Management of chronic venous insufficiency patients presenting an axial deep reflux in isolation orcombined with iliac vein obstruction
Sanno Hospital, Japan
Abstract
The pathology of chronic deep venous incompetence often involves postthrombotic syndrome, secondary to an episode of deep venous thrombosis. Obstruction of the iliac vein and valve incompetence of the femoral/popliteal veins produce symptoms caused by the resulting chronic venous hypertension in the legs. The clinical symptoms are common symptoms of chronic venous insufficiency: varicose vein formation, swelling of the lower-limbs, heaviness, venous claudication, stasis dermatitis/pigmentation, and ulceration; these are generally severe and refractory in cases of deep venous incompetence. Diagnosis and assessment are based on ultrasonography, similarly to general venous disease. As deep venous incompetence necessitates integrated assessment of the veins of the legs, including the inferior vena cava and iliac veins, assessment requires computed tomography, magnetic resonance imaging, venography, and even endovascular assessment with intravascular ultrasound. The initial approach for patients with chronic venous insufficiency is typically compression therapy, superficial venous surgery, and perforator surgery, which are effective for most patients. When these treatment modalities fail to heal, therapeutic options focus on the deep venous system. Endovascular treatment, such as venous stenting, is indicated for obstructive lesions of the iliac vein, and deep venous reconstructive surgery for valve reflux of the femoral/popliteal vein. Iliac vein stenting has become common in recent years, reportedly yielding the most favorable results.
Introduction
Deep venous intervention has been challenging, as it is performed in only a small number of centers; however, a recent increase in the use of venous stenting has resulted in this therapy becoming more common. Iliac vein stenting is a relatively new method of treatment, but it should be considered as an additional option to conventional compression therapy or common leg vein therapies, such as superficial vein surgery or perforator surgery.
Constructing a proper treatment strategy requires a clear understanding of the pathology, etiology, and knowledge of the indications and limitations of the various therapeutic techniques. With the introduction of new therapeutic methods, it is important to be aware of how these should be combined with conventional therapeutic methods, what methods of examination should be used for assessment, and for what kinds of cases these therapies are indicated. This paper provides an explanation thereof, along with an explanation of the management of patients with chronic venous insufficiency.
Pathology
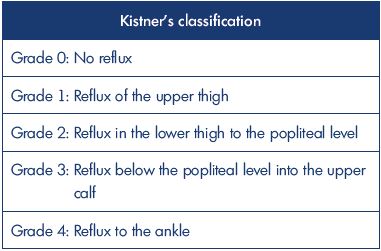
The main pathology of deep venous incompetence is defined as clinical symptoms caused by venous hypertension due to stenosis/obstruction of the iliac vein as well as valve incompetence of the femoral/popliteal veins.1-3 However, in patients with severe chronic venous insufficiency, venous reflux involves deep veins as an isolated abnormality in less than 10% of cases, but it is associated with superficial venous insufficiency and/or perforator incompetence in 46% of cases.1,4 The clinical symptoms of chronic venous insufficiency are classified in the CEAP classification (C0, no clinical signs; C1, telangiectasias or reticular veins; C2, varicose veins; C3, edema; C4a, pigmentation or eczema; C4b, lipodermatosclerosis or atrophic blanche; C5, healed venous ulcer; C6, active venous ulcer).5 These symptoms can also be caused by superficial venous insufficiency or perforator incompetence in isolation, but deep venous incompetence often presents with more severe clinical symptoms (Figure 1A). deep venous incompetence can also be implicated in the refractory varicose vein postoperative recurrence.
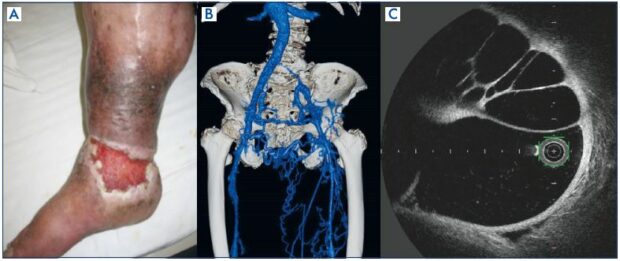
Figure 1. Postthrombotic syndrome.
Panel A. Stasis dermatitis, active ulcer.
Panel B. Computed tomography venography showing the obstruction of the left common iliac vein and the significant developed
collateral veins.
Panel C. Optical frequency domain imaging finding of the common iliac vein: intraluminal fibrotic septum (a honeycomb-like
multichannel form).
The pathology of postthrombotic syndrome, the main subject of this paper, can cause both iliac venous outflow obstruction and deep venous axial reflux and it is the most common form of deep venous incompetence. The pathology is secondary to an episode of deep venous thrombosis and is caused by a venous thrombus, fibrotic change in the chronic phase of deep venous thrombosis, with stenosis/obstruction of venous drainage and reflux due to venous valve damage causing stasis symptoms.6 Only 20% to 30% of iliac vein thrombi completely recanalize with anticoagulation therapy alone, while the remaining veins develop obstruction with variable collateralization.7-9 A fibrotic-changed thrombus may cause narrowing of the intravascular lumen, creating an obstructive lesion (Figure 1B), or form a septum that presents with a honeycomb-like multichannel form in the intravascular lumen (Figure 1C). The venous outflow obstruction causes several paths of collateral circulation to form, resulting in secondary varicosities, which, when reaching the finer venous vessels, results in open arteriovenous channels and formation of arteriovenous fistulae (Figure 2A and 2B). The fibrotic changes also cause valve destruction, resulting in downward venous reflux. Postthrombotic syndrome involves coexisting stenotic/obstructive and regurgitant lesions, and clinical symptoms tend to be especially severe in the presence of obstructions at the iliac vein level and valve incompetence at the femoral vein level. Clinical symptoms include chronic venous insufficiency, as already mentioned, and one known clinical classification is the Villalta scale.10
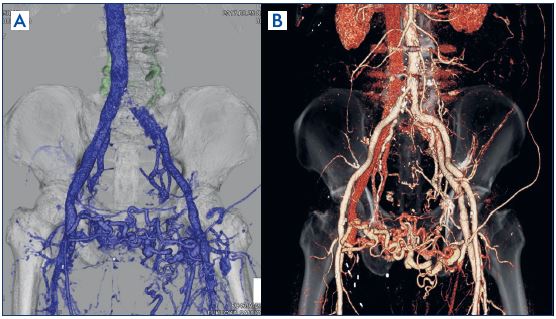
Figure 2. Postthrombotic syndrome.
Panel A. Contrast-enhanced
computed tomography (venous
phase) left common iliac vein
obstruction, much collateral
circulation observed.
Panel B. Contrast-enhanced
computed tomography (arterial
phase); arteriovenous fistula
formation at the site of the
collateral circulation.
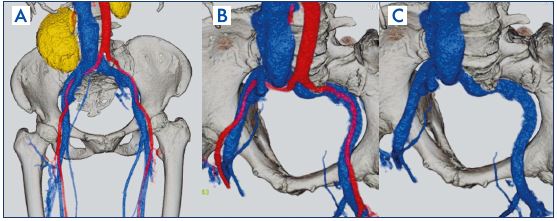
Figure 3. Nonthrombotic iliac vein
lesions case: findings from contrastenhanced
computed tomography.
Panels A and B. The left common
iliac vein is compressed between
the right common iliac artery and
the vertebral body.
Panel C. Same computed
tomography findings (image with
arterial portions subtracted).
Causes of iliac vein stenosis are not limited to thrombi and include being compressed between the iliac artery and a vertebral body, also known as May-Thurner syndrome after aomprehensive reporting from May and Thurner in 1957.11 Later, Raju et al described the same pathology around 2006 as nonthrombotic iliac vein lesions.12 The pathology is thought to present with clinical symptoms of chronic venous insufficiency and provoke deep venous thrombosis, as it involves venous outflow obstruction in the legs due to stenosis of the iliac vein. A common, well-known form is stenosis of the portion where the left common iliac vein is compressed between the right common iliac artery and the fifth lumbar vertebra (Figure 3A-3C). Such stenosis, however, is also reportedly often observed in many healthy individuals13 and the necessity remains a matter of debate.
Less common causes of chronic iliocaval obstruction include tumors, retroperitoneal fibrosis, iatrogenic injury, irradiation, and aneurysms. Primary deep valve incompetence and congenital valve malformation should also be considered as causes of deep venous valve incompetence.
Examination
Assessment points include iliac vein stenosis/obstruction and patency/reflux of the femoral vein, deep femoral vein, and popliteal vein, as well as superficial venous insufficiency and the perforator incompetence.
Ultrasonography Ultrasonography should be the first examination because it is minimally invasive and provides a high volume of information.
Iliac vein level
The assessment point is whether there are findings of stenosis/obstruction. If a deep location makes direct observation impossible, indirect assessment remains possible by using the respiratory maneuvers or the Valsalva maneuvers. As obstruction of the iliac vein results secondarily in collateral circulation at the saphenofemoral junction, thicken collateral paths at the saphenofemoral junction are regarded as a sign to suspect stenosis/obstruction of the iliac vein.
Femoral/popliteal vein level
Assessing reflux necessitates performing the examination in a standing posture. Venous reflux is assessed by compressing the calf, and is deemed positive if the reflux duration exceeds 1 second.14 A fibrotic-changed thrombus, also sometimes partially presenting with calcification, are overall highly echogenic (Figure 4A). Patency/reflux of the deep femoral vein can also be assessed, however, in some cases it may be difficult to investigate due to its deep location. Moreover, adequate great saphenous vein reflux can be observed. With postthrombotic syndrome, it is crucial that there are cases of secondary varicosities resulting merely from significant development of the saphenous veins (with no reflux) as collateral circulation due to deep vein occlusion.
Calf vein level
This will mainly be an assessment of incompetent perforators in the legs. Cases of deep venous incompetence caused by postthrombotic syndrome often have posterior tibial perforator incompetence, and thus assessment of incompetent perforators must be centered on this area. As with the great saphenous vein, reflux of the small saphenous vein is also assessed.
The leg is completely covered with an air-filled cuff, and volume changes in the leg are recorded as numerical values; this offers a completely noninvasive, quantitative assessment of arteriovenous return function in the legs. However, it is not able to differentiate between superficial venous insufficiency and deep venous incompetence or to assess reflux of an incompetent perforator. Additionally, it is difficult to assess the accurate deep venous incompetence from isolation data because the arterial blood flow rate has a major impact on the data.
Computed tomography and magnetic resonance imaging allow for regional assessments of deep vein stenosis/obstruction, as well as the formation of collateral circulation. Even plain computed tomography (no contrast agent is used) offers a certain degree of assessment of deep vein stenosis/obstruction and the aforementioned venous calcification can also be observed (Figure 4B). Contrast-enhanced computed tomography enables a more detailed assessment of stenosis/obstruction lesions. Offsetting the timing of imaging makes it possible to assess both the arterial phase and the venous phase, with stereoscopic image construction making it possible to build an overall image that is easier to understand (Figures 1B, 2, and 3). It is also possible to assess differential diseases, such as retroperitoneal fibrosis, tumors, congenital venous malformations, and arteriovenous fistulae. Similar assessments can also be made with magnetic resonance imaging; however, for the aforementioned reasons computed tomography is of greater utility.
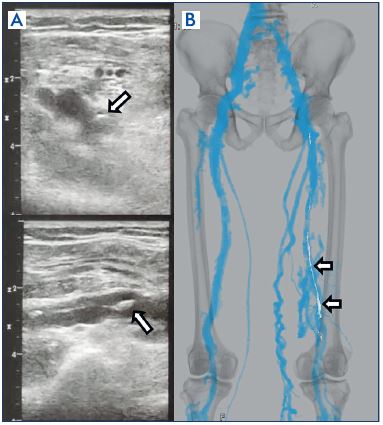
Figure 4. Postthrombotic syndrome.
Panel A. Ultrasound findings: fibrotic changed thrombus of the
popliteal vein, with high echogenicity and a portion (arrow)
presenting with calcification.
Panel B. Plain computed tomography findings: calcification
(arrow) observable in plain computed tomography as well.
Direct imaging of stenotic/obstructive lesions of the iliac vein make it possible to assess lesion sites, check for guide wire passage, and assess the development of collateral circulation. “Pancake-shaped” contrast imaging of stenosis of the iliac vein could potentially be missed with venography alone, hence, stenosis must be assessed from the intravascular lumen by intravascular ultrasound (Figure 5). Not only findings of iliac vein stenosis/obstruction, but also findings of femoral/popliteal vein axial reflux are an important assessment point. To assess femoral/popliteal vein reflux, descending venography is used with the Valsalva maneuver to assess how far the venous reflux descends (Figure 6). One known classification for reflux assessment is the Kistner classification (Table I).16,17 If deep axial reflux has been observed, assessing valve morphology and checking for their presence or absence enables differentiation as to whether the etiology is postthrombotic syndrome or primary deep valve incompetence, as well as congenital venous valve dysplasia/hypoplasia1; it is a crucial examination to perform when deep venous reconstructive surgery is being considered. It is optimal with oblique projection; however, a supine position is also possible. Direct measurement of venous pressure is not of particularly great significance if examination is done in the supine position. Proper valve assessment is not possible if the guide wire or catheter is located inside the femoral vein; therefore, there must be a case-by-case consideration of the ideal puncture site.
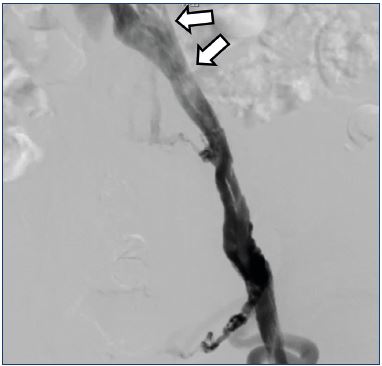
Figure 5. Postthrombotic syndrome.
Ascending venography findings: stenosis at the common iliac
vein, with a “pancake” shape at the arrow.
While the abovementioned examinations are crucial, completely asymptomatic cases that still have obstructive findings in the iliac vein and axial reflux findings in the femoral vein are not uncommon. Perrin has noted that, because various examination findings and a patient’s clinical symptoms may not be correlated, the decision of performing deep venous interventions should be based not only on examination data, but also on the patient’s clinical condition.4
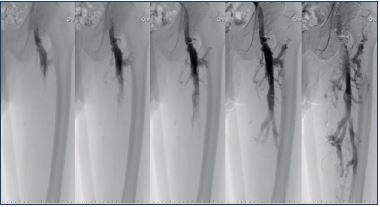
Figure 6. Postthrombotic syndrome.
Descending venography demonstrating axial reflux down to
the knee level.
Treatment
Comparable to compression therapy for ordinary venous diseases, compression therapy with elastic stockings or elastic bandages also lies at the center of treatment for deep venous incompetence. The fundamental role of compression in the treatment of chronic venous insufficiency is well recognized and has been validated by randomized controlled studies.18 Intermittent pneumatic compression pumps can also serve as useful auxiliary means.19 Several symptoms of chronic venous insufficiency improve with rest, elevation of the legs, and utilization of compression therapy. Weight loss, walking exercise, and physical therapy to improve the mobility of the ankle joint are also effective for symptom improvement.9 The pressure from compression with compressive garments in compression therapy must be higher with deep venous incompetence than with superficial venous insufficiency.20 Compression therapy is also a technique that must be performed simultaneously with the various treatments described below.
Even if deep venous incompetence coexists with superficial venous insufficiency or perforator incompetence, superficial vein surgery and perforator surgery should first be considered. These are both simple, but highly effective techniques. In a randomized controlled trial, superficial venous surgery significantly reduced the 12-month ulcer recurrence rate.21 In a subgroup analysis of this study, it was shown that superficial venous surgery might improve venous hemodynamics in legs with venous ulceration despite coexistent deep venous reflux.22 Surgical techniques for superficial venous insufficiency include endovascular ablation, foam sclerotherapy, and stripping. Patency of the femoral vein and popliteal vein is a requirement for performing these treatments. There is still debate on the efficacy of perforator surgery, but surgery on an incompetent perforator has shown favorable results, especially in cases with deep venous incompetence.22 There have been several techniques for eliminating incompetent perforators including the classic Linton procedure (rarely performed now), subfascial endoscopic perforator surgery, and, recently, perforator ablation and foam sclerotherapy.23
Deep venous interventions should be considered if these treatments fail to yield desired results.
Pattern 1: cases of iliac vein obstruction or iliac vein obstruction combined with femoral vein axial reflux
With pattern 1, revascularization of the obstructed iliac vein should be considered, as it is an important venous outflow of the leg veins. An obstructed iliac vein has been previously treated with bypass surgery, as with the Palma-Dale procedure, or surgical revascularization24; however, these are currently rarely performed due to poor long-term outcomes.
They have been replaced by endovascular therapy in the form of stent placement (Figure 7),25,26 with many recent reports showing favorable treatment outcomes.27,28 Despite the absence of objective hemodynamic outcome measures, the clinical efficacy of iliac vein stenting has been proven by alleviation of symptoms, such as pain and swelling, and by the high rate of ulcer healing. Objectively documented swelling is completely alleviated in approximately one-third of limbs and is significantly improved in others. About 50% of patients have complete relief of pain following stenting. Median venous clinical severity and disability scores also significantly improved (8.5 to 2 and 2 to 0, respectively).29 Despite the presence of untreated axial reflux, approximately half of ulcerated legs will heal, and stay healed for up to 2 years, following iliac vein stenting.30,31
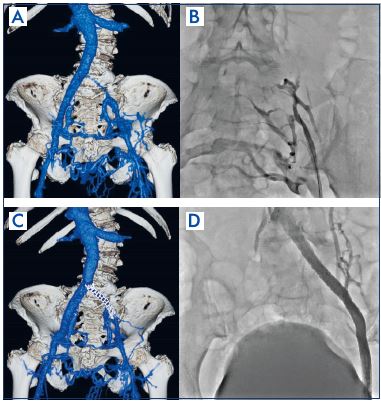
Figure 7. Iliac vein stenting in a postthrombotic syndrome case.
Panel A. Contrast-enhanced computed tomography finding:
before stenting.
Panel B. Ascending venography findings: before stenting.
Panel C. Contrast-enhanced computed tomography finding:
after stenting.
Panel D. Ascending venography findings: after stenting.
A large diameter stent is used: 14 mm to 16 mm at the common iliac vein, 12 mm to 14 mm at the external iliac vein, and 10 mm to 12 mm at the common femoral vein.28 Decision for the site of stent placement and the optimal stent size must take into consideration both findings from venography and intravascular ultrasound (Figure 8).
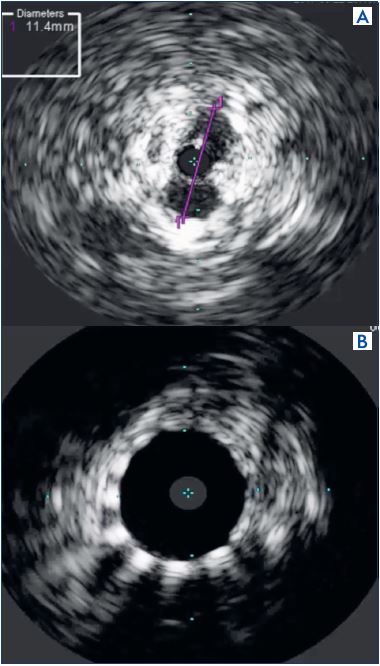
Figure 8. Intravascular ultrasound finding in a postthrombotic
syndrome case.
Panel A. Assessment/measurement of stenotic lesion; left
common iliac vein before stenting.
Panel B. Examining patency after stenting.
Especially with postthrombotic syndrome, fibrotic changes in the thrombus cause the intravascular lumen to have a honeycomb-like multichannel form, or to be completely obstructed, and thus intravascular ultrasound is very useful because the guide wire can be passed through the correct route. Meissner stated that several technical factors may improve patency including the use of adequately sized stents, the routine use of intravascular ultrasound, stenting all areas of disease, and assuring adequate inflow.28 It is well known that stent outcomes are poor if there is postthrombotic syndrome areas at the common femoral vein, and the risk of stent occlusion when extending stents across the inguinal ligament reportedly increases 3.8-fold.27 Femoral endovectomy is also performed at the same time as stenting, while conserving the deep femoral vein, which is recognized as an important inflow.32
The mortality rate following venous stenting is zero, with a morbidity rate of 1%. Cumulative secondary patency rates are about 90% at 4 to 6 years, and late occlusions are rare.29,30 Several patients have been followed for 5 years or more without precipitous deterioration of stent patency or clinical efficacy. However, limbs with postthrombotic syndrome fare significantly worse after stenting than those with nonthrombotic disease (primary, assisted-primary, and secondary cumulative patency rates at 36 months of 65%, 85%, and 88% with postthrombotic syndrome vs 89%, 100% and 100% with nonthrombotic disease).30,31
The data regarding the adjunct use of antiplatelet agents and anticoagulants in venous interventions is substantially less robust. Antiplatelet agents are likely most appropriate for patients with nonthrombotic disease, while anticoagulants likely have a greater role in postthrombotic disease, but there is no evidence yet.28 However, given the absence of evidence supporting their use, long-lasting anticoagulant therapy is regarded as necessary for cases with a previous history of deep venous thrombosis.
Pattern 2: Femoral vein axial reflux in isolation or if symptoms persist after stenting in patients with pattern 1
The treatment aims to surgically repair femoral/popliteal vein reflux. The most common etiology is postthrombotic syndrome (60% to 85% of cases), where the valve is completely or partially damaged by the postthrombotic fibrotic changes. Primary deep valve incompetence is less frequent, where the valve cusps are present, but malfunctioning; the abnormal, free edge of each valve results in valve prolapse, dilatation of the annulus with a widening of the commissures.1 A very rare cause of reflux is the congenital valve aplasia. Indication for deep venous reconstructive surgery is severe chronic venous insufficiency (C4b-C6) not controlled by any treatment modality (Figure 9) in patients with deep axial reflux (grade 3to 4 according to Kistner classification.1,4 Various surgeries have previously been performed; the surgical technique is selected in accordance with the etiology and the condition of the valve.
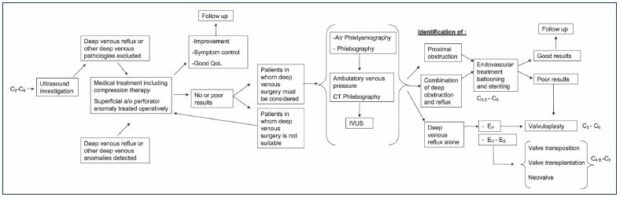
Figure 9. Diagram of the therapeutic strategy for deep vein insufficiency.
From reference 1: Maleti O, Perrin M. Eur J Vasc Endovasc Surg. 2011;41(6):837-848. © 2011, European Society for Vascular
Surgery.
Known as the “Kistner method,” this technique involves making a phlebotomy to expose the incompetent valve to reapproximate the valve leaflets.33 The whole valve apparatus is visualized, and the advantage is that the cusp can be repaired even with left/right asymmetry. The difficulty, however, is that valve competence cannot be checked before proximal clamp release.
With no phlebotomy, this technique involves transmural suturing of the valve commissure,34 and, in some cases, an angioscopy may be used (Figure 10). Though there is an advantage in that no phlebotomy is made, the repair is difficult and less precision.
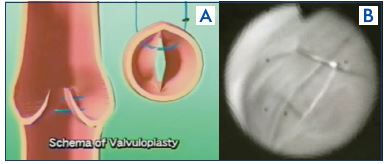
Figure 10. External valvuloplasty.
Panel A. External valvuloplasty schema.
Panel B. Transmural suturing with angioscopic supervision.
The use of an external sleeve of Dacron or PTFE wrapped around the incompetent valve has been applied to correct reflux.35 Despite an advantageously simple technique, there is a potential risk of vein lumen narrowing. This procedure can be used alone or in association with other reconstructive techniques.
The principle behind axillary vein transplants consists of inserting a segment of a competent valvulated vein in the incompetent deep venous network.36 The donor segment can be the axillary vein or brachial vein. However, 40% of axillary veins also have valve incompetence, and there is a high risk of early occlusion.
If the ipsilateral great saphenous vein or deep femoral vein has a proximal competent valve and adequate caliber, the transfer of a femoral vein distal to the competent valve can be performed (Figure 11).37 The advantage is that there is no direct action on the valve apparatus.
Neovalve
The neovalve technique is obtained by dissecting the vein wall to create a flap, which is positioned as a monocuspid or bicuspid valve.38 Since the principle of this procedure is creating a new valve in the deep venous system, this technique can not only be employed for postthrombotic syndrome, but also for primary deep valve incompetence and valve aplasia (Figure 12). The disadvantage of this procedure is that the technique cannot be standardized; it depends on the anatomical condition of the wall and, therefore, the most suitable option is decided only after phlebotomy.
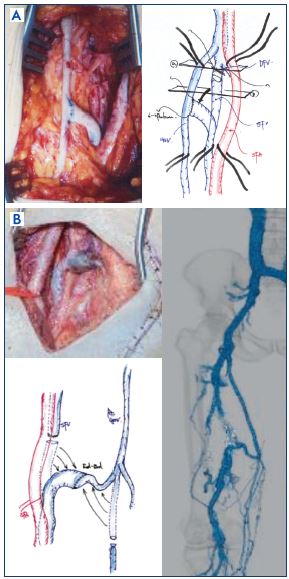
Figure 11. Femoral transposition.
The transfer of a femoral vein distal to the competent valve.
Panel A. Termino-lateral anastomosis of the femoral vein into
great saphenous vein (picture and scheme).
Panel B. End-to-end anastomosis of the femoral vein into the
great saphenous vein (picture, scheme, and contrast-enhanced
computed tomography finding).
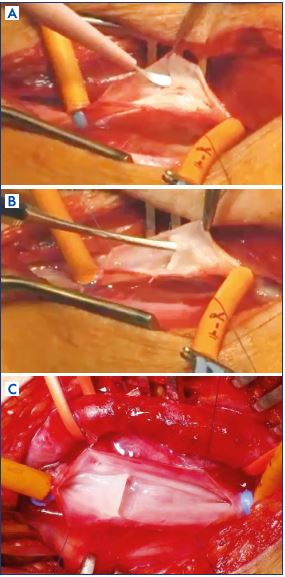
Figure 12. The Neovalve (monocusp).
Panel A. Neovalve in postthrombotic syndrome: partial incision
and parietal dissection.
Panel B. Neovalve in postthrombotic syndrome: dissecting the
vein wall to create a flap.
Panel C. Neovelve in primary deep valve incompetence.
It is difficult to evaluate the results of deep venous reconstructive surgery and, generally, the outcomes are based on pain relief, absence of ulcer recurrence, and restored valve competence.
In terms of outcomes of valvuloplasty for primary deep valve incompetence, clinical outcome and valve competence generally show an excellent correlation, and the 5-year follow-up surgical success rate has been around 70%.39-42
For postthrombotic syndrome, an axillary vein transplant has a clinical outcome of 33% to 82%, with valve competence of 16% to 87%,40,41,43,44 while, for femoral vein transposition, these figures are 50% to 70% and 40% to 77%, respectively.39,40,44 The neovalve technique has an 83% ulcer healing rate, with success rates in terms of valve competence of 68% (monocuspid) and 100% (bicuspid).45
In cases of primary deep valve incompetence, the recommended technique is internal valvuloplasty for the majority of authors.39,40,44 In postthrombotic syndrome, the techniques to be used, in order of recommendation, are femoral vein transposition, neovalve, and axillary vein transplant.1,4 Issues with deep venous reconstructive surgery include the difficulty of the technique, case-by-case differences in the extent of deformation and damage to the valve, differences in technique between a postthrombotic syndrome, a primary deep valve incompetence, or a congenital etiology, and the lack of long-term outcomes data; thus, the procedure is performed only in specialized and highly trained centers.
Conclusion
The severe and refractory clinical symptoms of chronic venous insufficiency are often correlated with deep venous incompetence. With these treatments for chronic venous insufficiency, each of the techniques do not exist on their own, but consideration must also be given to understanding the etiology, hemodynamics, and pathophysiology, and assessing all venous systems to consider the indications for therapy and the timing of treatment. For deep venous interventions, long-term anticoagulant therapy must be considered in most cases. Therefore, the interventions should be decided on only after careful consideration.

REFERENCES
1. Maleti O, Perrin M. Reconstructive surgery for deep vein reflux in the lower-limbs: techniques, results and indications. Eur J Vasc Endovasc Surg. 2011;41(6):837-848.
2. Prandoni P, Villalta S, Polistena P, et al. Symptomatic deep-vein thrombosis and the post-thrombotic syndrome. Haematologica. 1995;80(suppl 2):42- 48.
3. Ginsberg JS, Turkstra F, Buller HR, et al. Postthrombotic syndrome after hip or knee arthroplasty: a cross-sectional study. Arch Intern Med. 2000;160(5):669-672.
4. Perrin M. Surgery for deep venous reflux in the lower-limb. J Mal Vasc. 2004;29(2):73-87.
5. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders consensus statement. J Vasc Surg. 2004;40:1248- 1252.
6. Meissner MH, Manzo RA, Bergelin RO, et al. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18(4):596-605.
7. Akesson H, Brudin L, Dahlstrom JA, Eklof B, Ohlin P, Plate G. Venous function assessed during a 5-year period after acute iliofemoral venous thrombosis treated with anticoagulation. Eur J Vasc Surg. 1990;4:43-48.
8. Johnson BF, Manzo RA, Bergelin RO, Strandness DE. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower-limb deep vein thrombosis: a one- to six- year follow-up. J Vasc Surg. 1995;21:307-313.
9. Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg. 2007;46:68S-83S.
10. Villalta S, Bagatella P, Piccioli A, et al. Assessment of validity and reproducibility of a clinical scale for the post-thrombotic syndrome (abstract). Haemostasis. 1994;24:158a.
11. May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427.
12. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143;144.
13. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39:937-943.
14. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53:2-48.
15. Christopoulos DC, Nicolaides AN, Szendro G, et al. Air-plethysmography and the effect of elastic compression on venous hemodynamics of the leg. J Vasc Surg. 1987,5:148-159.
16. Ferris EB, Kistner RL. Femoral vein reconstruction in the management of chronic venous insufficiency. A 14-year experience. Arch Surg. 1982;117(12):1571-1579.
17. Ackroyd JS, Lea Thomas M, Browse NL. Deep vein reflux: an assessment by descending phlebography. Br J Surg. 1986;73(1):31-33.
18. Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression bandages and stocking for venous leg ulcers. Cochrane Database Syst Rev. 2000;(2):CD000265.
19. Kumar S, Samraj K, Nirujogi V, Budnik J, Walker MA. Intermittent pneumatic compression as an adjuvant therapy in venous ulcer disease. J Tissue Viability. 2002;12:42-44.
20. Partsch H, Flour M, Smith PC; International Compression Club. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Int Angiol. 2008;27(3):193-219.
21. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trial. Lancet. 2004;363:1854-1859.
22. Gohel MS, Barwell JR, Earnshaw JJ, et al. Randomized clinical trial of compression plus surgery versus compression alone in chronic venous ulceration (ESCHAR study)–haemodynamic and anatomical changes. Br J Surg. 2005;92:291-297.
23. Masuda EM, Kessler DM, Lurie F, Puggioni A, Kistner RL, Eklof B. The effect of ultrasound-guided sclerotherapy of incompetent perforator veins on venous clinical severity and disability scores. J Vasc Surg. 2006;43(3):551-557.
24. Jost CJ, Gloviczki P, Cherry KJ Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320-327.
25. Gloviczki P, Cho JS. Surgical treatment of chronic occlusions of the iliocaval veins. In: Rutherford RB, eds. Rutherford’s Vascular Surgery. 6th ed. Philadelphia, US: Elsevier; 2005:2303-2320.
26. Kalra M, Gloviczki P, Andrews JC, et al. Open surgical and endovascular treatment of superior vena cava syndrome caused by nonmalignant disease. J Vasc Surg. 2003;38:215-223.
27. Neglen P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979-990.
28. Meissner MH. Indications for platelet aggregation inhibitors after venous stents. Phlebology. 2013;28:91-98.
29. Hartung O, Otero A, Boufi M, et al. Midterm results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138- 1144.
30. Neglen P. Endovascular treatment of chronic iliofemoral venous obstruction – a review. Phlebolymphology. 2003;43:204-211.
31. Raju S, Owen S Jr, Neglen P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8-15.
32. Vogal D, Comerota AJ, Al-Jabouri M, Assi ZI. Common femoral endovenectomy with iliocaval endoluminal recanalization improves symptoms and quality of life in patients with postthrombotic iliofemoral obstruction. J Vasc Surg. 2012;55:129- 135.
33. Kistner RL. Surgical repair of a venous valve. Straub Clin Proc. 1968; 24:41-43.
34. Gloviczki P, Merrell SW, Bower TC. Femoral vein valve repair under direct vision without venotomy: a modified technique with use of angioscopy. J Vasc Surg. 1991;14:645-648.
35. Hallberg D. A method for repairing incompetent valves in deep veins. Acta Chir Scand. 1972;138:143-145.
36. Raju S, Neglén P, Doolittle J, et al. Axillary vein transfer in trabeculated postthrombotic veins. J Vasc Surg. 1999;29:1050-1064.
37. Kistner RL, Sparkuhl MD. Surgery in acute and chronic venous disease. Surgery. 1979;85:31-43.
38. Maleti O, Lugli M. Neovalve construction in postthrombotic syndrome. J Vasc Surg. 2006;43:794-799.
39. Masuda EM, Kistner RL. Long-term results of venous valve reconstruction: a four to twenty-one year follow-up. J Vasc Surg. 1994;19:391-403.
40. Perrin M. Reconstructive surgery for deep venous reflux. A report on 144 cases. Cardiovasc Surg. 2000;8:246-255.
41. Raju S, Fredericks R, Neglen P, Bass JD. Durability of venous valve reconstruction for primary and post-thrombotic reflux. J Vasc Surg. 1996;23:357-367.
42. Rosales A, Slagsvold CE, Kroese AJ, Stranden E, Risum O, Jorgensen JJ. External venous valveplasty (EVVp) in patients with primary chronic venous insufficiency (PVCI). Eur J Vasc Endovasc Surg. 2006;32:570-576.
43. Rosales A, Jorgensen JJ, Slagsvold CE, Stranden E, Risum O, Kroese AJ. Venous valve reconstruction in patients with secondary chronic venous insufficiency. Eur J Vasc Endovasc Surg. 2008;36:466- 472.
44. Sottiurai VS. Current surgical approaches to venous hypertension and valvular reflux. Int J Angiol. 1996;5:49-54.
45. Lugli M, Guerzoni S, Garofalo M, Smedile G, Maleti O. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49:156-162.