. J Vasc Surg 2009;49:498-501.
9. Ramelet AA, Boisseau MR, Allegra C, et al. Veno-active drugs in the management of chronic venous disease. An international consensus statement: current medical position, prospective views and final resolution. Clin Hemorheol Microcirc 2005;33:309- 319.
10. Ramelet AA, Perrin M, Kern P, Bounameaux H. Phlebology. 5th ed. Issy les Moulineaux, France: Elsevier Masson; 2008. 11. Bergan JJ, Schmid-Schönbein G, Coleridge-Smith P, Nicolaides A, Boisseau M, Eklof B. Chronic venous disease. N Engl J Med 2006;355:488-498.
12. Ono T, Bergan JJ, Schmid-Schönbein GN, Takase S. Monocyte infiltration into venous valves. J Vasc Surg 1998;27:158-166.
13. Takase S, Bergan JJ, Schmid- Schönbein GW. Expression of adhesion molecules and cytokines on saphenous veins in chronic venous insufficiency. Ann Vasc Surg 2000;14:427-435.
14. Takase S, Lerond L, Bergan JJ, Schmid- Schönbein GW. Enhancement of reperfusion injury by elevation of microvascular pressures. Am J Physiol Heart Circ 2002;282:H1387-H1394.
15. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg 2004;28:484-493.
16. Pascarella L, Lulic D, Penn AH, et al. Mechanisms in Experimental Venous Valve Failure and their Modification by MPFF at a dose of 500 mg. Eur J Vasc Endovasc Surg 2008;35:102-110.
17. Danziger N. Hypothesis on the origin of pain. Phlebolymphology 2008;15:107- 114.
18. Amiel M, Barbe R. Etude du délai et de la durée d’action de MPFF at a dose of 500 mg. JIM 1987;88:22-24.
19. Barbe R, Amiel M. Pharmacodynamic properties and therapeutic efficacy of MPFF at a dose of 500 mg. Phlebology 1992;7 (suppl 2):41-44.
20. Amiel M, Barbe R. Etude de l’activité pharmacodynamique de MPFF at a dose of 500 mg. Ann Cardiol Angéiol 1998;47:185-188.
21. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology 1997;48:45-49.
22. Behar A, Lagrue G, Cohen-Boulakia F, et al. Study of capillary filtration by double labelling I131-albumin and Tc99m red cells. Application to the pharmacodynamic activity of MPFF at a dose of 500 mg. Int Angiol 1988;7(2 suppl):35- 38.
23. Cesarone MR, Laurora G, De Sanctis MT, et al. Capillary filtration and ankle edema in patients with venous hypertension: effects of Daflon. Angiology 1993;44:57-61.
24. Struckmann JR. Clinical efficacy of micronized purified flavonoid fraction: an overview. J Vasc Res 1999;36(Suppl 1):37-41.
25. Allegra C, Bartolo MJr, Carioti B, et al. Microlymphography: assessment of MPFF at a dose of 500 mg activity in patients with chronic venous insufficiency. Lymphology 1997;31(Suppl):12-16.
26. Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol 2008;27:1-59.
27. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg 2005;30:198-208.
28. Kearon C, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest 2008;133:454S–545S.
29. Coleridge Smith PD. Drug treatment of varicose veins, venous edema, and ulcers. In: Gloviczki P, ed. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 3rd ed. London, UK: Hodder Arnold; 2009: 359-365.
30. Chassignolle J-F, Amiel M, Lanfranchi G, et al. Activité thérapeutique de MPFF at a dose of 500 mg dans l’insuffisance veineuse fonctionnelle. J Int Med 1987;99(Suppl):32-37.
31. Gilly R, Pillion G, Frileux C. Evaluation of a new venoactive micronized flavonoid fraction (S 5682) in symptomatic disturbances of the venolymphatic circulation of the lower limb: a double-blind, placebocontrolled study. Phlebology 1994;9:67-70.
32. Cospite M, Dominici A. Double blind study of the pharmacodynamic and clinical activities of 5682 SE in venous insufficiency. Advantages of the new micronized form. Int Angiol 1989;8(4 Suppl):61-65.
33. Pokrovsky AV, Saveljev VS, Kirienko AI et al. Surgical correction of varicose vein disease under micronized diosmin protection (results of the Russian multicenter controlled trial DEFANS). Angiol Sosud Khir. 2007;13(2):47-55.
34. Pokrovsky AV, Saveljev VS, Kirienko AI et al. Stripping of the great saphenous vein under micronized purified flavonoid fraction (MPFF) protection (results of the Russian multicenter controlled trial DEFANCE). Phlebolymphology. 2008;15:45-51.
35. Veverkova L, Kalac J, Jedlicka V et al. Analysis of surgical procedures on the vena saphena magna in the Czech Republic and an effect of Detralex during its stripping. Rozhl Chir. 2005;84:410-12.
36. Veverkova L, Kalac J, Jedlicka V et al. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of MPFF at a dose of 500 mg to postoperative symptoms. Phlebolymphology 2006;13:195-201.
37. Simsek M, Burak F, Taskin O. Effects of micronized purified flavonoid fraction on pelvic pain in women with laparoscopically diagnosed pelvic congestion syndrome: a randomized crossover trial. Clin Exp Obstet Gynecol. 2007;34:96-98.
38. Laurent R, Gilly R, Frileux C. Clinical evaluation of a venotropic drug in man. Example of MPFF at a dose of 500 mg. Int Angiol. 1988;7(Suppl):S34-S43.
39. Blume J, Langenbahn H, de Champvallins M. Quantification of oedema using the volometer technique: therapeutic application of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology 1992; (Suppl 2):37-40.
40. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF study. Angiology 2002;53:245-256.
41. Glinski W, Chodynicka B, Roszkiewicz J, et al. The beneficial augmentative effects of micronised purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open multicenter, controlled, randomized study. Phlebology 1999;14:151-157.
42. Roztocil K, Stvrtinova V, Strejcek J. Efficacy of a 6-month treatment with MPFF at a dose of 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol 2003;122:24-31.
43. Guilhou JJ, Dereure O, Marzin L, et al. Efficacy of MPFF at a dose of 500 mg in venous ulcer healing: a double-blind, randomized, controlled versus placebo trial in 107 patients. Angiology 1997;48:77-85.
